Effects of Non-Invasive Ventilation Combined with Oxygen Supplementation on Exercise Performance in COPD Patients with Static Lung Hyperinflation and Exercise-Induced Oxygen Desaturation: A Single Blind, Randomized Cross-Over Trial
Abstract
1. Introduction
2. Methods
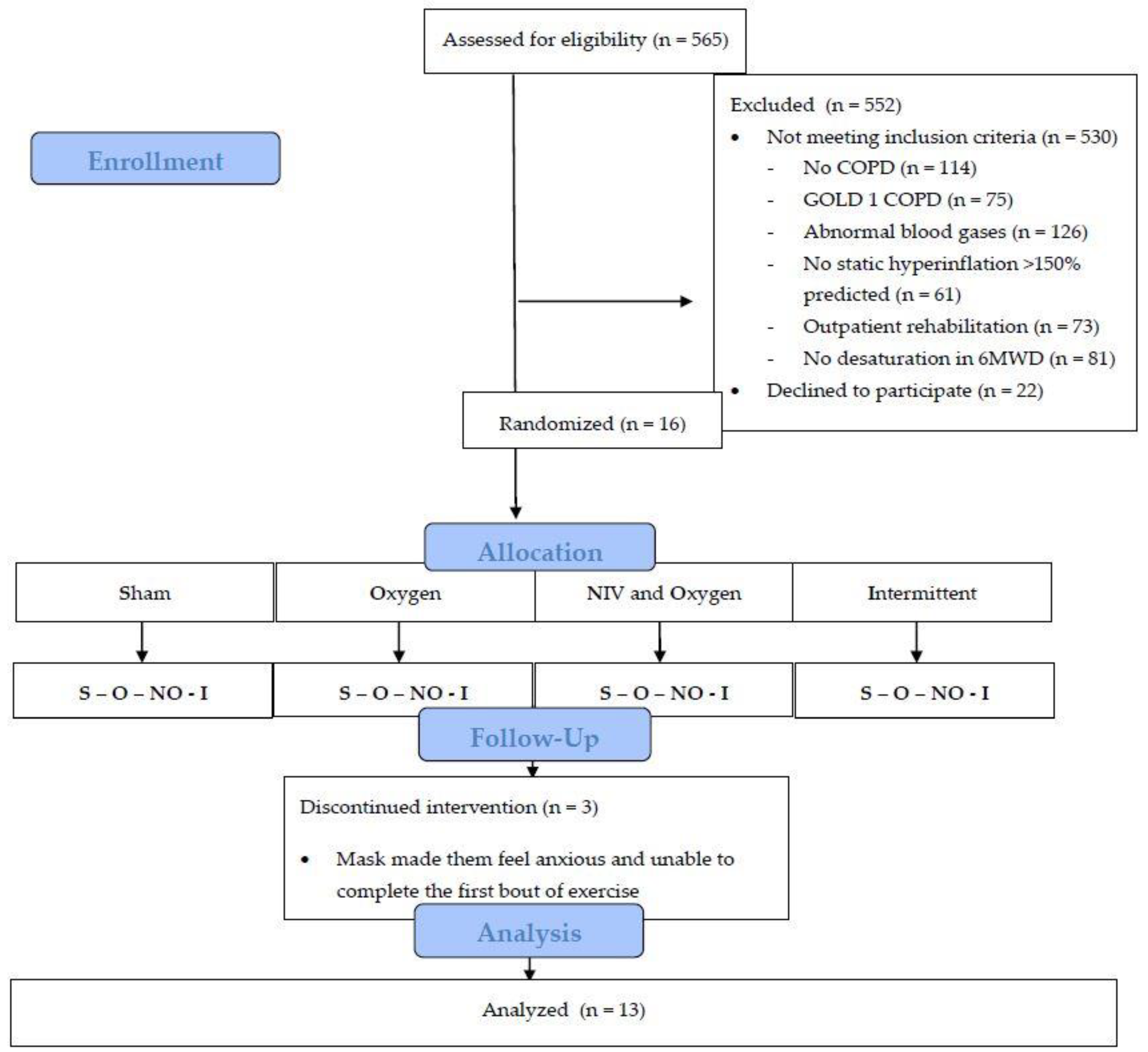
2.1. Patients
2.2. Sample Size
2.3. Baseline Assessment
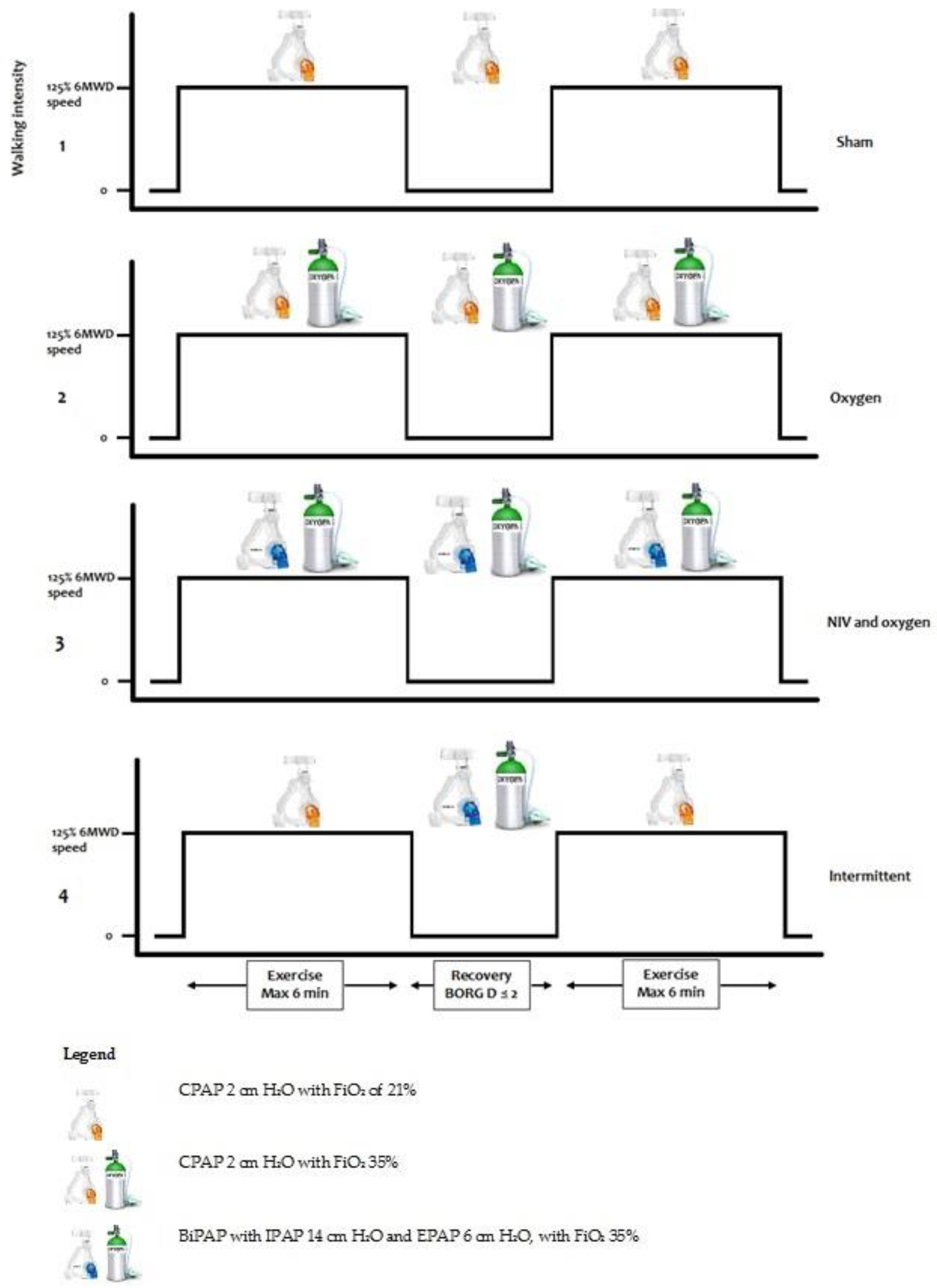
2.4. Interventions
- (1)
- Setting 1 (‘Sham’): Continuous positive airway pressure (CPAP) 2 cm H2O with an inspired oxygen fraction (FiO2) of 21%.
- (2)
- Setting 2 (‘Oxygen’): CPAP 2 cm H2O with FiO2 35%.
- (3)
- Setting 3 (‘NIV and oxygen’): BiPAP with inspiratory positive airway pressure of 14 cm H2O and expiratory positive airway pressure of 6 cm H2O, with FiO2 35%.
- (4)
- Setting 4 (‘Intermittent’): Setting 1 during walking (2 cm H2O), when the patient had an unintended stop the setting was switched to setting 3 (IPAP 14, EPAP 6 cm H2O). When the patient started walking again, the setting was switched back to setting 1.
2.5. Statistical Analysis
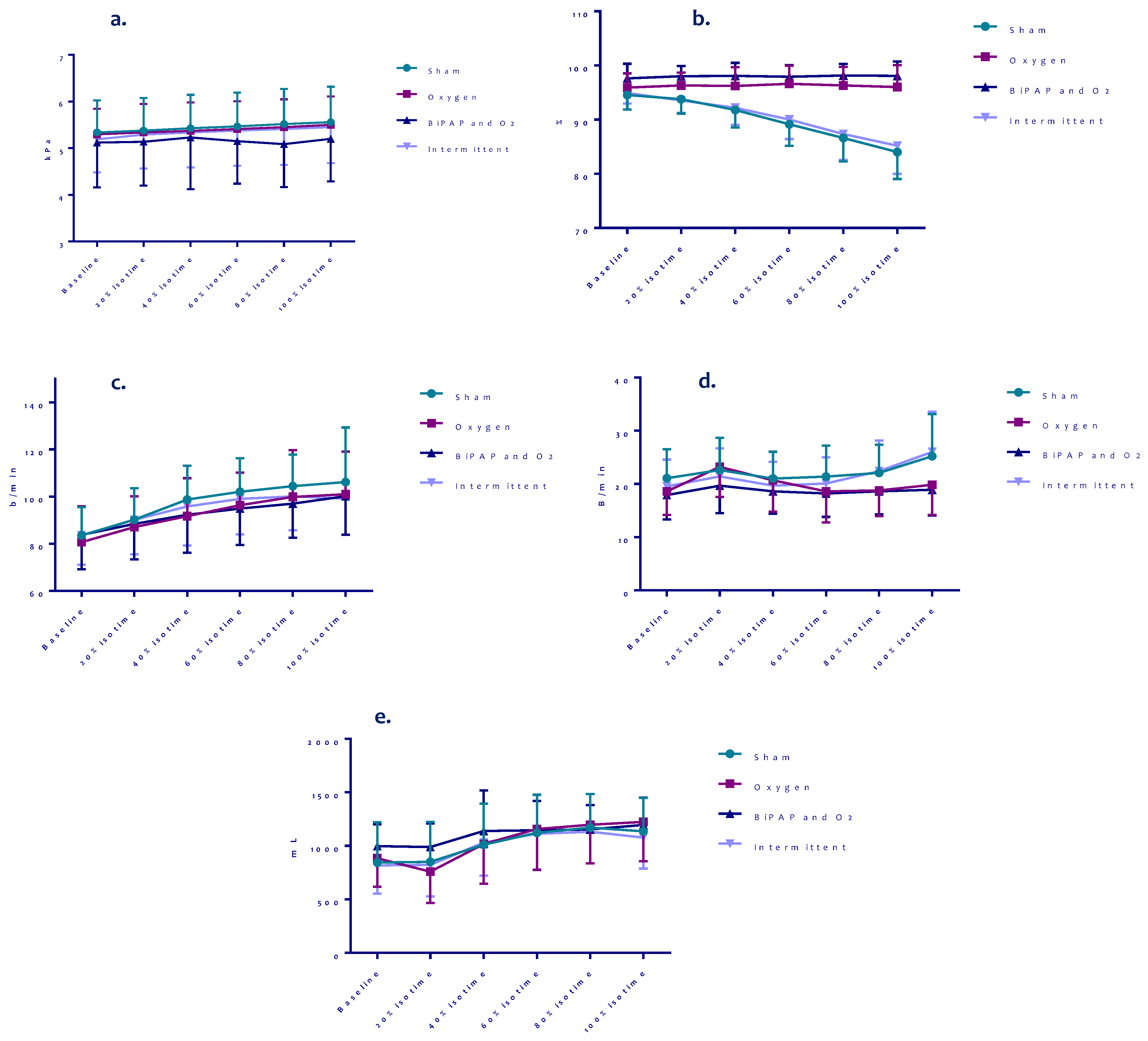
3. Results
Treadmill Test Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Donnell, D.E.; Webb, K.A. The major limitation to exercise performance in COPD is dynamic hyperinflation. J. Appl. Physiol. (1985) 2008, 105, 753–755, discussion 755–757. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e1364. [Google Scholar] [CrossRef]
- Rocha, A.; Arbex, F.F.; Sperandio, P.A.; Mancuso, F.; Marillier, M.; Bernard, A.C.; Alencar, M.C.N.; O’Donnell, D.E.; Neder, J.A. Exercise intolerance in comorbid COPD and heart failure: The role of impaired aerobic function. Eur. Respir. J. 2019, 53, 2018. [Google Scholar] [CrossRef]
- American Thoracic, S.; American College of Chest, P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Force, E.R.S.T.; Palange, P.; Ward, S.A.; Carlsen, K.H.; Casaburi, R.; Gallagher, C.G.; Gosselink, R.; O’Donnell, D.E.; Puente-Maestu, L.; Schols, A.M.; et al. Recommendations on the use of exercise testing in clinical practice. Eur. Respir. J. 2007, 29, 185–209. [Google Scholar] [CrossRef]
- Caviedes, I.R.; Delgado, I.; Soto, R. Ventilatory inefficiency as a limiting factor for exercise in patients with COPD. Respir. Care 2012, 57, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Whipp, B.J. The bioenergetic and gas exchange basis of exercise testing. Clin. Chest Med. 1994, 15, 173–192. [Google Scholar] [PubMed]
- Andrianopoulos, V.; Celli, B.R.; Franssen, F.M.; Pinto-Plata, V.M.; Calverley, P.M.; Vanfleteren, L.E.; Vogiatzis, I.; Vestbo, J.; Agusti, A.; Bakke, P.S.; et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: Results from the ECLIPSE study. Respir. Med. 2016, 119, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Augustin, I.M.L.; Spruit, M.A.; Houben-Wilke, S.; Franssen, F.M.E.; Vanfleteren, L.; Gaffron, S.; Janssen, D.J.A.; Wouters, E.F.M. The respiratory physiome: Clustering based on a comprehensive lung function assessment in patients with COPD. PLoS ONE 2018, 13, e0201593. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Gascon, M.; Sanchez, A.; Gallego, B.; Celli, B.R. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 1395–1399. [Google Scholar] [CrossRef]
- Gloeckl, R.; Andrianopoulos, V.; Stegemann, A.; Oversohl, J.; Schneeberger, T.; Schoenheit-Kenn, U.; Hitzl, W.; Dreher, M.; Rembert Koczulla, A.; Kenn, K. High-pressure non-invasive ventilation during exercise in COPD patients with chronic hypercapnic respiratory failure: A randomized, controlled, cross-over trial. Respirology 2018. [Google Scholar] [CrossRef] [PubMed]
- Vitacca, M.; Kaymaz, D.; Lanini, B.; Vagheggini, G.; Ergun, P.; Gigliotti, F.; Ambrosino, N.; Paneroni, M. Non-invasive ventilation during cycle exercise training in patients with chronic respiratory failure on long-term ventilatory support: A randomized controlled trial. Respirology 2017. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hul, A.; Kwakkel, G.; Gosselink, R. The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease: A systematic review. J. Cardiopulm. Rehabil. 2002, 22, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hul, A.; Gosselink, R.; Hollander, P.; Postmus, P.; Kwakkel, G. Acute effects of inspiratory pressure support during exercise in patients with COPD. Eur. Respir. J. 2004, 23, 34–40. [Google Scholar] [CrossRef]
- Borghi-Silva, A.; Carrascosa, C.; Oliveira, C.C.; Barroco, A.C.; Berton, D.C.; Vilaca, D.; Lira-Filho, E.B.; Ribeiro, D.; Nery, L.E.; Neder, J.A. Effects of respiratory muscle unloading on leg muscle oxygenation and blood volume during high-intensity exercise in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 2465–2472. [Google Scholar] [CrossRef]
- Babcock, M.A.; Pegelow, D.F.; Harms, C.A.; Dempsey, J.A. Effects of respiratory muscle unloading on exercise-induced diaphragm fatigue. J. Appl. Physiol. (1985) 2002, 93, 201–206. [Google Scholar] [CrossRef]
- Mahadevan, A.; Brouqueyre, L.; Cain, C.; Cropp, A.; ZuWallack, R. Philips Respironics. Impact of Positive Airway Pressure in Post-Exercise Recovery from Dyspnea in COPD Patients. In Proceedings of the ATS, San Diego, CA, USA; Available online: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A2648 (accessed on 2 May 2015).
- Emtner, M.; Porszasz, J.; Burns, M.; Somfay, A.; Casaburi, R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am. J. Respir. Crit. Care Med. 2003, 168, 1034–1042. [Google Scholar] [CrossRef]
- Garrod, R.; Paul, E.A.; Wedzicha, J.A. Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemia. Thorax 2000, 55, 539–543. [Google Scholar] [CrossRef]
- Jarosch, I.; Gloeckl, R.; Damm, E.; Schwedhelm, A.L.; Buhrow, D.; Jerrentrup, A.; Spruit, M.A.; Kenn, K. Short-term Effects of Supplemental Oxygen on 6-Min Walk Test Outcomes in Patients With COPD: A Randomized, Placebo-Controlled, Single-blind, Crossover Trial. Chest 2017, 151, 795–803. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Bain, D.J.; Webb, K.A. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am. J. Respir. Crit. Care Med. 1997, 155, 530–535. [Google Scholar] [CrossRef]
- Rodrigues, M.K.; Oliveira, M.F.; Soares, A.; Treptow, E.; Neder, J.A. Additive effects of non-invasive ventilation to hyperoxia on cerebral oxygenation in COPD patients with exercise-related O2 desaturation. Clin. Physiol. Funct. Imaging 2013, 33, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Vanderhoven-Augustin, I.; Janssen, P.P.; Wouters, E.F. Integration of pulmonary rehabilitation in COPD. Lancet 2008, 371, 12–13. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Stocks, J.; Quanjer, P.H. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur. Respir. J. 1995, 8, 492–506. [Google Scholar] [CrossRef]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Porszasz, J.; Cao, R.; Morishige, R.; van Eykern, L.A.; Stenzler, A.; Casaburi, R. Physiologic effects of an ambulatory ventilation system in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 334–342. [Google Scholar] [CrossRef]
- Brochard, L. Intrinsic (or auto-) positive end-expiratory pressure during spontaneous or assisted ventilation. Intensive Care Med. 2002, 28, 1552–1554. [Google Scholar] [CrossRef]
- Aliverti, A.; Dellaca, R.L.; Lotti, P.; Bertini, S.; Duranti, R.; Scano, G.; Heyman, J.; Lo Mauro, A.; Pedotti, A.; Macklem, P.T. Influence of expiratory flow-limitation during exercise on systemic oxygen delivery in humans. Eur. J. Appl. Physiol. 2005, 95, 229–242. [Google Scholar] [CrossRef]
- Aliverti, A.; Macklem, P.T. The major limitation to exercise performance in COPD is inadequate energy supply to the respiratory and locomotor muscles. J. Appl. Physiol. (1985) 2008, 105, 749–751, discussion 755-747. [Google Scholar] [CrossRef] [PubMed]
- Keilty, S.E.; Ponte, J.; Fleming, T.A.; Moxham, J. Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary disease. Thorax 1994, 49, 990–994. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, D.E.; Guenette, J.A.; Maltais, F.; Webb, K.A. Decline of resting inspiratory capacity in COPD: The impact on breathing pattern, dyspnea, and ventilatory capacity during exercise. Chest 2012, 141, 753–762. [Google Scholar] [CrossRef]
- Guenette, J.A.; Webb, K.A.; O’Donnell, D.E. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur. Respir. J. 2012, 40, 322–329. [Google Scholar] [CrossRef]
- Hajian, B.; De Backer, J.; Sneyers, C.; Ferreira, F.; Barboza, K.C.; Leemans, G.; Vos, W.; De Backer, W. Pathophysiological mechanism of long-term noninvasive ventilation in stable hypercapnic patients with COPD using functional respiratory imaging. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2197–2205. [Google Scholar] [CrossRef][Green Version]
- Wuyam, B.; Payen, J.F.; Levy, P.; Bensaidane, H.; Reutenauer, H.; Le Bas, J.F.; Benabid, A.L. Metabolism and aerobic capacity of skeletal muscle in chronic respiratory failure related to chronic obstructive pulmonary disease. Eur. Respir. J. 1992, 5, 157–162. [Google Scholar]
- Layec, G.; Hart, C.R.; Trinity, J.D.; Kwon, O.S.; Rossman, M.J.; Broxterman, R.M.; Le Fur, Y.; Jeong, E.K.; Richardson, R.S. Oxygen delivery and the restoration of the muscle energetic balance following exercise: Implications for delayed muscle recovery in patients with COPD. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E94–E104. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Sanii, R.; Younes, M. Improvement in exercise endurance in patients with chronic airflow limitation using continuous positive airway pressure. Am. Rev. Respir. Dis. 1988, 138, 1510–1514. [Google Scholar] [CrossRef]
- Petrof, B.J.; Calderini, E.; Gottfried, S.B. Effect of CPAP on respiratory effort and dyspnea during exercise in severe COPD. J. Appl. Physiol. (1985) 1990, 69, 179–188. [Google Scholar] [CrossRef]
- Contal, O.; Vignaux, L.; Combescure, C.; Pepin, J.L.; Jolliet, P.; Janssens, J.P. Monitoring of noninvasive ventilation by built-in software of home bilevel ventilators: A bench study. Chest 2012, 141, 469–476. [Google Scholar] [CrossRef]

| Baseline Characteristics | Mean |
|---|---|
| Age, years | 61.5 ± 6.8 |
| Female, n (%) | 9 (69) |
| BMI, kg/m2 | 25.4 ± 5.0 |
| mMRC score, median (IQR) | 3.0 (2.0) |
| Ex-smoker, n (%) | 13 (100%) |
| Packyears | 40.8 ± 15.2 |
| FEV1, % predicted | 34.0 ± 10.2 |
| FEV1/FVC, % | 27.9 ± 4.2 |
| FRC, % predicted | 171.9 ± 18.3 |
| RV, % predicted | 193.4 ± 32.0 |
| TLC, % predicted | 128.4 ± 13.5 |
| RV/TLC ratio | 0.59 ± 0.08 |
| DLCO, % predicted (n = 12) | 46.7 ± 12.8 |
| PaO2, kPa | 9.2 ± 1.5 |
| PaCO2, kPa | 5.6 ± 0.6 |
| 6MWD, m | 403.2 ± 73.6 |
| 6MWD, % predicted | 66.1 ± 13.2 |
| 6MWD SpO2 start, % | 92.2 ± 3.2 |
| 6MWD SpO2 stop, % | 82.5 ± 5.1 |
| 6MWD HR start, b/min | 83.0 ± 13.6 |
| 6MWD HR stop, b/min | 108.2 ± 13.1 |
| 6MWD Borg D start | 2.3 ± 1.3 |
| 6MWD Borg D stop | 5.4 ± 1.8 |
| 6MWD Borg F start | 2.2 ± 1.4 |
| 6MWD Borg F stop | 4.5 ± 1.1 |
| Exercise Bout One | Exercise Bout Two | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 Sham | 2 Oxygen | 3 NIV and Oxygen | 4 Intermittent | 1 Sham | 2 Oxygen | 3 NIV and Oxygen | 4 Intermittent | |
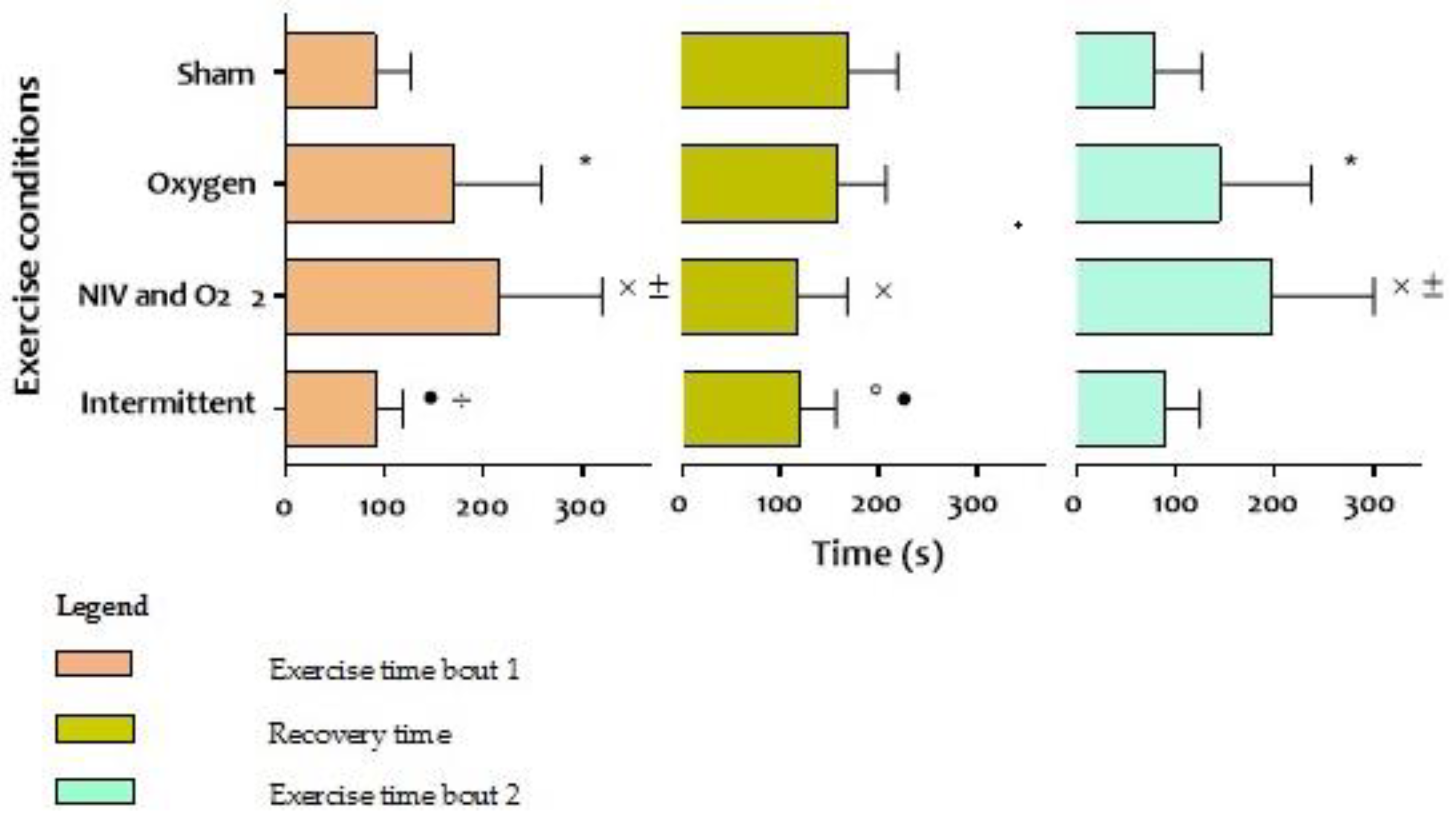
| Total distance walked, m | 120 (50) | 225 (107) * | 283 (128) ˟ ± | 123 (38) • ÷ | 107 (69) | 188 (109) * | 261 (136) ˟ ± | 119 (45) • ÷ |
| Endurance time, seconds | 91 (36) | 171 (88) * | 216 (105) ˟ ± | 91 (27) • ÷ | 80 (47) | 145 (92) * | 198 (103) ˟ ± | 89 (34) • ÷ |
| Recovery time, seconds | 170 (49) | 150 (48) | 117 (51) ˟ | 119 (37) º • | ||||
| Pre-test HR, bpm | 84 (12.2) | 84 (14.4) | 83 (14.8) | 84 (13.2) | 91 (15.4) | 91 (14.3) | 92 (16.1) | 89 (13.0) |
| Post- test HR, bpm | 100 (19.9) | 105 (16.7) | 105 (19) | 104 (15.5) | 106 (13.8) | 107 (17.6) | 110 (17.5) | 102 (13.6) |
| Recovery HR, bpm | 91 (15.4) | 91 (14.3) | 92 (16.1) | 89 (13.0) | 92 (10.6) | 92 (16.0) | 94 (16.9) | 93 (15.1) |
| Pre-test BF, bpm | 21 (5.0) | 21 (6.5) | 18 (4.1) | 20 (4.7) ÷ | 21 (8.0) | 24 (5.6) | 24 (4.3) | 23 (4.5) |
| Post-test BF, bpm | 31 (10) | 28 (7.8) | 27 (6.7) | 32 (5.4) ÷ | 30 (7.7) | 29 (8.1) | 28 (5.8) | 32 (8.7) |
| Recovery BF, bpm | 21 (8.0) | 24 (5.6) | 24 (4.3) | 23 (4.5) | 22 (5.2) | 23 (5.7) | 22 (5.5) | 24 (4.5) |
| Pre-test SpO2, % | 94 (3.0) | 96 (2.5) | 98 (2.6) | 95 (2.1) | 93 (4.4) | 98 (2.7) * | 99 (1.0) ˟ | 99 (1.0) º |
| Post-test SPO2, % | 84 (6.6) | 96 (5.2) * | 98 (1.2) ˟ | 85 (4.6) • | 84 (7.0) | 97 (3.2) * | 99 (1.5) º | 85 (4.6) • ÷ |
| Recovery SpO2, % | 93 (4.4) | 98 (2.7) * | 99 (1.0) ˟ | 99 (1.0) º | 90 (5.0) | 98 (2.6) * | 99 (0.8) ˟ | 90 (5.9) • ÷ |
| Pre-test tcPaCO2, kPa (n = 1) | 5.3 (0.7) | 5.4 (0.7) | 5.1 (0.9) | 5.2 (0.7) | 6.1 (0.9) | 6.7 (0.9) * | 6.3 (1.2) | 5.9 (0.7) • |
| Post-test tcPaCO2, kPa (n = 11) | 5.6 (0.8) | 6.3 (1.1) * | 5.9 (1.1) | 5.5 (0.7) • | 6.1 (0.9) | 6.6 (1.4) | 6.5 (1.3) | 6.0 (0.9) |
| Δ tcPaCO2, kPa (n = 11) | 0.3 (0.2) | 1.0 (0.9) * | 0.9 (0.6) ˟ | 0.3 (0.3) ÷ | 6.4 (0.9) | 7.1 (1.2) * | 6.6 (1.2) | 6.4 (0.9) • |
| Recovery tcPaCO2, kPa (n = 11) | 6.1 (0.9) | 6.7 (0.9) * | 6.3 (1.2) | 5.9 (0.7) • | 0.02 (0.1) | -0.06 (0.8) | 0.1 (0.3) | 0.1 (0.2) |
| Pre-test tidal volume, mL (Median (IQR)) | 808 (354) (696 (398)) | 822 (383) (699 (479)) | 954 (214) (919 (242)) | 800 (298) (709 (255)) | 1059 (276) (1031 (405)) | 921 (290) * (849 (463)) | 1130 (284) ± (1075 (424)) | 1020 (245) (954 (252)) |
| Post-test tidal volume, mL (Median (IQR)) | 1075 (296) (1034 (336)) | 1145(338) (1051 (519)) | 1166 (257) (1263 (441)) | 1119 (249) (1066 (407)) | 1128 (291) (1018 (381)) | 1122 (304) (1083 (440)) | 1141 (410) (1161 (463)) | 1039 (289) (977 (412)) |
| Δ tidal volume, mL (Median (IQR)) | 267 (241) (360 (448)) | 323 (336) (306 (530)) | 212 (256) (166 (321)) | 318 (228) (313 (260)) | 68 (170) (117 (257)) | 201 (234) (166 (302)) | 11 (287) (29 (207)) | 19 (165) (−18 (229)) |
| Recovery tidal volume, mL (Median (IQR)) | 1059 (276) (1031 (405)) | 921 (290) * (849 (463)) | 1130 (284) ± (1075 (424)) | 1020 (245) (954 (252)) | 1208 (421) (1143 (588)) | 1124 (437) (981 (862)) | 1285 (348) (1223 (357)) | 1202 (334) (1169 (515)) |
| Pre-test ventilation, L/min | 15.9 (3.2) | 15.4 (3.1) | 16.8 (4.0) | 15.3 (2.6) | 22.0 (8.7) | 20.7 (4.7) | 26.5 (5.7) ± | 22.6 (5.5) ÷ |
| Post-test ventilation, L/min | 31.1 (9.8) | 30.6 (8.4) | 30.9 (9.3) | 35.9 (10.3) | 32.1 (8.0) | 30.9 (8.2) | 32.3 (12.2) | 31.7 (8.2) |
| Pre-test Borg-D, points (Median (IQR)) | 0.7 (1.0) (0.5 (1.5)) | 0.8 (0.9) (0.5 (2.0)) | 0.5 (0.6) (0.5 (1.0)) | 0.8 (1.2) (0.0 (1.5)) | 1.8 (0.6) (2.0 (0.0)) | 1.9 (0.4) (2.0 (0.0)) | 1.8 (0.4) (2.0 (0.0)) | 1.7 (0.7) (2.0 (0.0)) |
| Post-test Borg-D, points (Median (IQR)) | 4.1 (2.1) (5.0 (2.0)) | 5.2 (2.1) (4.0 (3.0)) | 4.3 (1.4) ± (4.0 (1.0)) | 5.2 (1.4) (5.0 (3.0)) | 4.9 (2.4) (4.0 (3.0)) | 4.8 (2.2) (4.0 (3.5)) | 4.7 (1.8) (4.0 (2.5)) | 5.1 (1.7) (5.0 (2.0)) |
| Recovery Borg-D, points (Median (IQR)) | 1.8 (0.6) (2.0 (0.0)) | 1.9 (0.4) (2.0 (0.0)) | 1.8 (0.4) (2.0 (0.0)) | 1.7 (0.7) (2.0 (0.0)) | 1.9 (0.6) (3.0 (0.5)) | 1.8 (0.5) (2.0 (0.0)) | 1.8 (0.6) (2.0 (0.5)) | 2.0 (0.4) (2.0 (0.5)) |
| Pre-test Borg-F, points (Median (IQR)) | 1.0 (1.1) (0.5 (2.0)) | 1.0 (1.1) (0.25 (2.0)) | 0.6 (0.6) (0.75 (1.0)) | 0.6 (0.8) (0.25 (1.0)) | 1.7 (0.9) (2.0 (1.0)) | 1.8 (1.0) (2.0 (2.0)) | 1.7 (1.2) (1.0 (2.0)) | 0.6 (0.8) º • ÷ (0.0 (1.0)) |
| Post-test Borg-F, points (Median (IQR)) | 2.8 (1.9) (2.0 (2.5)) | 3.3 (1.6) (3.0 (1.0)) | 3.1 (1.5) (3.0 (2.0)) | 2.6 (2.0) (3.0 (2.8)) | 2.4 (1.4) (2.0 (2.5)) | 2.8 (1.3) (3.0 (1.0)) | 3.2 (1.9) (3.0 (2.5)) | 2.3 (1.6) (2.0 (2.3)) |
| Recovery Borg-F, points (Median (IQR)) | 1.7 (0.9) (2.0 (1.0)) | 1.8 (1.0)(2.0 (2.0)) | 1.7 (1.2) (1.0 (2.0)) | 0.6 (0.8) º • ÷ (0.0 (1.0)) | 1.9 (0.8) (2.0 (1.0)) | 1.7 (1.1) (2.0 (2.0)) | 1.8 (1.0) (1.0 (0.5)) | 1.6 (0.8) (1.0 (1.0)) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koopman, M.; Spruit, M.A.; Franssen, F.M.E.; Delbressine, J.; Wouters, E.F.M.; Mathew, D.; Vink, A.; Vanfleteren, L.E.G.W. Effects of Non-Invasive Ventilation Combined with Oxygen Supplementation on Exercise Performance in COPD Patients with Static Lung Hyperinflation and Exercise-Induced Oxygen Desaturation: A Single Blind, Randomized Cross-Over Trial. J. Clin. Med. 2019, 8, 2012. https://doi.org/10.3390/jcm8112012
Koopman M, Spruit MA, Franssen FME, Delbressine J, Wouters EFM, Mathew D, Vink A, Vanfleteren LEGW. Effects of Non-Invasive Ventilation Combined with Oxygen Supplementation on Exercise Performance in COPD Patients with Static Lung Hyperinflation and Exercise-Induced Oxygen Desaturation: A Single Blind, Randomized Cross-Over Trial. Journal of Clinical Medicine. 2019; 8(11):2012. https://doi.org/10.3390/jcm8112012
Chicago/Turabian StyleKoopman, Maud, Martijn A. Spruit, Frits M.E. Franssen, Jeannet Delbressine, Emiel F.M. Wouters, Denny Mathew, Anton Vink, and Lowie E.G.W. Vanfleteren. 2019. "Effects of Non-Invasive Ventilation Combined with Oxygen Supplementation on Exercise Performance in COPD Patients with Static Lung Hyperinflation and Exercise-Induced Oxygen Desaturation: A Single Blind, Randomized Cross-Over Trial" Journal of Clinical Medicine 8, no. 11: 2012. https://doi.org/10.3390/jcm8112012
APA StyleKoopman, M., Spruit, M. A., Franssen, F. M. E., Delbressine, J., Wouters, E. F. M., Mathew, D., Vink, A., & Vanfleteren, L. E. G. W. (2019). Effects of Non-Invasive Ventilation Combined with Oxygen Supplementation on Exercise Performance in COPD Patients with Static Lung Hyperinflation and Exercise-Induced Oxygen Desaturation: A Single Blind, Randomized Cross-Over Trial. Journal of Clinical Medicine, 8(11), 2012. https://doi.org/10.3390/jcm8112012





