Evaluation of the Sex-and-Age-Specific Effects of PM2.5 on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Air Pollution Measures
2.3. Primary Outcome Measure
2.4. Statistical Analysis
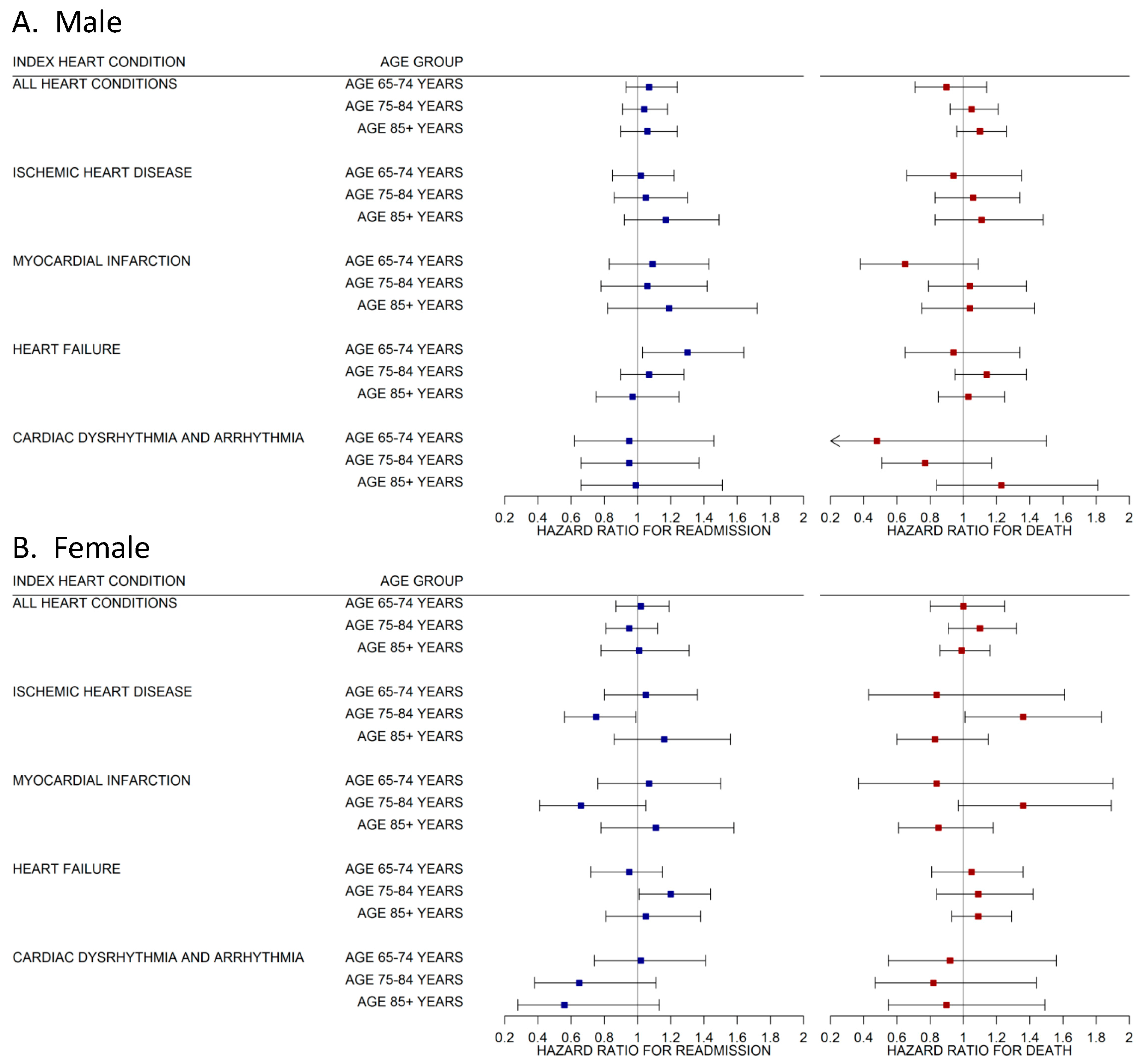
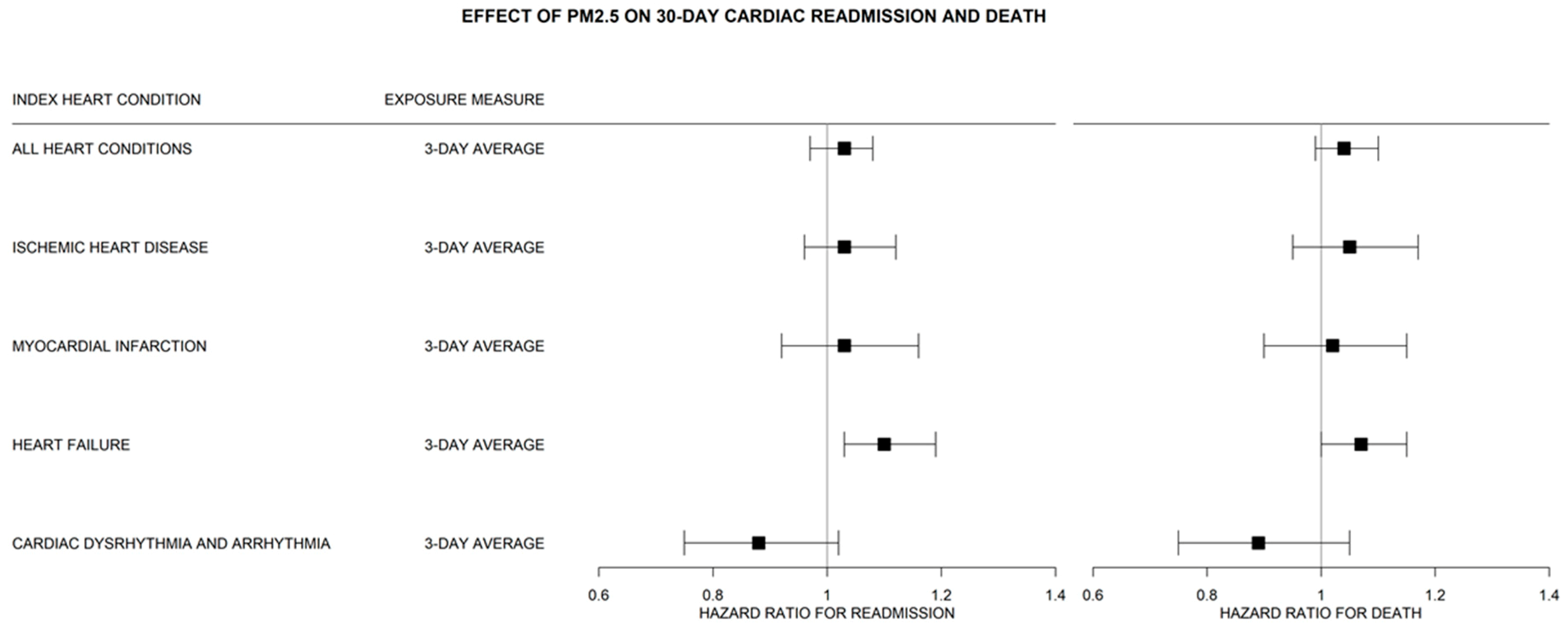
3. Results
Supplementary Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburton, D.E.R.; Bredin, S.S.D.; Shellington, E.M.; Cole, C.; de Faye, A.; Harris, J.; Kim, D.D.; Abelsohn, A. A systematic review of the short-term health effects of air pollution in persons living with coronary heart disease. J. Clin. Med. 2019, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominci, F.; Schwartz, J.D. Air pollution and mortality in the Medicare population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Brook, R.; Pope, C.A., 3rd. Air pollution and cardiovascular disease. Curr. Probl. Cardiol. 2015, 40, 207–238. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A., III; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Renlund, D.G.; Kfoury, A.G.; May, H.T.; Horne, B.D. Relation of heart failure hospitalization to exposure to fine particulate air pollution. Am. J. Cardiol. 2008, 102, 1230–1234. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Shumake, K.L.; Sacks, J.D.; Lee, J.S.; Johns, D.O. Susceptibility of older adults to health effects induced by ambient air pollutants regulated by the European Union and the United States. Aging Clin. Exp. Res. 2013, 25, 3–8. [Google Scholar] [CrossRef]
- Sandström, T.; Frew, A.J.; Svartengren, M.; Viegi, G. The need for a focus on air pollution research in the elderly. Eur. Respir. J. Suppl. 2003, 40, 92s–95s. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Mukherjee, P.S.; Reynoso-Robles, R.; Pérez-Guilléc, B.; Gayosso-Chávez, C.; Torres-Jardón, R.; Cross, J.V.; Ahmed, I.A.M.; Karloukovski, V.V.; et al. Combustion-and-friction derived magnetic air pollution nanoparticles in human hearts. Environ. Res. 2019, 176, 108567. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Y.; Yu, Z.; Lu, R. PM2.5 and cardiovascular diseases in the elderly: An overview. Int. J. Environ. Res. Public Health 2015, 12, 8187–8197. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.D.; Stanek, L.W.; Luben, T.J.; Johns, D.O.; Buckley, B.J.; Brown, J.S.; Ross, M. Particulate matter–induced health effects: Who is susceptible? Environ. Health Perspect. 2011, 119, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; London, S.J.; Chen, G.; Zhang, Y.; Song, G.; Zhao, N.; Jiang, L.; Chen, B. Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The public health and air pollution in Asia (PAPA) Study. Environ. Health Perspect. 2008, 116, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Clougherty, J.E. A growing role for gender analysis in air pollution epidemiology. Environ. Health Perspect. 2010, 118, 167–176. [Google Scholar] [CrossRef]
- Becklake, M.; Kauffmann, F. Gender differences in airway behaviour over the human life span. Thorax 1999, 54, 1119–1138. [Google Scholar] [CrossRef]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among patients in the Medicare Fee-for-Service Program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef]
- Boccuti, C.; Casillas, G. Aiming for Fewer Hospital U-Turns: The Medicare Hospital Readmission Reduction Program. Henry J Kaiser Family Foundation. Available online: https://www.kff.org/medicare/issue-brief/aiming-for-fewer-hospital-u-turns-the-medicare-hospital-readmission-reduction-program (accessed on 10 November 2018).
- Medicare Payment Advisory Commission (U.S.). Report to the Congress: Promoting Greater Efficiency in Medicare; Medicare Payment Advisory Commission: Washington, DC, USA, 2007.
- Barrett, M.L.; Wier, L.M.; Jiana, H.J.; Steiner, C.A. All-Cause Readmissions by Payer and Age, 2009–2013; Healthcare Cost and Utilization Project: Rockville, MD, USA, 2015.
- McIlvennan, C.K.; Eapen, Z.J.; Allen, A.A. Hospital Readmissions Reduction Program. Circulation 2015, 13, 1796–1803. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Koton, S.; Molshatzi, N.; Yuval; Meyers, V.; Broday, D.M.; Drory, Y.; Steinberg, D.M.; Gerber, Y. Cumulative exposure to particulate matter air pollution and long-term post-myocardial infarction outcomes. Prev. Med. 2013, 57, 339–344. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Cao, Y.; Song, J.; Huang, C.; Xiang, X.; Li, M.; Hu, Y. Fine particulate air pollution and hospital admissions and readmissions for acute myocardial infarction in 26 Chinese cities. Chemosphere 2018, 192, 282–288. [Google Scholar] [CrossRef]
- Von Klot, S.; Peters, A.; Aalto, P.; Bellander, T.; Berglind, N.; D’Ippoliti, D.; Elosua, R.; Hörmann, A.; Kulmala, M.; Lanki, T.; et al. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation 2005, 112, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. Particulate air pollution, progression, and survival after myocardial infarction. Environ. Health Perspect. 2007, 115, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.O.; Jones, P.G.; Chan, P.S.; Peri-Okonny, P.A.; Hejjaji, V.; Spertus, J.A. Association of long-term exposure to particulate matter and ozone with health status and mortality in patients after myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005598. [Google Scholar] [CrossRef]
- Berry, S.D.; Ngo, L.; Samelson, E.J.; Kiel, D.P. Competing risk of death: An important consideration in studies of older adults. J. Am. Geriatr. Soc. 2010, 58, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Ellimoottil, C.; Khouri, R.K.; Apoorv, D.; Hou, H.; Miller, D.C.; Dupree, J.M. An opportunity to improve Medicare’s planned readmission measure. J. Hosp. Med. 2017, 12, 840–842. [Google Scholar] [CrossRef] [PubMed]
- AQS Data Mart. United States Environmental Protection Agency. Available online: https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html (accessed on 24 February 2017).
- Pirozzi, C.S.; Jones, B.E.; VanDerslice, J.A.; Zhang, Y.; Paine, R., 3rd; Dean, N.C. Short-term air pollution and incident pneumonia. A case–crossover study. Ann. Am. Thorac. Soc. 2018, 15, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Climate Data Online. National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/cdo-web/ (accessed on 25 September 2018).
- Finder, A.F. Advanced Search. Available online: https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t (accessed on 5 January 2018).
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in healthy aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 640–649. [Google Scholar] [CrossRef]
- Limaye, V.; Patz, J. Climate change and health risks to the elderly from heat and air pollution. Gerontologist 2015, 55, 460. [Google Scholar]
- Orru, H.; Ebi, K.L.; Forsberg, B. The interplay of climate change and air pollution on health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.M.; Naik, V.; Leibensperger, E.M. Air quality and climate connections. J. Air Waste Manag. Assoc. 2015, 65, 645–685. [Google Scholar] [CrossRef] [PubMed]
- Pallin, D.J.; Allen, M.B.; Espinola, J.A.; Camargo, C.A., Jr.; Bohan, J.S. Population aging and emergency departments: Visits will not increase, lengths-of-stay and hospitalizations will. Health Aff. Millwood 2013, 32, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Pallin, D.J.; Espinola, J.A.; Camargo, C.A. US population aging and demand for inpatient services. J. Hosp. Med. 2014, 9, 193–196. [Google Scholar] [CrossRef] [PubMed]
| No. of Admissions | 30,510 |
|---|---|
| No. of Individuals | 19,602 |
| Age Group | |
| 65–74 years | 9148(29.98%) |
| 75–84 years | 13,954(45.74%) |
| 85 years and older | 7408(24.28%) |
| Sex | |
| Male | 15,411(50.51%) |
| Female | 15,099(49.49%) |
| Index Admission (not mutually exclusive) | |
| Myocardial Infarction | 4077 |
| Heart Failure | 8378 |
| Ischemic Heart Disease | 11,964 |
| Cardiac Dysrhythmia and Arrhythmia | 6146 |
| Dual Enrollment Status | |
| Yes | 1459(4.78%) |
| No | 29,051(95.22%) |
| Charlson Comorbidity Index Category | |
| 0 | 8832(28.95%) |
| 1 | 7197(23.59%) |
| 2+ | 14,481(47.46%) |
| Maximum Daily Temperature | |
| Mean (Std dev) (degrees F) | 58.03(18.76) |
| Range (degrees F) | 13.98–98.05 |
| Median Household Income | |
| Mean (Std dev) (US dollars) | 49,572.83(11,363.21) |
| Range (US dollars) | 22,219.00–87,515.00 |
| PM2.5 Measure | |
| Lag 0 | |
| Mean (Std dev) (μg/m3) | 10.96(10.45) |
| Range (μg/m3) | 0.05–97.68 |
| Lag 1 | |
| Mean (Std dev) (μg/m3) | 10.95(10.43) |
| Range (μg/m3) | 0.05–10.43 |
| 3-Day Average | |
| Mean (Std dev) (μg/m3) | 10.96(9.60) |
| Range (μg/m3) | 0.05–97.68 |
| 7-Day Average | |
| Mean (Std dev) (μg/m3) | 10.79(10.43) |
| Range (μg/m3) | 0.39–79.50 |
| Duration | |
| Mean(Std dev) (days) | 28.07(7.62) |
| Range(days) | 1–30 |
| Outcome | |
| Cardiac Readmission | 2032(6.75%) |
| Other Cause Readmission | 2587(8.48%) |
| Death | 1420(4.65%) |
| No Readmission | 24,471(80.12%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiser, C.L.; Smith, K.R.; VanDerslice, J.A.; Glotzbach, J.P.; Farrell, T.W.; Hanson, H.A. Evaluation of the Sex-and-Age-Specific Effects of PM2.5 on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009. J. Clin. Med. 2019, 8, 2114. https://doi.org/10.3390/jcm8122114
Leiser CL, Smith KR, VanDerslice JA, Glotzbach JP, Farrell TW, Hanson HA. Evaluation of the Sex-and-Age-Specific Effects of PM2.5 on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009. Journal of Clinical Medicine. 2019; 8(12):2114. https://doi.org/10.3390/jcm8122114
Chicago/Turabian StyleLeiser, Claire L, Ken R Smith, James A VanDerslice, Jason P Glotzbach, Timothy W Farrell, and Heidi A Hanson. 2019. "Evaluation of the Sex-and-Age-Specific Effects of PM2.5 on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009" Journal of Clinical Medicine 8, no. 12: 2114. https://doi.org/10.3390/jcm8122114
APA StyleLeiser, C. L., Smith, K. R., VanDerslice, J. A., Glotzbach, J. P., Farrell, T. W., & Hanson, H. A. (2019). Evaluation of the Sex-and-Age-Specific Effects of PM2.5 on Hospital Readmission in the Presence of the Competing Risk of Mortality in the Medicare Population of Utah 1999–2009. Journal of Clinical Medicine, 8(12), 2114. https://doi.org/10.3390/jcm8122114






