Pharmaceutical Aspects of Artificial Nutrition
Abstract
1. Introduction
2. Accesses for Artificial Nutrition
3. Handling of Feeding Tubes and Catheters
4. Complications of Enteral Nutrition
4.1. Gastrointestinal Complications
4.2. Mechanical Complications
4.3. Infectious Complications
4.4. Metabolic Complication
5. Complications of Parenteral Nutrition
5.1. Mechanical Complications
5.2. Infectious Complications
5.3. Metabolic Complications
5.3.1. Refeeding Syndrome
5.3.2. Hyperglycemia
5.3.3. Liver-Associated Complications
5.3.4. Thrombosis
6. The Role of the Pharmacist and Specificities of Pharmaceutical Management
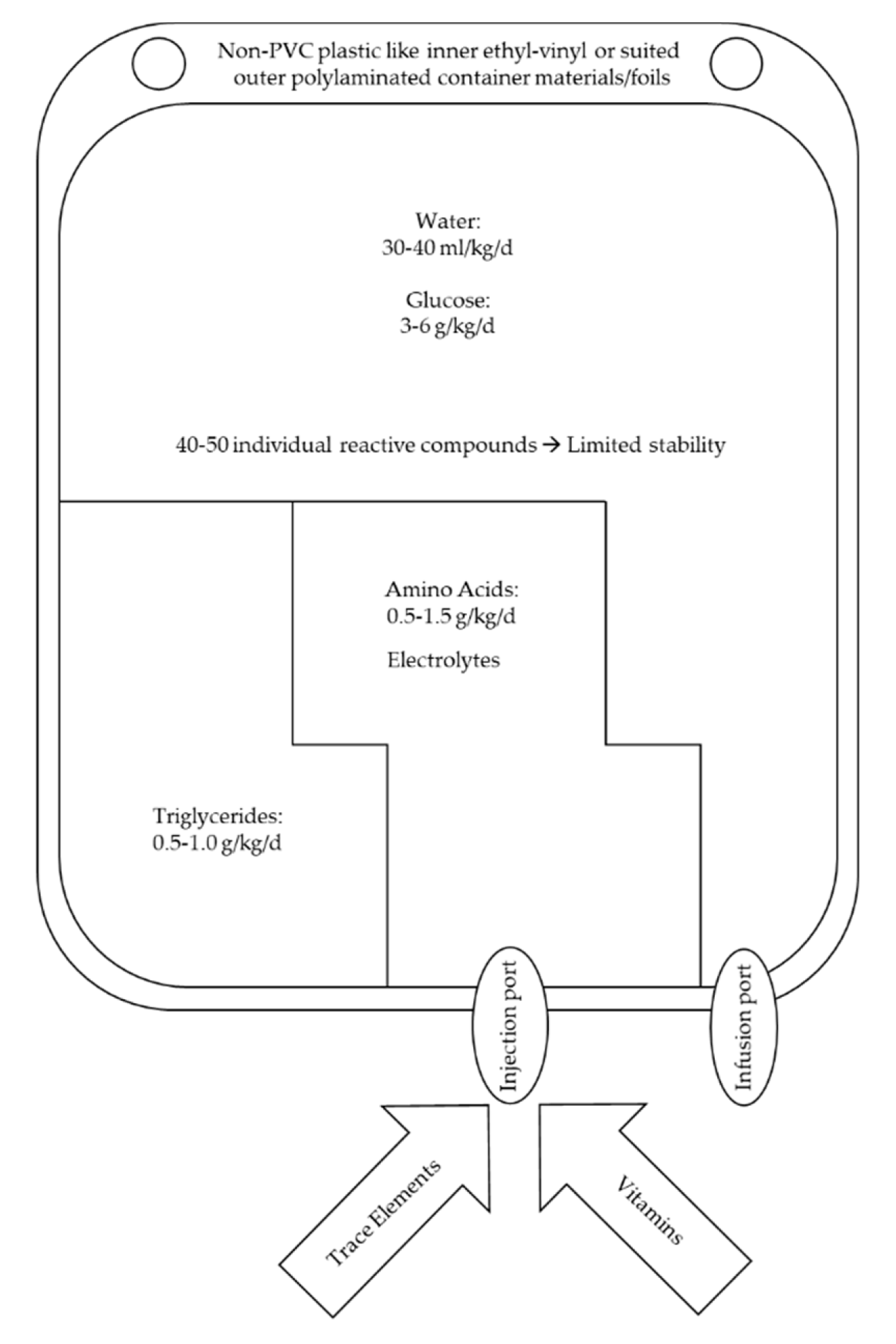
7. Components of Artificial Nutrition
7.1. Amino Acids
7.2. Glucose
7.3. Lipids
7.4. Fluids and Electrolytes
7.5. Micronutrients
7.6. The All-In-One Concept as the Pharmaceutical Formulation of Choice
8. Stability and Compatibility
8.1. Vitamin Stability
8.2. Trace Elements Compatibility
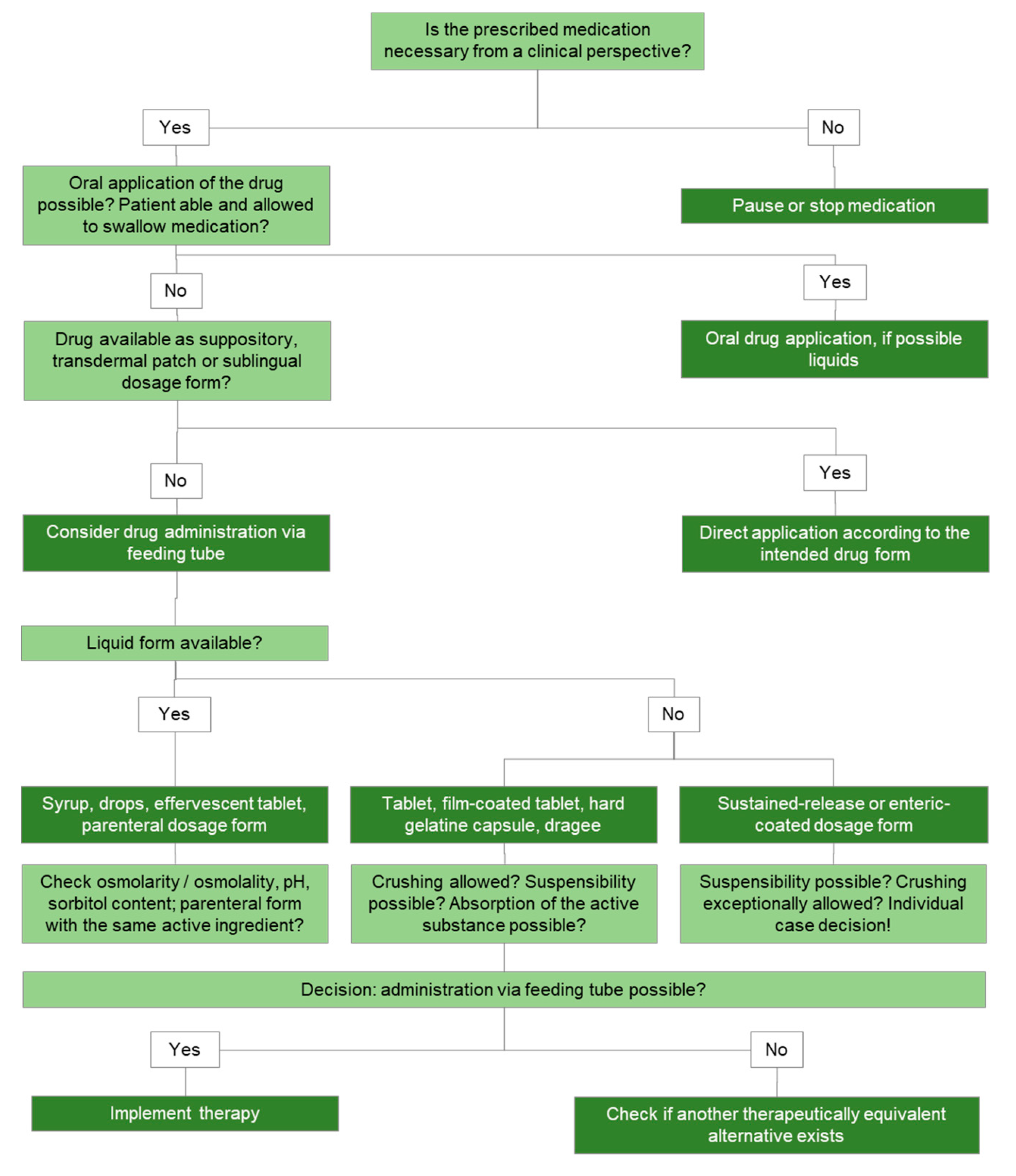
9. Artificial Nutrition and Drug Admixture
9.1. Drug Administration via Feeding Tube
9.1.1. Administration of Drugs via Feeding Tube
9.1.2. Safety Issues in the Administration of Drugs via Tube
9.2. Drug Admixture to PN
- Check if the medication is really needed.
- Ask for pharmaceutical advice, ideally from the nutrition support team when therapy regimen are complicated or when drug admixing to PN admixture is considered.
- Admix compatible drugs to PN only just before administration in order to minimize interactions.
- Document procedure, creating a database to control and reference drug-PN admixing interventions.
- If possible collect samples for later analysis and evaluation.
- Use alternative infusion lines (inclusive of other catheter lumen) for drug administration whenever possible. In absence of a separate line, intermittent intravenous drug administration in saline or glucose solutions may be considered. Sufficient catheter rinsing before and after drug administration is mandatory. Special attention is needed for metabolic adverse effects when stopping PN administration because of intermittent drug administration. Insulin stimulation induced by glucose infusion may for example be reduced by lowering the PN administration rates over the last half hour before stopping.
10. Monitoring of Artificial Nutrition
11. Home Artificial Nutrition
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pironi, L.; Steiger, E.; Brandt, C.; Joly, F.; Wanten, G.; Chambrier, C.; Aimasso, U.; Sasdelli, A.S.; Zeraschi, S.; Kelly, D.; et al. Home parenteral nutrition provision modalities for chronic intestinal failure in adult patients: An international survey. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, S. Diets and Diet Therapy: Parenteral Nutrition. In Encyclopedia of Food Security and Sustainablility (FOSS); Ferranti, P., Berry, E., Anderson, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–142. [Google Scholar] [CrossRef]
- National Collaborating Centre for Acute Care. Nutrition Support in Adults Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition; National Collaborating Centre for Acute Care: London, UK, 2006. [Google Scholar]
- Mühlebach, S.; Driscoll, H.; Hardy, G. Pharmaceutical Aspects of Parenteral Nutrition Support. In Basics in Clinical Nutrition, 4th ed.; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2011; pp. 373–400. [Google Scholar]
- Aeberhard, C.; Mühlebach, S. Parenteral Nutrition—Basics and Good Practice. Aktuel Ernahr. 2017, 42, 53–76. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Arends, J.; Dörje, F.; Engeser, P.; Hanke, G.; Köchling, K.; Leischkeret, A.H.; Mühlebach, S.; Schneider, A.; Seipt, A.; et al. S3-Leitlinie der Deutschen Gesellschaft für Ernährungsmedizin (DGEM) in Zusammenarbeit mit der GESKES und der AKE. Aktuel Ernahrungsmed 2013, 38, e101–e154. [Google Scholar] [CrossRef]
- Wanten, G.; Calder, P.C.; Forbes, A. Managing adult patients who need home parenteral nutrition. BMJ 2011, 342, d1447. [Google Scholar] [CrossRef] [PubMed]
- Dudrick, S.J. Early developments and clinical applications of total parenteral nutrition. J. Parenter. Enter. Nutr. 2003, 27, 291–299. [Google Scholar] [CrossRef]
- Brennan, G.T.; Ha, I.; Hogan, C.; Nguyen, E.; Jamal, M.M.; Bechtold, M.L.; Nguyen, D.L. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn’s disease patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 997–1002. [Google Scholar] [CrossRef]
- Dreznik, Y.; Horesh, N.; Gutman, M.; Gravetz, A.; Amiel, I.; Jacobi, H.; Zmora, O.; Rosin, D. Preoperative Nutritional Optimization for Crohn’s Disease Patients Can Improve Surgical Outcome. Dig. Surg. 2018, 35, 442–447. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Anderson, A.D.; Palmer, D.; MacFie, J. Peripheral parenteral nutrition. Br. J. Surg. 2003, 90, 1048–1054. [Google Scholar] [CrossRef]
- Gura, K.M. Is there still a role for peripheral parenteral nutrition? Nutr. Clin. Pract. 2009, 24, 709–717. [Google Scholar] [CrossRef]
- Omotani, S.; Tani, K.; Nagai, K.; Hatsuda, Y.; Mukai, J.; Myotoku, M. Water Soluble Vitamins Enhance the Growth of Microorganisms in Peripheral Parenteral Nutrition Solutions. Int. J. Med. Sci. 2017, 14, 1213–1219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugrue, D.; Jarrell, A.S.; Kruer, R.; Davis, S.; Johnson, D.; Tsui, E.; Snyder, S.; Crow, J. Appropriateness of peripheral parenteral nutrition use in adult patients at an academic medical center. Clin. Nutr. ESPEN 2018, 23, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Farrer, K.; Donaldson, E.; Blackett, B.; Lloyd, H.; Forde, C.; Garside, G.; Paine, P.; Lal, S. Quality and safety impact on the provision of parenteral nutrition through introduction of a nutrition support team. Eur. J. Clin. Nutr. 2014, 68, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Mariani, L.; Bertinet, D.B.; Chiavenna, G.; Crose, N.; De Cicco, M.; Gigli, G.; Micklewright, A.; Moreno Villares, J.M.; Orban, A.; et al. Central venous catheter complications in 447 patients on home parenteral nutrition: An analysis of over 100.000 catheter days. Clin. Nutr. 2002, 21, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Briz, E.; Ruiz Garcia, V.; Cabello, J.B.; Bort-Marti, S.; Carbonell Sanchis, R.; Burls, A. Heparin versus 0.9% sodium chloride locking for prevention of occlusion in central venous catheters in adults. Cochrane Database Syst. Rev. 2018, 7, Cd008462. [Google Scholar] [CrossRef] [PubMed]
- Raupp, P.; von Kries, R.; Schmidt, E.; Pfahl, H.G.; Gunther, O. Incompatibility between fat emulsion and calcium plus heparin in parenteral nutrition of premature babies. Lancet 1988, 1, 700. [Google Scholar] [CrossRef]
- Wouters, Y.; Theilla, M.; Singer, P.; Tribler, S.; Jeppesen, P.B.; Pironi, L.; Vinter-Jensen, L.; Rasmussen, H.H.; Rahman, F.; Wanten, G.J.A. Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment. Pharmacol. Ther. 2018, 48, 410–422. [Google Scholar] [CrossRef]
- Muehlebach, S.; Driscoll, D.; Aeberhard, C.; Stanga, Z. Stability and compatibility of parenteral nutrition (PN) admixtures. In Basics in Clinical Nutrition, 5th ed.; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2019; pp. 354–361. [Google Scholar]
- McKinnon, B.T. FDA safety alert: Hazards of precipitation associated with parenteral nutrition. Nutr. Clin. Pract. 1996, 11, 59–65. [Google Scholar] [CrossRef]
- Jauch, K.W.; Schregel, W.; Stanga, Z.; Bischoff, S.C.; Brass, P.; Hartl, W.; Muehlebach, S.; Pscheidl, E.; Thul, P.; Volk, O. Access technique and its problems in parenteral nutrition—Guidelines on Parenteral Nutrition, Chapter 9. GMS Ger. Med. Sci. 2009, 7, Doc19. [Google Scholar] [CrossRef]
- Ayers, P.; Adams, S.; Boullata, J.; Gervasio, J.; Holcombe, B.; Kraft, M.D.; Marshall, N.; Neal, A.; Sacks, G.; Seres, D.S. ASPEN parenteral nutrition safety consensus recommendations. J. Parenter. Enter. Nutr. 2014, 38, 296–333. [Google Scholar] [CrossRef]
- Pirlich, M.; Bodoky, G.; Kent-Smith, L. Complications of enteral nutrition. In Basics in Clinical Nutrition, 5th ed.; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2019; pp. 319–323. [Google Scholar]
- Montejo, J.C. Enteral nutrition-related gastrointestinal complications in critically ill patients: A multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit. Care Med. 1999, 27, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hebuterne, X.; Pertkiewicz, M.; et al. ESPEN Guidelines on Parenteral Nutrition: Home parenteral nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Aubry, E.; Friedli, N.; Schuetz, P.; Stanga, Z. Refeeding syndrome in the frail elderly population: Prevention, diagnosis and management. Clin. Exp. Gastroenterol. 2018, 11, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Friedli, N.; Stanga, Z.; Culkin, A.; Crook, M.; Laviano, A.; Sobotka, L.; Kressig, R.W.; Kondrup, J.; Mueller, B.; Schuetz, P. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018, 47, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Laesser, C.; Cumming, P.; Reber, E.; Stanga, Z.; Muka, T.; Bally, L. Management of Glucose Control in Noncritically Ill, Hospitalized Patients Receiving Parenteral and/or Enteral Nutrition: A Systematic Review. J. Clin. Med. 2019, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Hellman, R.; Korytkowski, M.T.; Kosiborod, M.; Maynard, G.A.; Montori, V.M.; Seley, J.J.; Van den Berghe, G. Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 16–38. [Google Scholar] [CrossRef] [PubMed]
- Muehlebach, S.; Driscoll, D.F.; Hardy, G. How to prepare parenteral nutrition (PN) admixtures, and the role and function of the pharmacis. In Basics in Clinical Nutrition, 5th ed.; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2019; pp. 345–353. [Google Scholar]
- Gales, B.J.; Riley, D.G. Improved total parenteral nutrition therapy management by a nutritional support team. Hosp. Pharm. 1994, 29, 469–470. [Google Scholar]
- Taxis, K.; Barber, N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003, 326, 684. [Google Scholar] [CrossRef]
- American Diabetes Association. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S173–S181. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Dao, D.T.; Baker, M.A.; Fell, G.L.; Puder, M.; Gura, K.M. Intravenous Fat Emulsion Formulations for the Adult and Pediatric Patient: Understanding the Differences. Nutr. Clin. Pract. 2016, 31, 596–609. [Google Scholar] [CrossRef]
- Steger, P.J.; Muhlebach, S.F. Lipid peroxidation of i.v. lipid emulsions in TPN bags: The influence of tocopherols. Nutrition 1998, 14, 179–185. [Google Scholar] [CrossRef]
- Steger, P.J.; Muhlebach, S.F. Lipid peroxidation of intravenous lipid emulsions and all-in-one admixtures in total parenteral nutrition bags: The influence of trace elements. J. Parenter. Enter. Nutr. 2000, 24, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Lewington, A.; Allison, S. Basic Concepts of Fluid and Electrolyte Therapy. Available online: https://www.researchgate.net/publication/249625074_Basic_Concepts_of_Fluid_and_Electrolyte_Balance (accessed on 1 January 2013).
- Muhlebach, S. Practical aspects of multichamber bags for total parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Evering, V.H.; Andriessen, P.; Duijsters, C.E.; Brogtrop, J.; Derijks, L.J. The Effect of Individualized Versus Standardized Parenteral Nutrition on Body Weight in Very Preterm Infants. J. Clin. Med. Res. 2017, 9, 339–344. [Google Scholar] [CrossRef]
- Yailian, A.L.; Serre, C.; Fayard, J.; Faucon, M.; Thomare, P.; Filali, S.; Pivot, C.; Vetele, F.; Pirot, F.; Olivier, E. Production and stability study of a hospital parenteral nutrition solution for neonates. J. Pharm. Anal. 2019, 9, 83–90. [Google Scholar] [CrossRef]
- Mühlebach, S. Incompatibility reactions in drug therapy–preventable medication errors. Eur. Hosp. Pharm. J. Pract. 2007, 13, 30–31. [Google Scholar]
- Driscoll, D. Examination of selection of light-scattering and light-obscuration acceptance criteria for lipid injectable emulsions. Pharm. Forum. 2004, 30, 2244–2253. [Google Scholar]
- Schmutz, C.W. Zubereitung Parenteraler Ernährungsmischungen in der Spitalapotheke: Untersuchungen zur Pharmazeutischen Qualität und Stabilität. Ph.D. Thesis, Universität Basel, Basel, Switzerland, 1993. [Google Scholar]
- Washington, C. The stability of intravenous fat emulsions in total parenteral nutrition mixtures. Int. J. Pharm. 1990, 66, 1–21. [Google Scholar] [CrossRef]
- Davis, S. The stability of fat emulsions for intravenous administration. In Advances in Clinical Nutrition; Springer: Berlin, Germany, 1983; pp. 213–239. [Google Scholar]
- Newton, D.W.; Driscoll, D.F. Calcium and phosphate compatibility: Revisited again. Am. J. Health Syst. Pharm. 2008, 65, 73–80. [Google Scholar] [CrossRef]
- Gräflein, C. Parenterale Ernährung mit Stabilitätsgeprüften, Modularen Standardlösungen in der Neonatologie. Ph.D. Thesis, Philosophisch-Naturwissenschaftliche Fakultät der Universität Basel, Basel, Switzerland, 2004. [Google Scholar]
- Aeberhard, C.; Steuer, C.; Saxer, C.; Huber, A.; Stanga, Z.; Muhlebach, S. Physicochemical stability and compatibility testing of levetiracetam in all-in-one parenteral nutrition admixtures in daily practice. Eur. J. Pharm. Sci. 2017, 96, 449–455. [Google Scholar] [CrossRef]
- Pharmacopea, U. Globule size distribution in lipid injectable emulsions. U. S. Pharm. Rockv. MD US Pharm. 2011, 1, 297–299. [Google Scholar]
- Ribeiro, D.O.; Pinto, D.C.; Lima, L.M.; Volpato, N.M.; Cabral, L.M.; de Sousa, V.P. Chemical stability study of vitamins thiamine, riboflavin, pyridoxine and ascorbic acid in parenteral nutrition for neonatal use. Nutr. J. 2011, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, S.; Franken, C.; Stanga, Z. Practical handling of AIO admixtures—Guidelines on Parenteral Nutrition, Chapter 10. GMS Ger. Med. Sci. 2009, 7, Doc18. [Google Scholar] [CrossRef] [PubMed]
- Allwood, M.C.; Martin, H.; Greenwood, M.; Maunder, M. Precipitation of trace elements in parenteral nutrition mixtures. Clin. Nutr. 1998, 17, 223–226. [Google Scholar] [CrossRef]
- Laborie, S.; Lavoie, J.C.; Pineault, M.; Chessex, P. Contribution of multivitamins, air, and light in the generation of peroxides in adult and neonatal parenteral nutrition solutions. Ann. Pharmacother. 2000, 34, 440–445. [Google Scholar] [CrossRef]
- Allwood, M.C.; Kearney, C. Compatibility and stability of additives in parenteral nutrition admixtures. Nutrition 1998, 14, 697–706. [Google Scholar] [CrossRef]
- Muehlebach, S.; Aeberhard, C.; Stanga, Z. Drugs and Nutritional Admixtures. In Basics in Clinical Nutrition; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2019; p. 362. [Google Scholar]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Prohaska, E.S.; King, A.R. Administration of antiretroviral medication via enteral tubes. Am. J. Health Syst. Pharm. 2012, 69, 2140–2146. [Google Scholar] [CrossRef]
- Schier, J.G.; Howland, M.A.; Hoffman, R.S.; Nelson, L.S. Fatality from administration of labetalol and crushed extended-release nifedipine. Ann. Pharmacother. 2003, 37, 1420–1423. [Google Scholar] [CrossRef]
- Subotic, U.; Hannmann, T.; Kiss, M.; Brade, J.; Breitkopf, K.; Loff, S. Extraction of the plasticizers diethylhexylphthalate and polyadipate from polyvinylchloride nasogastric tubes through gastric juice and feeding solution. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 71–76. [Google Scholar] [CrossRef]
- Stein, J.; Dormann, A. Sonden-und Applikationstechniken. In Praxishandbuch Klinische Ernährung und Infusionstherapie; Springer: Berlin, Germany, 2003; pp. 291–310. [Google Scholar]
- Treleano, A.; Wolz, G.; Brandsch, R.; Welle, F. Investigation into the sorption of nitroglycerin and diazepam into PVC tubes and alternative tube materials during application. Int. J. Pharm. 2009, 369, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Ikuno, Y.; Tsubakimoto, Y.; Hoshino, N.; Minouchi, T.; Yoshio, K.; Inoue, T.; Taga, T.; Ando, A.; Hodohara, K.; et al. Adsorption and pharmacokinetics of cyclosporin A in relation to mode of infusion in bone marrow transplant patients. Bone Marrow Transplant. 2000, 25, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Griese-Mammen, N.; Hersberger, K.E.; Messerli, M.; Leikola, S.; Horvat, N.; van Mil, J.W.F.; Kos, M. PCNE definition of medication review: Reaching agreement. Int. J. Clin. Pharm. 2018, 40, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Muehlebach, S.; Aeberhard, C.; Stanga, Z. Pharmaceutical aspects in enteral feeding and drugs. In Basics in Clinical Nutrition; Sobotka, L., Ed.; Galen: Prague, Czech Republic, 2019; p. 314. [Google Scholar]
- Mühlebach, S. Basics in clinical nutrition: Drugs and nutritional admixtures. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2009, 3, e134–e136. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Van Gossum, A.; Bakker, H.; De Francesco, A.; Ladefoged, K.; Leon-Sanz, M.; Messing, B.; Pironi, L.; Pertkiewicz, M.; Shaffer, J.; Thul, P.; et al. Home parenteral nutrition in adults: A multicentre survey in Europe in 1993. Clin. Nutr. 1996, 15, 53–59. [Google Scholar] [CrossRef]
| Type of Chronic Intestinal Failure | Underlying Disease |
|---|---|
| Benign chronic intestinal failure (n = 2919, 90.1%) | Crohn’s disease (22.4%) Mesenteric ischemia (17.7%) Surgical complications (15.8%) Primary chronic intestinal pseudo-obstruction (9.7%) Post-radiation enteritis (7.3%) Others (21.3%, with <3% each-one) Not reported (5.9%) |
| Malignant chronic intestinal failure (n = 320, 9.9%) | Type of active cancer not specified (62%) Gastrointestinal cancer (28%) Extra-abdominal cancer (10%) Concurrent enteritis due to radio- or chemotherapy (5%) Peritoneal carcinomatosis (12%) |
| Type | Rates Measures Per Catheter Year (95% Confidence Interval) |
|---|---|
| Catheter sepsis | 0.34 (0.32–0.37) |
| Catheter occlusion | 0.07 (0.06–0.08) |
| Central vein thrombosis | 0.03 (0.02–0.03) |
| Liver/biliary issues | |
| Mild | 0.42 (0.27–0.63) |
| Severe | 0.02 (0.01–0.06) |
| Metabolic bone disease | 0.05 (0.01–0.15) |
| Advice Not to Admix |
|
| Admixing May be Possible |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reber, E.; Messerli, M.; Stanga, Z.; Mühlebach, S. Pharmaceutical Aspects of Artificial Nutrition. J. Clin. Med. 2019, 8, 2017. https://doi.org/10.3390/jcm8112017
Reber E, Messerli M, Stanga Z, Mühlebach S. Pharmaceutical Aspects of Artificial Nutrition. Journal of Clinical Medicine. 2019; 8(11):2017. https://doi.org/10.3390/jcm8112017
Chicago/Turabian StyleReber, Emilie, Markus Messerli, Zeno Stanga, and Stefan Mühlebach. 2019. "Pharmaceutical Aspects of Artificial Nutrition" Journal of Clinical Medicine 8, no. 11: 2017. https://doi.org/10.3390/jcm8112017
APA StyleReber, E., Messerli, M., Stanga, Z., & Mühlebach, S. (2019). Pharmaceutical Aspects of Artificial Nutrition. Journal of Clinical Medicine, 8(11), 2017. https://doi.org/10.3390/jcm8112017





