Encouraging Split Liver Transplantation for Two Adult Recipients to Mitigate the High Incidence of Wait-List Mortality in the Setting of Extreme Shortage of Deceased Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Recipient Cohort
2.2. Donor Evaluation
2.3. Liver Graft Preparation
2.4. Recipients and Liver Graft Implantation
2.5. Postoperative Care and Statistical Analysis
3. Results
3.1. Clinical Features of Donors
3.2. Outcome Analysis of Recipients
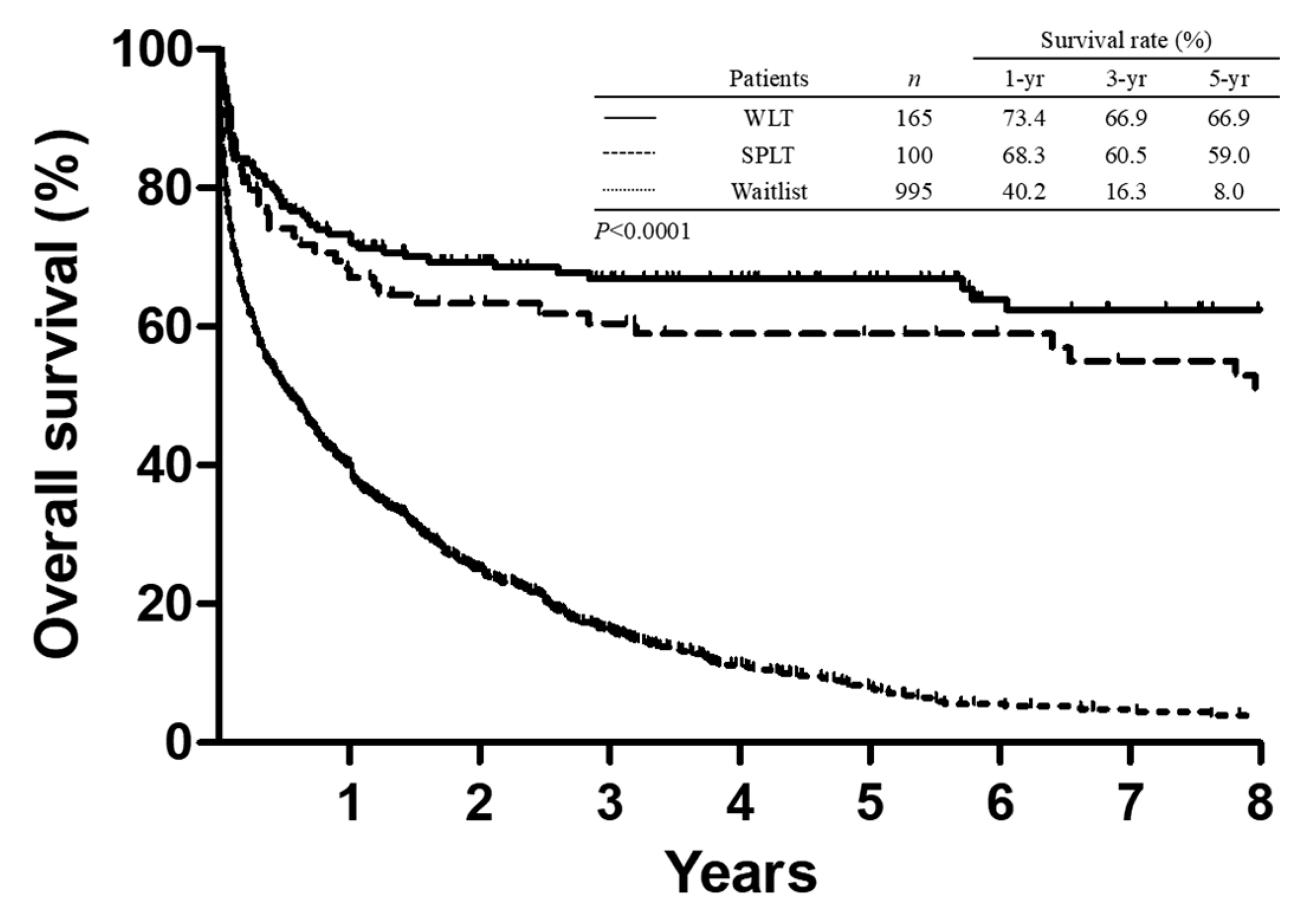
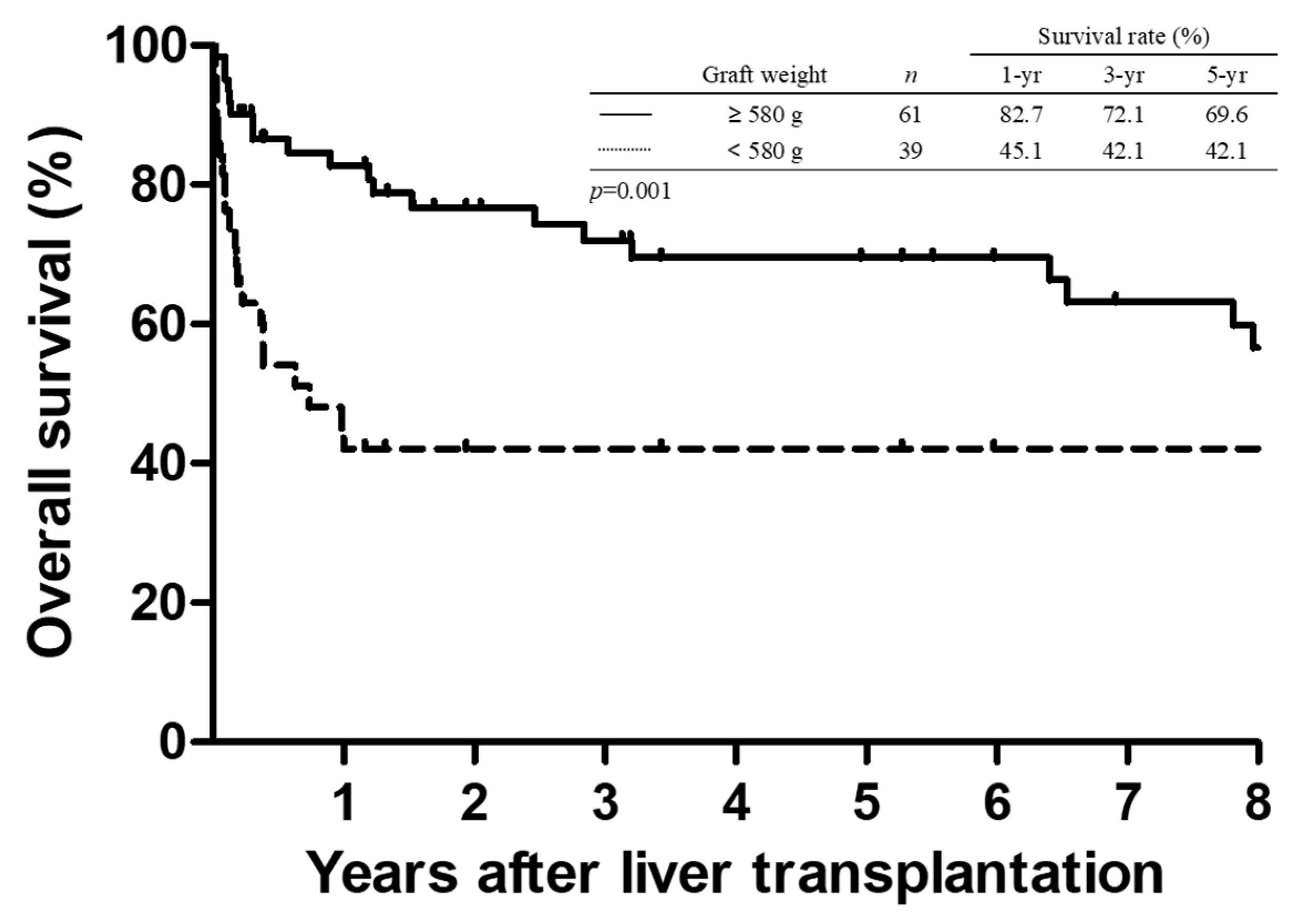
3.3. Survival Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jadlowiec, C.C.; Taner, T. Liver transplantation: Current status and challenges. World J. Gastroenterol. 2016, 22, 4438–4445. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J. An update on liver transplantation: A critical review. J. Autoimmun. 2016, 66, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pichlmayr, R.; Ringe, B.; Gubernatis, G.; Hauss, J.; Bunzendahl, H. Transplantation of a donor liver to 2 recipients (splitting transplantation)—A new method in the further development of segmental liver transplantation. Langenbecks Arch. Chir. 1988, 373, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.C.; Yersiz, H.; Farmer, D.G.; Duffy, J.P.; Ghobrial, R.M.; Nonthasoot, B.; Collins, T.E.; Hiatt, J.R.; Busuttil, R.W. Longterm outcomes for whole and segmental liver grafts in adult and pediatric liver transplant recipients: A 10-year comparative analysis of 2,988 cases. J. Am. Coll. Surg. 2009, 208, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Yersiz, H.; Renz, J.F.; Farmer, D.G.; Hisatake, G.M.; McDiarmid, S.V.; Busuttil, R.W. One hundred in situ split-liver transplantations: A single-center experience. Ann. Surg. 2003, 238, 496–505. [Google Scholar] [CrossRef]
- Mogul, D.B.; Luo, X.; Bowring, M.G.; Chow, E.K.; Massie, A.B.; Schwarz, K.B.; Cameron, A.M.; Bridges, J.F.P.; Segev, D.L. Fifteen-Year Trends in Pediatric Liver Transplants: Split, Whole Deceased, and Living Donor Grafts. J. Pediatr. 2018, 196, 148–153.e2. [Google Scholar] [CrossRef]
- Bismuth, H.; Morino, M.; Castaing, D.; Gillon, M.C.; Descorps Declere, A.; Saliba, F.; Samuel, D. Emergency orthotopic liver transplantation in two patients using one donor liver. Br. J. Surg. 1989, 76, 722–724. [Google Scholar] [CrossRef]
- Colledan, M.; Andorno, E.; Valente, U.; Gridelli, B. A new splitting technique for liver grafts. Lancet 1999, 353, 1763. [Google Scholar] [CrossRef]
- Boillot, O.; Delafosse, B.; Mechet, I.; Boucaud, C.; Pouyet, M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet 2002, 359, 406–407. [Google Scholar] [CrossRef]
- Collett, D.; Friend, P.J.; Watson, C.J. Factors Associated With Short- and Long-term Liver Graft Survival in the United Kingdom: Development of a UK Donor Liver Index. Transplantation 2017, 101, 786–792. [Google Scholar] [CrossRef]
- Feng, S.; Goodrich, N.P.; Bragg-Gresham, J.L.; Dykstra, D.M.; Punch, J.D.; DebRoy, M.A.; Greenstein, S.M.; Merion, R.M. Characteristics associated with liver graft failure: The concept of a donor risk index. Am. J. Transplant. 2006, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Lee, P.C.; Chiang, Y.J. Taiwan’s organ donation and transplantation: Observation from national registry point of view. J. Formos. Med. Assoc. 2017, 116, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Chan, K.M.; Chou, H.S.; Wu, T.J.; Lee, C.F.; Soong, R.S.; Wu, T.H.; Lee, C.S. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann. Surg. 2013, 258, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lee, C.S.; Soong, R.S.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M. Split liver transplantation in adults: Preoperative estimation of the weight of right and left hemiliver grafts. Liver Transplant. 2011, 17, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Urata, K.; Kawasaki, S.; Matsunami, H.; Hashikura, Y.; Ikegami, T.; Ishizone, S.; Momose, Y.; Komiyama, A.; Makuuchi, M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995, 21, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, K.D.; Chan, K.M.; Wu, T.J.; Lee, C.F.; Lee, W.C. Split-liver transplantation in 2 adults: Significance of caudate lobe outflow reconstruction in left lobe recipient: Case report. Transplant. Proc. 2009, 41, 3937–3940. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Lee, W.C. “Left at right” liver transplantation with heterotopic implantation of left liver graft in the right subphrenic space: Reappraisal and technical concerns for decision making. Med. Baltim. 2019, 98, e16415. [Google Scholar] [CrossRef]
- Chan, K.M.; Eldeen, F.Z.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Wu, T.H.; Soong, R.S.; Lee, W.C. “Left at right” adult liver transplantation: The feasibility of heterotopic implantation of left liver graft. Am. J. Transplant. 2012, 12, 1511–1518. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010, 16, 943–949. [Google Scholar] [CrossRef]
- Chan, K.M.; Lee, C.S.; Wu, T.J.; Lee, C.F.; Chen, T.C.; Lee, W.C. Clinical perspective of acute humoral rejection after blood type-compatible liver transplantation. Transplantation 2011, 91, e29–e30. [Google Scholar] [CrossRef]
- Taylor, A.L.; Gibbs, P.; Bradley, J.A. Acute graft versus host disease following liver transplantation: The enemy within. Am. J. Transplant. 2004, 4, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Kabiling, C.S.; Concejero, A.M. Why does living donor liver transplantation flourish in Asia? Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.; Schmidt, K.M.; Susal, C.; Dohler, B.; Zidek, M.; Schlitt, H.J. Split liver transplantation: Current developments. World J. Gastroenterol. 2018, 24, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Quintini, C.; Aucejo, F.N.; Fujiki, M.; Diago, T.; Watson, M.J.; Kelly, D.M.; Winans, C.G.; Eghtesad, B.; Fung, J.J.; et al. Split liver transplantation using Hemiliver graft in the MELD era: A single center experience in the United States. Am. J. Transplant. 2014, 14, 2072–2080. [Google Scholar] [CrossRef]
- Aseni, P.; De Feo, T.M.; De Carlis, L.; Valente, U.; Colledan, M.; Cillo, U.; Rossi, G.; Mazzaferro, V.; Donataccio, M.; De Fazio, N.; et al. A prospective policy development to increase split-liver transplantation for 2 adult recipients: Results of a 12-year multicenter collaborative study. Ann. Surg. 2014, 259, 157–165. [Google Scholar] [CrossRef]
- Merion, R.M.; Rush, S.H.; Dykstra, D.M.; Goodrich, N.; Freeman, R.B., Jr.; Wolfe, R.A. Predicted lifetimes for adult and pediatric split liver versus adult whole liver transplant recipients. Am. J. Transplant. 2004, 4, 1792–1797. [Google Scholar] [CrossRef]
- Renz, J.F.; Emond, J.C.; Yersiz, H.; Ascher, N.L.; Busuttil, R.W. Split-liver transplantation in the United States: Outcomes of a national survey. Ann. Surg. 2004, 239, 172–181. [Google Scholar] [CrossRef]
- Lee, T.C.; Barshes, N.R.; Washburn, W.K.; Halff, G.A.; Carter, B.A.; Karpen, S.J.; Bristow, L.J.; Scott, J.D.; Goss, J.A. Split-liver transplantation using the left lateral segment: A collaborative sharing experience between two distant centers. Am. J. Transplant. 2005, 5, 1646–1651. [Google Scholar] [CrossRef]
- Azoulay, D.; Castaing, D.; Adam, R.; Savier, E.; Delvart, V.; Karam, V.; Ming, B.Y.; Dannaoui, M.; Krissat, J.; Bismuth, H. Split-liver transplantation for two adult recipients: Feasibility and long-term outcomes. Ann. Surg. 2001, 233, 565–574. [Google Scholar] [CrossRef]
- Busuttil, R.W.; Goss, J.A. Split liver transplantation. Ann. Surg. 1999, 229, 313–321. [Google Scholar] [CrossRef]
- Rogiers, X.; Sieders, E. Split-liver transplantation: An underused resource in liver transplantation. Transplantation 2008, 86, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.; Urbach, D.R.; Kumar, M.; Li, Q.; Murray, B.J.; Juurlink, D.; Kennedy, E.; Gagliardi, A.; Sutradhar, R.; Baxter, N.N. Outcomes of Daytime Procedures Performed by Attending Surgeons after Night Work. N. Engl. J. Med. 2015, 373, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.M.; Keohane, C.A.; Rogers, S.; Gardner, R.; Lipsitz, S.R.; Salzberg, C.A.; Yu, T.; Yoon, C.S.; Williams, D.H.; Wien, M.F.; et al. Risks of complications by attending physicians after performing nighttime procedures. JAMA 2009, 302, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Kashfi, A.; Cattral, M.S.; Selzner, N.; Greig, P.D.; Lilly, L.; McGilvray, I.D.; Therapondos, G.; Adcock, L.E.; Ghanekar, A.; et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transplant. 2009, 15, 1776–1782. [Google Scholar] [CrossRef]
- Broering, D.C.; Wilms, C.; Lenk, C.; Schulte, E.J., 2nd; Schonherr, S.; Mueller, L.; Kim, J.S.; Helmke, K.; Burdelski, M.; Rogiers, X. Technical refinements and results in full-right full-left splitting of the deceased donor liver. Ann. Surg. 2005, 242, 802–812. [Google Scholar] [CrossRef]
- Humar, A.; Ramcharan, T.; Sielaff, T.D.; Kandaswamy, R.; Gruessner, R.W.; Lake, J.R.; Payne, W.D. Split liver transplantation for two adult recipients: An initial experience. Am. J. Transplant. 2001, 1, 366–372. [Google Scholar] [CrossRef]
- Wang, F.; Pan, K.T.; Chu, S.Y.; Chan, K.M.; Chou, H.S.; Wu, T.J.; Lee, W.C. Preoperative estimation of the liver graft weight in adult right lobe living donor liver transplantation using maximal portal vein diameters. Liver Transplant. 2011, 17, 373–380. [Google Scholar] [CrossRef]
- Zambelli, M.; Andorno, E.; De Carlis, L.; Rossi, G.; Cillo, U.; De Feo, T.; Carobbio, A.; Giacomoni, A.; Bottino, G.; Colledan, M. Full-right-full-left split liver transplantation: The retrospective analysis of an early multicenter experience including graft sharing. Am. J. Transplant. 2012, 12, 2198–2210. [Google Scholar] [CrossRef]
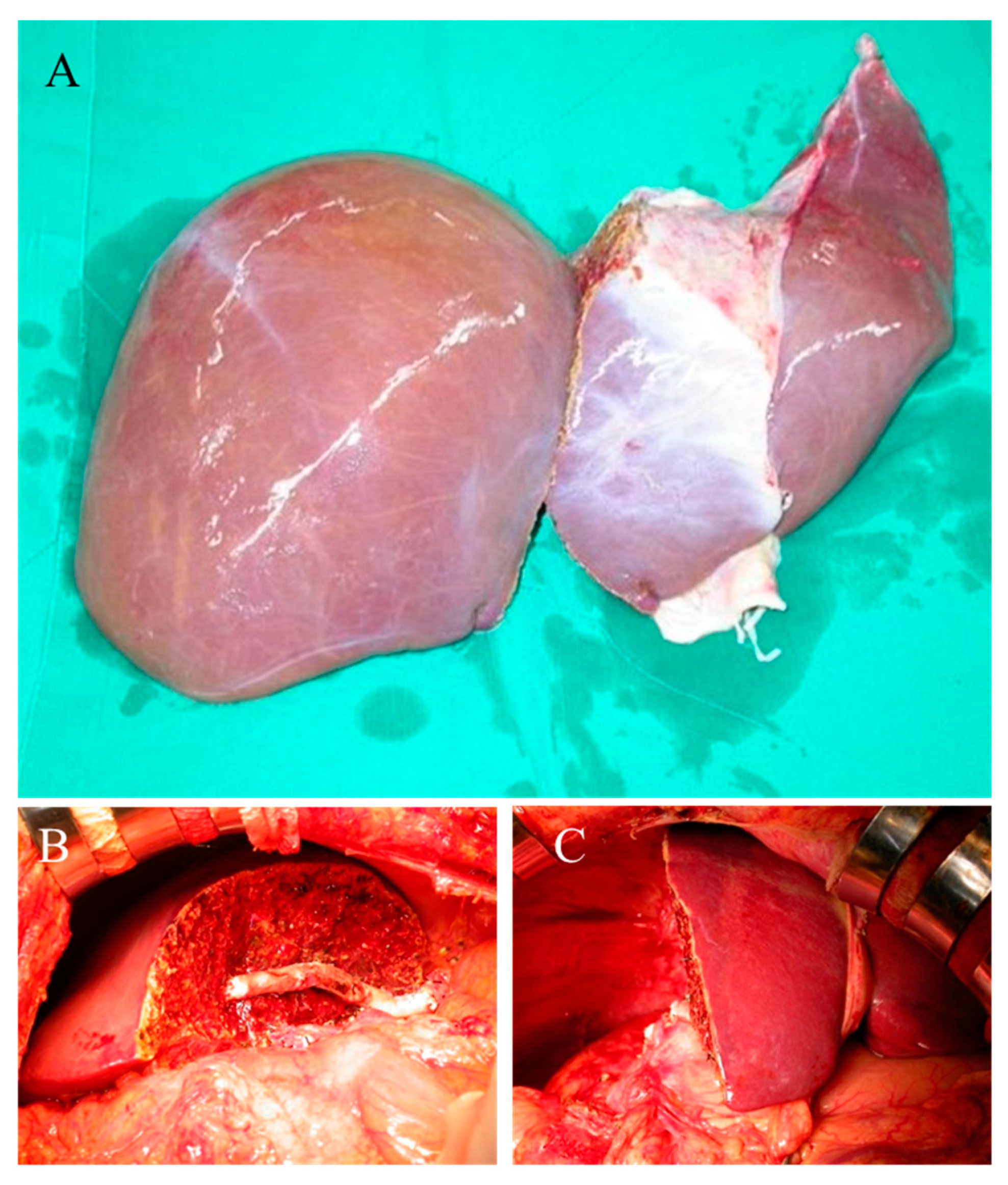
| Total Donors | n = 54 |
|---|---|
| Male:female | 39:15 |
| Age (years) | 27 (15–53) |
| Body height (cm) | 170 (150–187) |
| Body weight (kg) | 67 (45–97) |
| Estimated standard liver volume (mL) | 1274 (929–1533) |
| Actual liver graft weight (gm) * | 1380 (990–2100) |
| Discrepancy of graft estimation (%) | –4.5% (–32.8%–37.1%) |
| Hospital stay (days) | 5 (1–36) |
| CPCR history | 9 |
| Cause of brain death | |
| CVA | 29 |
| Traumatic head injury | 17 |
| Others | 8 |
| Graft recovery | |
| In the institute (n = 45) | |
| Two hemi-live graft | 41 (82 hemi-liver graft) |
| One hemi-liver graft | 4 hemi-liver graft |
| Other institute (n = 9) | |
| Two hemi-liver graft | 5 (10 hemi-liver graft) |
| One hemi-liver graft | 4 hemi-liver graft |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Characteristics | Yes, n = 21 | No, n = 79 | p-Value | OR (95% CI) | p-Value |
| Donor factors | |||||
| Age, median (range) | 32 (15–49) | 27 (15–53) | 0.611 | — | |
| Sex (Male:Female) | 13:8 | 62:17 | 0.119 | — | |
| Cause of brain death | |||||
| CVA | 16 (76.2) | 58 (73.4) | 0.848 | — | |
| Traumatic head injury | 4 (19.0) | 13 (16.5) | |||
| Others | 1 (4.8) | 8 (10.1) | |||
| Hospital stay (days) | 5 (1–36) | 5 (1–36) | 0.835 | — | |
| Graft factors | |||||
| Graft type | 0.004 | 1.30 (0.31–5.38) | 0.710 | ||
| Left hemi-liver | 16 (76.2) | 32 (40.5) | |||
| Right hemi-liver | 5 (23.8) | 47 (59.5) | |||
| Graft weight (gm) | 520 (335–1100) | 695 (350–1500) | 0.004 | 0.99 (0.98–0.99) | 0.036 |
| Cold ischemia time (min) | 474 (102–763) | 337 (69–779) | 0.207 | — | |
| Warm ischemia time (min) | 39 (31–57) | 45 (27–65) | 0.116 | — | |
| Recipient factors | |||||
| Age, median (range) | 49 (39–65) | 51 (33–65) | 0.929 | — | |
| Sex (Male:Female) | 12:9 | 51:28 | 0.532 | — | |
| Main indication of transplantation | 0.353 | — | |||
| Alcoholic Liver cirrhosis | 3 (14.3) | 20 (25.3) | |||
| Virus-related liver cirrhosis | 7 (33.3) | 24 (30.4) | |||
| Hepatocellular carcinoma | 5 (23.8) | 24 (30.4) | |||
| Others | 6 (28.6) | 11 (13.9) | |||
| Viral hepatitis | 0.902 | — | |||
| HBV | 10 (47.6) | 35 (44.3) | |||
| HCV | 4 (19.0) | 15 (19.0) | |||
| HBV + HCV | 0 | 2 (2.5) | |||
| None | 7 (33.3) | 27 (34.2) | |||
| Child classification | 0.220 | — | |||
| A, B | 13 (61.9) | 37 (46.8) | |||
| C | 8 (38.1) | 42 (53.2) | |||
| Chronic kidney disease staging | 0.462 | — | |||
| Grade 1,2 | 14 (66.7) | 59 (74.7) | |||
| Grade 3,4,5 | 7 (33.3) | 20 (25.3) | |||
| Hemodialysis before transplantation | 3 (14.3) | 5 (6.3) | 0.232 | — | |
| MELD score, median (range) | 18 (7–42) | 19 (7–45) | 0.842 | — | |
| Biochemical factors | |||||
| Sodium (Na, ng/mL) | 137 (121–151) | 138 (124–152) | 0.176 | — | |
| Total Bilirubin (ng/mL) | 3.7 (0.5–34.3) | 4.1 (0.5–38.2) | 0.956 | — | |
| Neutrophil-lymphocyte ratio | 4.4 (2.3–47.0) | 5.3 (0.9–169.7) | 0.545 | — | |
| GRWR | 1.0 (0.8–2.0) | 1.1 (0.6–2.0) | 0.054 | 4.14 (0.27–62.90) | 0.305 |
| Transplantation factors | |||||
| Transplantation period | 0.362 | — | |||
| Before 2008 | 8 (38.1) | 22 (27.8) | |||
| After 2008 (2008/01/01) | 13 (61.9) | 57 (72.2) | |||
| Time of Transplantation | 0.588 | — | |||
| Daytime transplantation | 18 (85.7) | 71 (89.9) | |||
| Nighttime transplantation | 3 (14.3) | 8 (10.1) | |||
| Total operation time (hours) | 10.9 (5.1–18.0) | 10.2 (5.6–15.5) | 0.681 | — | |
| Operative blood loss (L) | 3.0 (0.2–18.7) | 2.0 (0.1–19.0) | 0.130 | — | |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Characteristics | Yes, n = 31 | No, n = 58 | p-Value | OR (95% CI) | p-Value |
| Donor factors | |||||
| Age, median (range) | 32 (15–52) | 27 (15–53) | 0.482 | — | |
| Sex (Male:Female) | 20:11 | 46:12 | 0.129 | — | |
| Cause of brain death | 0.641 | — | |||
| CVA | 24 (77.4) | 41 (70.7) | |||
| Traumatic head injury | 6 (19.4) | 11 (19.0) | |||
| Others | 1 (3.2) | 6 (10.3) | |||
| Hospital stay (days) | 5 (1–36) | 5 (1–18) | 0.706 | — | |
| Graft factors | |||||
| Graft type | <0.001 | 1.64 (0.38–7.10) | 0.507 | ||
| Left hemi-liver | 23 (74.2) | 20 (34.5) | |||
| Right hemi-liver | 8 (25.8) | 38 (65.5) | |||
| Graft weight (gm) | 520 (335–1100) | 745 (390–1500) | <0.001 | 0.99 (0.98–1.00) | 0.070 |
| Cold ischemia time (min) | 513 (102–763) | 271 (69–779) | 0.039 | 1.00 (1.00–1.01) | 0.069 |
| Warm ischemia time (min) | 40 (31–58) | 45 (27–65) | 0.262 | — | |
| Recipient factors | |||||
| Age, median (range) | 50 (39–65) | 51 (33–65) | 0.368 | — | |
| Sex (Male:Female) | 18:13 | 39:19 | 0.390 | — | |
| Main indication of transplantation | 0.173 | — | |||
| Alcoholic Liver cirrhosis | 6 (19.4) | 14 (24.1) | |||
| Virus-related liver cirrhosis | 11 (35.5) | 18 (31.0) | |||
| Hepatocellular carcinoma | 6 (19.4) | 20 (34.5) | |||
| Others | 8 (25.8) | 6 (10.3) | |||
| Viral hepatitis | 0.409 | — | |||
| HBV | 16 (51.6) | 25 (43.1) | |||
| HCV | 4 (12.9) | 14 (24.1) | |||
| HBV + HCV | 0 | 2 (3.4) | |||
| None | 11 (35.5) | 17 (29.3) | |||
| Child classification | 0.885 | — | |||
| A, B | 15 (48.4) | 29 (50.0) | |||
| C | 16 (51.6) | 29 (50.0) | |||
| Chronic kidney disease staging | 0.312 | — | |||
| Grade 1, 2 | 21 (67.7) | 45 (77.6) | |||
| Grade 3, 4, 5 | 10 (32.3) | 13 (22.4) | |||
| Hemodialysis before transplantation | 3 (9.7) | 3 (5.2) | 0.419 | — | |
| MELD score, median (range) | 18 (7–42) | 19 (7–45) | 0.887 | — | |
| Biochemical factors | |||||
| Sodium (Na, ng/mL) | 137 (121–151) | 138 (124–152) | 0.455 | — | |
| Total Bilirubin (ng/mL) | 4.1 (0.5–34.3) | 4.6 (0.5–38.2) | 0.925 | — | |
| Neutrophil-lymphocyte ratio | 4.4 (1.6–169.7) | 5.4 (0.9–46.0) | 0.535 | — | |
| GRWR | 1.0 (0.7–2.0) | 1.1 (0.6–2.0) | 0.007 | 3.33 (0.21–51.53) | 0.388 |
| Transplantation factors | |||||
| Transplantation period | 0.832 | — | |||
| Before 2008 | 10 (32.3) | 20 (34.5) | |||
| After 2008 (2008/01/01) | 21 (67.7) | 38 (65.5) | |||
| Time of Transplantation | 0.574 | — | |||
| Daytime transplantation | 28 (90.3) | 50 (86.2) | |||
| Nighttime transplantation | 3 (9.7) | 8 (13.8) | |||
| Total operation time (hours) | 10.8 (5.1–18.0) | 10.3 (6.4–15.5) | 0.856 | — | |
| Operative blood loss (L) | 3.0 (0.2–18.7) | 2.0 (0.1–19.0) | 0.210 | — | |
| Recipient No. | Age/sex | Indication of Transplantation | MELD | Hemi-liver graft | Graft weight (g)/GRWR (%) | Major events | Mortality (Days) |
|---|---|---|---|---|---|---|---|
| 85 | 48/F | Autoimmune hepatitis | 38 | Left | 460/0.79 | Graft dysfunction, infection | 61 |
| 111 | 49/M | HBV, ESLD | 21 | Left | 425/0.85 | Graft dysfunction, infection | 26 |
| 126 | 39/M | HBV, ESLD | 18 | Left | 520/0.84 | Acute renal failure | 9 |
| 209 | 65/F | HCV, ESLD | 11 | Left | 410/0.85 | Graft dysfunction, infection | 13 |
| 221 | 62/F | Wilson‘s disease | 17 | Left | 520/0.91 | Graft dysfunction, infection | 7 |
| 254 | 50/M | HBV, HCC | 9 | Left | 525/1.17 | Graft dysfunction, infection | 32 |
| 342 | 54/M | HBV, HCC | 11 | Left | 480/0.81 | Antibody mediated Rejection | 18 |
| 399 | 48/M | Alcoholic liver cirrhosis | 16 | Left | 335/0.76 | Pneumonia | 64 |
| 426 | 49/M | HBV, HCC | 17 | Right | 550/0.95 | Antibody mediated rejection | 9 |
| 537 | 47/M | HBV, ESLD | 42 | Right | 570/0.97 | Graft dysfunction, infection | 11 |
| 538 | 45/F | PBC | 17 | Left | 450/0.90 | Graft dysfunction, infection | 31 |
| 790 | 43/F | Alcoholic liver cirrhosis | 27 | Left | 430/0.96 | Acute graft versus host disease | 46 |
| 848 | 60/F | HCV, HCC | 7 | Left | 520/0.81 | HCV relapse | 81 |
| 978 | 61/F | HCV, HCC | 28 | Left | 390/0.86 | Intra-operative massive bleeding | 1 |
| 1033 | 54/F | PBC | 29 | Left | 520/1.18 | Intracranial hemorrhage | 68 |
| Waitlist | SPLT | WLT | ||
|---|---|---|---|---|
| n = 995(%) | n = 100(%) | n = 165(%) | p-Value | |
| Age (years), median (range) | 53 (25–66) | 50 (33–65) | 53 (19–65) | 0.285 |
| Male: female | 750:245 | 63:37 | 132:33 | 0.006 |
| Hepatitis status | 0.061 | |||
| HBV | 476 (47.8) | 45 (45.0) | 93 (56.3) | |
| HCV | 167 (16.8) | 19 (19.0) | 30 (18.2) | |
| HBV + HCV | 35 (3.5) | 2 (2.0) | 9 (5.5) | |
| None | 317 (31.9) | 34 (34.0) | 33 (20) | |
| Main indications for LT | 0.116 | |||
| Acute hepatic failure | 48 (4.8) | 1 (1.0) | 2 (1.2) | |
| Hepatocellular carcinoma | 261 (26.2) | 29 (29.0) | 55 (33.4) | |
| Viral cirrhosis | 379 (38.1) | 30 (30.0) | 81 (49.1) | |
| Alcoholic cirrhosis | 172 (17.3) | 24 (24.0) | 20 (12.1) | |
| Others | 135 (13.6) | 16 (16.0) | 7 (4.2) | |
| MELD score, median (range) | 14 (3–42) | 19 (7–45) | 22 (7–54) | <0.0001 |
| Medical priority | 0.289 | |||
| Status 1 | 4 (0.4) | 0 (0.0) | 2 (1.2) | |
| Non-status 1 | 991 (99.6) | 100 (100.0) | 163 (98.8) | |
| 1-year mortality | 593 (59.6) | 31 (31.0) | 43 (26.0) | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.-M.; Wang, Y.-C.; Wu, T.-H.; Cheng, C.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Lee, W.-C. Encouraging Split Liver Transplantation for Two Adult Recipients to Mitigate the High Incidence of Wait-List Mortality in the Setting of Extreme Shortage of Deceased Donors. J. Clin. Med. 2019, 8, 2095. https://doi.org/10.3390/jcm8122095
Chan K-M, Wang Y-C, Wu T-H, Cheng C-H, Lee C-F, Wu T-J, Chou H-S, Lee W-C. Encouraging Split Liver Transplantation for Two Adult Recipients to Mitigate the High Incidence of Wait-List Mortality in the Setting of Extreme Shortage of Deceased Donors. Journal of Clinical Medicine. 2019; 8(12):2095. https://doi.org/10.3390/jcm8122095
Chicago/Turabian StyleChan, Kun-Ming, Yu-Chao Wang, Tsung-Han Wu, Chih-Hsien Cheng, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, and Wei-Chen Lee. 2019. "Encouraging Split Liver Transplantation for Two Adult Recipients to Mitigate the High Incidence of Wait-List Mortality in the Setting of Extreme Shortage of Deceased Donors" Journal of Clinical Medicine 8, no. 12: 2095. https://doi.org/10.3390/jcm8122095
APA StyleChan, K.-M., Wang, Y.-C., Wu, T.-H., Cheng, C.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., & Lee, W.-C. (2019). Encouraging Split Liver Transplantation for Two Adult Recipients to Mitigate the High Incidence of Wait-List Mortality in the Setting of Extreme Shortage of Deceased Donors. Journal of Clinical Medicine, 8(12), 2095. https://doi.org/10.3390/jcm8122095





