Clinical Utility of Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Diagnostic Tool and Potential Therapeutic Target in Prostate Cancer
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Samples
- Cohort 1:
- healthy volunteers (n = 97) that donated urine and blood samples.
- Cohort 2:
- Patients with suspect of PCa but negative results in the biopsy (n = 549).
- Cohort 3:
- Patients diagnosed with PCa (biopsy-proven, n = 347). Specifically, this cohort was divided in patients with non-significant PCa (NonSigPCa; defined as Gleason score of 6 in the biopsy; n = 143; cohort 3a), and in patients with significant PCa (SigPCa; defined as Gleason score ≥ 7 on the biopsy; n = 204; cohort 3b).
2.2. GOAT and PSA Determinations
2.3. Cell Culture and Reagents
2.4. Transient Transfection with siRNAs
2.5. Stable Transfection with Plasmids
2.6. Cell Proliferation
2.7. RNA Extraction and Retrotranscription
2.8. Real-Time qPCR
2.9. Xenograft Assay
2.10. Statistical Analyses
3. Results
3.1. Description of the Cohort
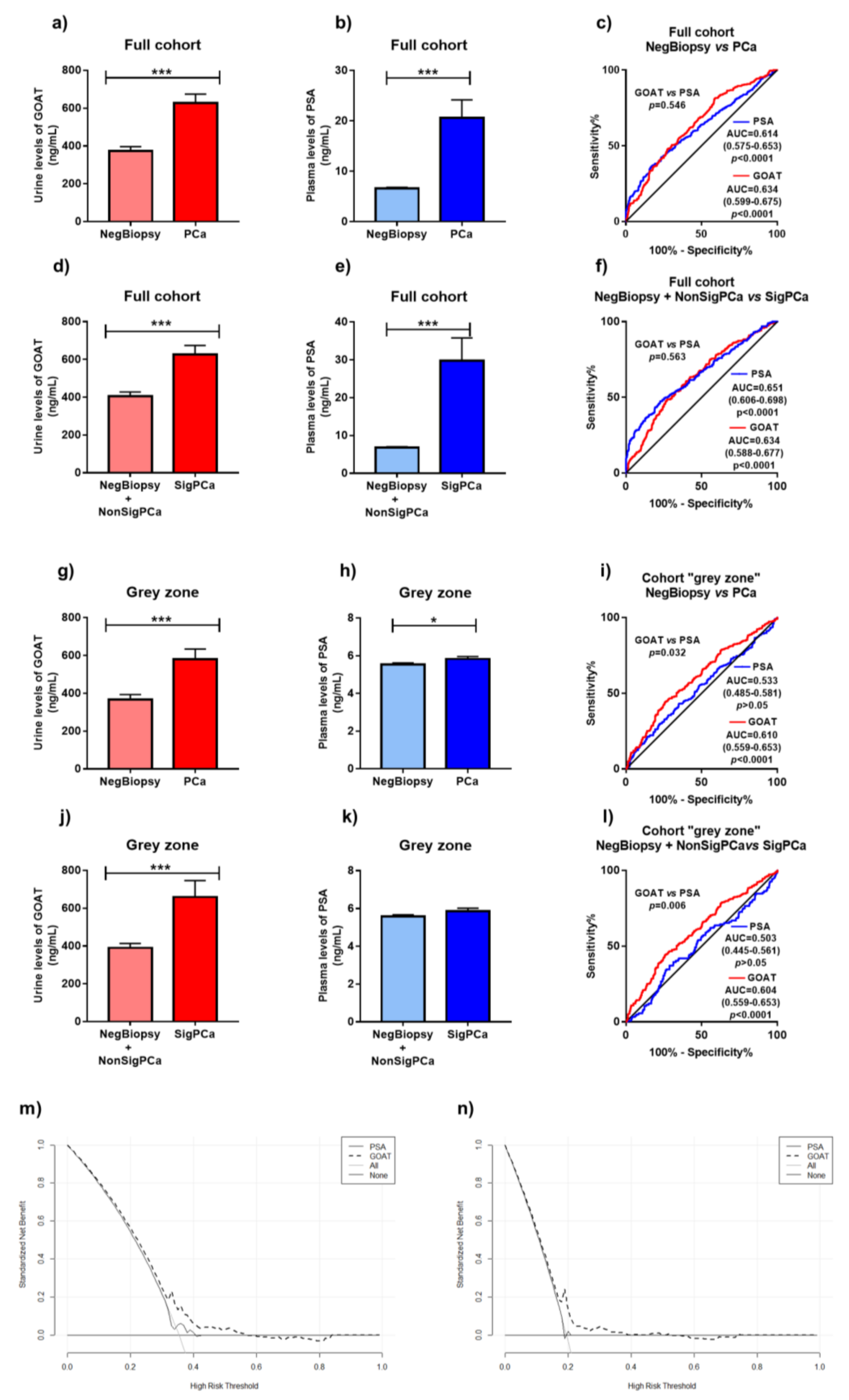
3.2. Levels of GOAT in Non-Invasive Samples from Patients with and without PCa
3.3. Comparison of Diagnostic Capacity of Urine GOAT and Plasma PSA
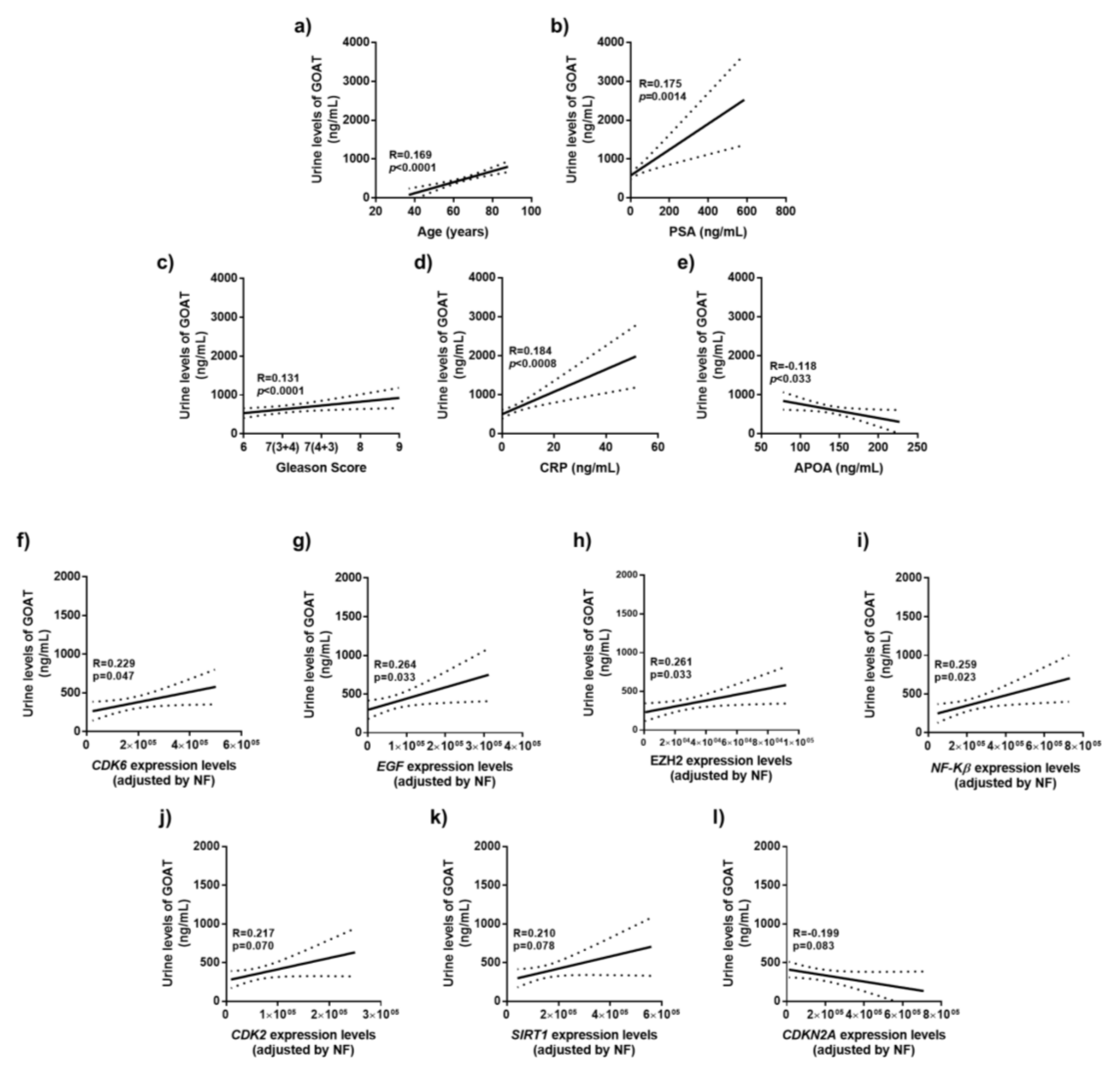
3.4. Correlation of Urine GOAT Levels with Clinical and Molecular Parameters of Tumor Aggressiveness
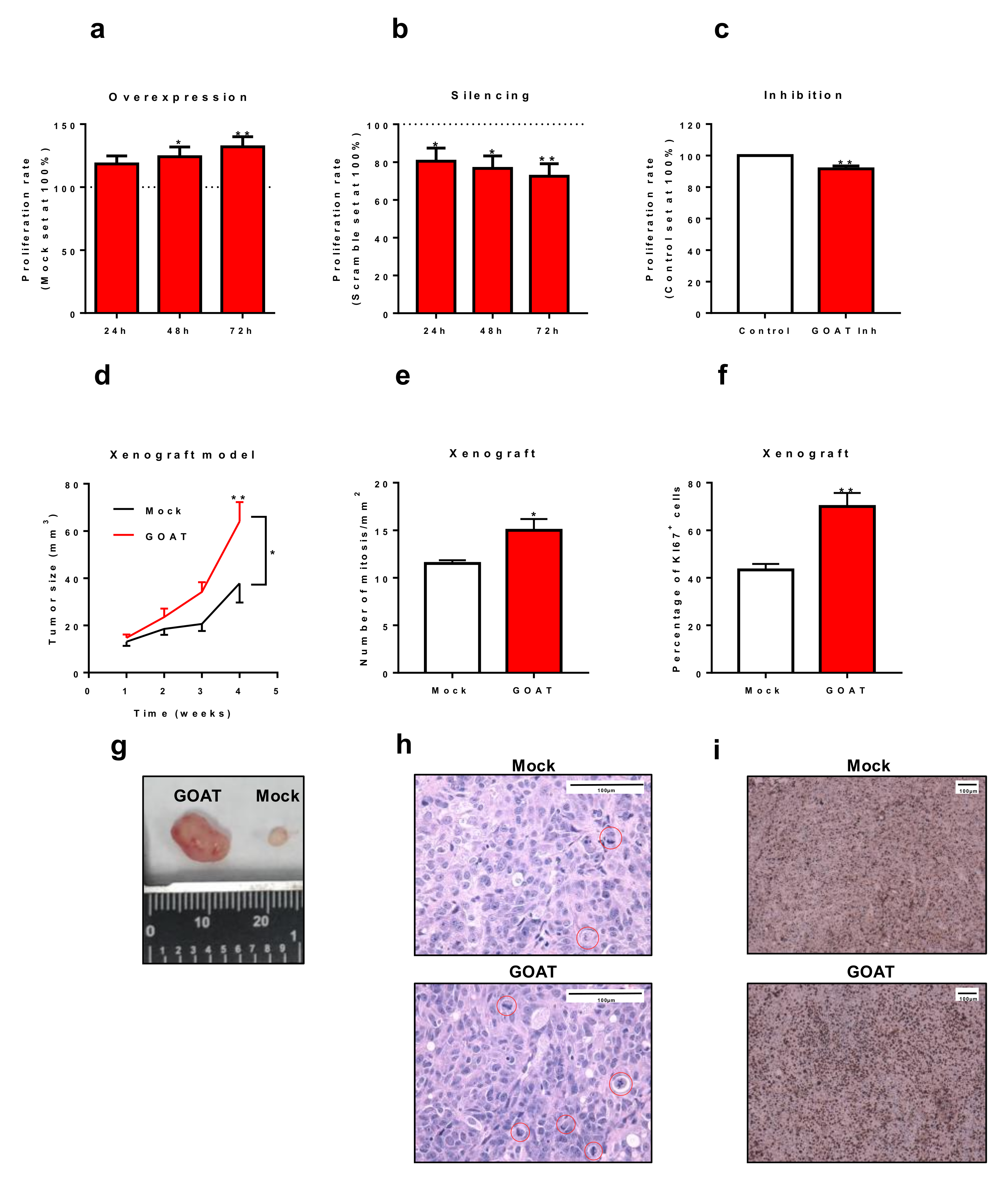
3.5. In Vitro and In Vivo Effects of the Modulation of GOAT Expression and/or Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.B.; Humphrey, P.A.; Smith, D.S.; Catalona, W.J.; Ratliff, T.L. Effect of inflammation and benign prostatic hyperplasia on elevated serum prostate specific antigen levels. J. Urol. 1995, 154, 407–413. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, R.J.; van Oort, I.M.; Schalken, J.A. Blood-based and urinary prostate cancer biomarkers: A review and comparison of novel biomarkers for detection and treatment decisions. Prost. Cancer Prost. Dis. 2017, 20, 12–19. [Google Scholar] [CrossRef]
- Chopin, L.K.; Seim, I.; Walpole, C.M.; Herington, A.C. The ghrelin axis—Does it have an appetite for cancer progression? Endocr. Rev. 2012, 33, 849–891. [Google Scholar] [CrossRef]
- Gahete, M.D.; Rincon-Fernandez, D.; Villa-Osaba, A.; Hormaechea-Agulla, D.; Ibanez-Costa, A.; Martinez-Fuentes, A.J.; Gracia-Navarro, F.; Castano, J.P.; Luque, R.M. Ghrelin gene products, receptors, and GOAT enzyme: Biological and pathophysiological insight. J. Endocrinol. 2014, 220, R1–R24. [Google Scholar] [CrossRef]
- Yeh, A.H.; Jeffery, P.L.; Duncan, R.P.; Herington, A.C.; Chopin, L.K. Ghrelin and a novel preproghrelin isoform are highly expressed in prostate cancer and ghrelin activates mitogen-activated protein kinase in prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 8295–8303. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Gahete, M.D.; Jimenez-Vacas, J.M.; Gomez-Gomez, E.; Ibanez-Costa, A.; Fernando, L.-L.; Rivero-Cortes, E.; Sarmento-Cabral, A.; Valero-Rosa, J.; Carrasco-Valiente, J.; et al. The oncogenic role of the In1-ghrelin splicing variant in prostate cancer aggressiveness. Mol. Cancer 2017, 16, 146. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef]
- Gahete, M.D.; Cordoba-Chacon, J.; Salvatori, R.; Castano, J.P.; Kineman, R.D.; Luque, R.M. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol. Cell. Endocrinol. 2010, 317, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hormaechea-Agulla, D.; Gomez-Gomez, E.; Ibanez-Costa, A.; Carrasco-Valiente, J.; Rivero-Cortes, E.; Fernando, L.-L.; Pedraza-Arevalo, S.; Valero-Rosa, J.; Sanchez-Sanchez, R.; Ortega-Salas, R.; et al. Ghrelin O-acyltransferase (GOAT) enzyme is overexpressed in prostate cancer, and its levels are associated with patient’s metabolic status: Potential value as a non-invasive biomarker. Cancer Lett. 2016, 383, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, E.; Jimenez-Vacas, J.M.; Carrasco-Valiente, J.; Herrero-Aguayo, V.; Blanca-Pedregosa, A.M.; Leon-Gonzalez, A.J.; Valero-Rosa, J.; Fernandez-Rueda, J.L.; Gonzalez-Serrano, T.; Lopez-Miranda, J.; et al. Plasma ghrelin O-acyltransferase (GOAT) enzyme levels: A novel non-invasive diagnosis tool for patients with significant prostate cancer. J. Cell. Mol. Med. 2018, 22, 5688–5697. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Allsbrook, W.C., Jr.; Amin, M.B.; Egevad, L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Vacas, J.M.; Herrero-Aguayo, V.; Gomez-Gomez, E.; Leon-Gonzalez, A.J.; Saez-Martinez, P.; Alors-Perez, E.; Fuentes-Fayos, A.C.; Martinez-Lopez, A.; Sanchez-Sanchez, R.; Gonzalez-Serrano, T.; et al. Spliceosome Component SF3B1 as Novel Prognostic Biomarker and Therapeutic Target for Prostate Cancer. Transl. Res. J. Lab. Clin. Med. 2019, 212, 89–103. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Jimenez-Vacas, J.M.; Gomez-Gomez, E.; Fernando, L.-L.; Carrasco-Valiente, J.; Valero-Rosa, J.; Moreno, M.M.; Sanchez-Sanchez, R.; Ortega-Salas, R.; Gracia-Navarro, F.; et al. The oncogenic role of the spliced somatostatin receptor sst5TMD4 variant in prostate cancer. FASEB. J. 2017, 31, 4682–4696. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.P.; Hwang, Y.; Taylor, M.S.; Kirchner, H.; Pfluger, P.T.; Bernard, V.; Lin, Y.Y.; Bowers, E.M.; Mukherjee, C.; Song, W.J.; et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 2010, 330, 1689–1692. [Google Scholar] [CrossRef]
- Duran-Prado, M.; Gahete, M.D.; Hergueta-Redondo, M.; Martinez-Fuentes, A.J.; Cordoba-Chacon, J.; Palacios, J.; Gracia-Navarro, F.; Moreno-Bueno, G.; Malagon, M.M.; Luque, R.M.; et al. The new truncated somatostatin receptor variant sst5TMD4 is associated to poor prognosis in breast cancer and increases malignancy in MCF-7 cells. Oncogene 2012, 31, 2049–2061. [Google Scholar] [CrossRef]
- Ibanez-Costa, A.; Gahete, M.D.; Rivero-Cortes, E.; Rincon-Fernandez, D.; Nelson, R.; Beltran, M.; de la Riva, A.; Japon, M.A.; Venegas-Moreno, E.; Galvez, M.A.; et al. In1-ghrelin splicing variant is overexpressed in pituitary adenomas and increases their aggressive features. Sci. Rep. 2015, 5, 8714. [Google Scholar] [CrossRef]
- Luque, R.M.; Ibanez-Costa, A.; Neto, L.V.; Taboada, G.F.; Hormaechea-Agulla, D.; Kasuki, L.; Venegas-Moreno, E.; Moreno-Carazo, A.; Galvez, M.A.; Soto-Moreno, A.; et al. Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas. Cancer Lett. 2015, 359, 299–306. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Principe, S.; Kim, Y.; Fontana, S.; Ignatchenko, V.; Nyalwidhe, J.O.; Lance, R.S.; Troyer, D.A.; Alessandro, R.; Semmes, O.J.; Kislinger, T.; et al. Identification of prostate-enriched proteins by in-depth proteomic analyses of expressed prostatic secretions in urine. J. Proteome Res. 2012, 11, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. PSA as a marker for prostate cancer: A critical review. Ann. Clin. Biochem. 1996, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Friend, C.; Dreher, A.; Allgar, V.; Macleod, U. The diagnostic test accuracy of rectal examination for prostate cancer diagnosis in symptomatic patients: A systematic review. BMC Fam. Pract. 2018, 19, 79. [Google Scholar] [CrossRef]
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur. Urol. 2016, 70, 740–748. [Google Scholar] [CrossRef]
- Gronberg, H.; Adolfsson, J.; Aly, M.; Nordstrom, T.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Clements, M.; et al. Prostate cancer screening in men aged 50-69 years (STHLM3): A prospective population-based diagnostic study. Lancet Oncol. 2015, 16, 1667–1676. [Google Scholar] [CrossRef]
- Calvocoressi, L.; Uchio, E.; Ko, J.; Radhakrishnan, K.; Aslan, M.; Concato, J. Prostate cancer aggressiveness and age: Impact of p53, BCL-2 and microvessel density. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2018, 66, 1142–1146. [Google Scholar] [CrossRef]
- Thurner, E.M.; Krenn-Pilko, S.; Langsenlehner, U.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur. J. Cancer 2015, 51, 610–619. [Google Scholar] [CrossRef]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and cdks in development and cancer: A perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, L.; Zhu, W.; Yao, X. Meta-analysis of CDKN2A methylation to find its role in prostate cancer development and progression, and also to find the effect of CDKN2A expression on disease-free survival (PRISMA). Medicine 2018, 97, e0182. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q.; Li, L. Functional and therapeutic significance of EZH2 in urological cancers. Oncotarget 2017, 8, 38044–38055. [Google Scholar] [CrossRef] [PubMed]
- Jung-Hynes, B.; Nihal, M.; Zhong, W.; Ahmad, N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J. Biol. Chem. 2009, 284, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; Hu, Y.; Carter, T.; Arumainayagam, N.; Lecornet, E.; Freeman, A.; Hawkes, D.; Barratt, D.C.; Emberton, M. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. J. Urol. 2011, 186, 458–464. [Google Scholar] [CrossRef] [PubMed]
| Variable | Healthy n = 97 Cohort 1 | NegBiopsy n = 549 Cohort 2 | PCa n = 347 | ||
|---|---|---|---|---|---|
| All n = 347 Cohort 3 | NonSigPCa n = 143 Cohort 3a | SigPCa n = 204 Cohort 3b | |||
| Age Median (IQR) | 62 (57–67) | 63 (57–69) | 67 (61–72) | 65 (59–69) | 69 (63–75) |
| PSA level (ng/mL) Median (IQR) | 0.82 (0.57–1.33) | 5.27 (3.84–7.39) | 6.64 (4.49–11.32) | 5.62 (3.79–9.09) | 7.44 (4.83–16.09) |
| BMI | 28.41 (25.54–32.09) | 28.31 (25.96–30.86) | 28.62 (26.30–31.63) | 28.41 (26.44–31.15) | 28.72 (26.15–32.05) |
| Patients with previous negative biopsy | - | 174 (31.7) | 66 (19.0) | 35(24.5) | 31 (15.2) |
| DRE (Abnormal) | - | 59 (10.7) | 125 (36.0) | 34 (23.8) | 91 (44.6) |
| 5 alpha reductase inhibitors | 20 (3.6) | 5 (1.4) | 1(0.7) | 4 (0.2) | |
| Family History | 106 (19.3) | 55 (15.9) | 25 (17.5) | 30 (14.7) | |
| GS < 7 | - | 0 | 143 (41.2) | ||
| GS ≥ 7 | - | 0 | 204 (58.8) | ||
| Metastasis (%) | - | 0 | 18 (5.2) | 0 | 18 (8.8) |
| Variable | OR | Bootstrap CI 95% | p Value |
|---|---|---|---|
| Age | 1.036 | 1.014, 1.058 | <0.001 |
| PSA | 1.047 | 1.019, 1.073 | <0.001 |
| Prior biopsy | 0.522 | 0.3137, 0.7134 | <0.001 |
| GOAT | 1.514 | 1.253, 1.755 | <0.001 |
| DRE | 3.139 | 1.804, 4.307 | <0.001 |
| Variable | OR | Bootstrap CI 95% | p Value |
|---|---|---|---|
| Age | 1.052 | 1.025, 1.079 | <0.001 |
| PSA | 1.051 | 1.023, 1.075 | <0.001 |
| Prior biopsy | 0.452 | 0.233, 0.662 | <0.001 |
| GOAT | 1.477 | 1.171, 1.753 | <0.001 |
| DRE | 2.878 | 1.639, 3.996 | <0.001 |
| Variable | OR | Bootstrap CI 95% | p Value |
|---|---|---|---|
| Age | 1.039 | 1.013, 1.064 | <0.001 |
| PSA | 1.144 | 1.014, 1.271 | 0.011 |
| Prior biopsy | 0.431 | 0.217, 0.633 | <0.001 |
| GOAT | 1.553 | 1.226, 1.838 | <0.001 |
| DRE | 1.553 | 1.212, 4.080 | <0.001 |
| Variable | OR | Bootstrap CI 95% | p Value |
|---|---|---|---|
| Age | 1.0532 | 1.020, 1.085 | <0.001 |
| PSA | 1.119 | 0.958, 1.275 | 0.07 |
| Prior biopsy | 0.366 | 0.121, 0.596 | 0.001 |
| GOAT | 1.526 | 1.144, 1.859 | <0.001 |
| DRE | 2.497 | 1.017, 3.762 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Vacas, J.M.; Gómez-Gómez, E.; Montero-Hidalgo, A.J.; Herrero-Aguayo, V.; L-López, F.; Sánchez-Sánchez, R.; Guler, I.; Blanca, A.; Méndez-Vidal, M.J.; Carrasco, J.; et al. Clinical Utility of Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Diagnostic Tool and Potential Therapeutic Target in Prostate Cancer. J. Clin. Med. 2019, 8, 2056. https://doi.org/10.3390/jcm8122056
Jiménez-Vacas JM, Gómez-Gómez E, Montero-Hidalgo AJ, Herrero-Aguayo V, L-López F, Sánchez-Sánchez R, Guler I, Blanca A, Méndez-Vidal MJ, Carrasco J, et al. Clinical Utility of Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Diagnostic Tool and Potential Therapeutic Target in Prostate Cancer. Journal of Clinical Medicine. 2019; 8(12):2056. https://doi.org/10.3390/jcm8122056
Chicago/Turabian StyleJiménez-Vacas, Juan M., Enrique Gómez-Gómez, Antonio J. Montero-Hidalgo, Vicente Herrero-Aguayo, Fernando L-López, Rafael Sánchez-Sánchez, Ipek Guler, Ana Blanca, María José Méndez-Vidal, Julia Carrasco, and et al. 2019. "Clinical Utility of Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Diagnostic Tool and Potential Therapeutic Target in Prostate Cancer" Journal of Clinical Medicine 8, no. 12: 2056. https://doi.org/10.3390/jcm8122056
APA StyleJiménez-Vacas, J. M., Gómez-Gómez, E., Montero-Hidalgo, A. J., Herrero-Aguayo, V., L-López, F., Sánchez-Sánchez, R., Guler, I., Blanca, A., Méndez-Vidal, M. J., Carrasco, J., Lopez-Miranda, J., Requena-Tapia, M. J., Castaño, J. P., Gahete, M. D., & Luque, R. M. (2019). Clinical Utility of Ghrelin-O-Acyltransferase (GOAT) Enzyme as a Diagnostic Tool and Potential Therapeutic Target in Prostate Cancer. Journal of Clinical Medicine, 8(12), 2056. https://doi.org/10.3390/jcm8122056








