Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro
Abstract
1. Introduction
2. Experimental
2.1. Donor Specifications
2.2. ASC Isolation and Expansion
2.3. Phenotypic Analysis
2.4. Differentiation Potential
2.5. Senescence-Associated β Galactosidase (SA-β-Gal) Staining
2.6. Cell Cycle Analysis
2.7. Analysis of ASC Secretome
2.8. qRT-PCR Analysis
2.9. Co-Culture Conditions
2.10. Western Blot Analysis
2.11. Collagen Content Quantification
2.12. Tube Formation Assays
2.13. Spheroid-Based Sprouting Assay
2.14. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. ASC Characterization
3.2.1. Morphology and Phenotype
3.2.2. Differentiation
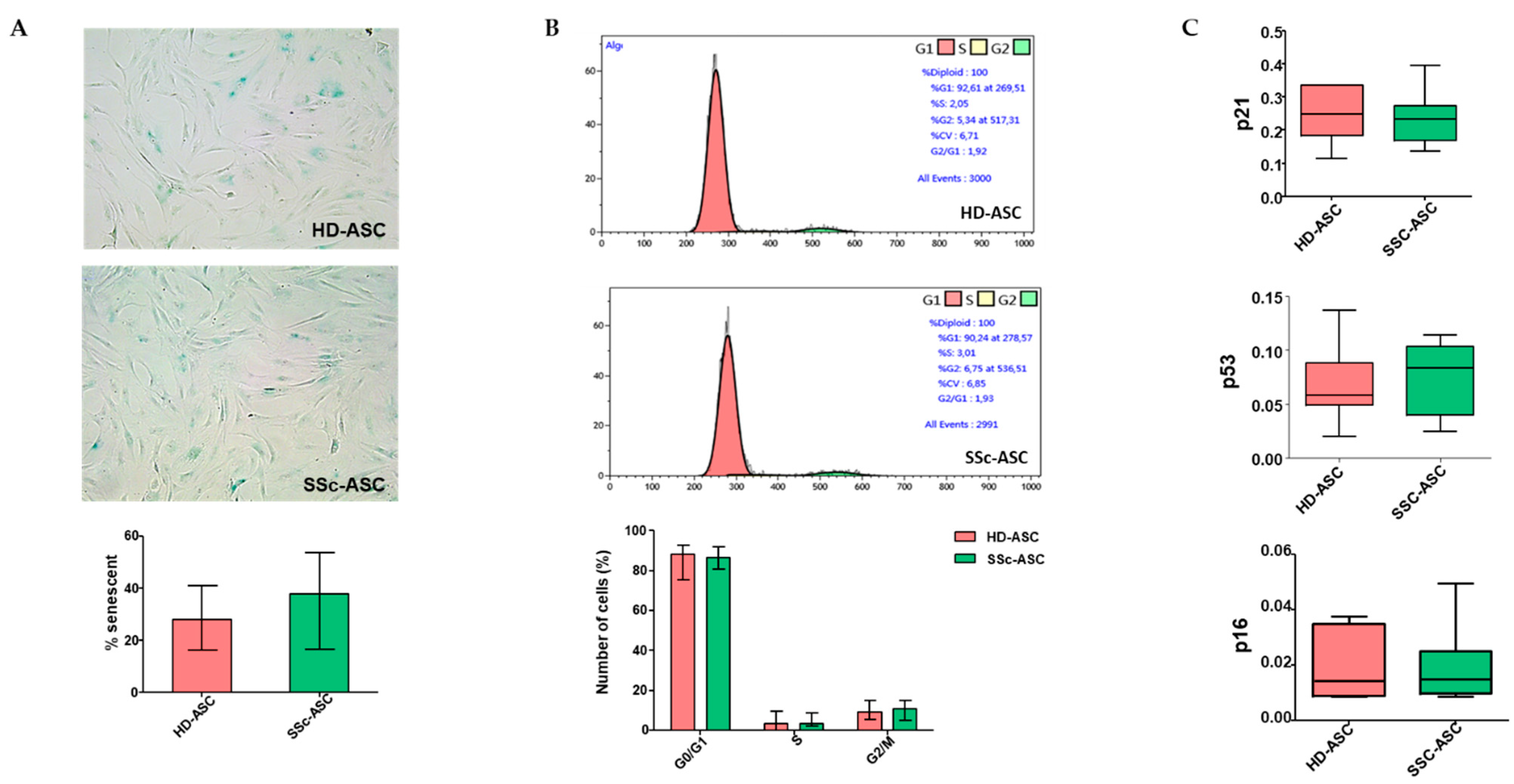
3.3. ASC Senescence Markers
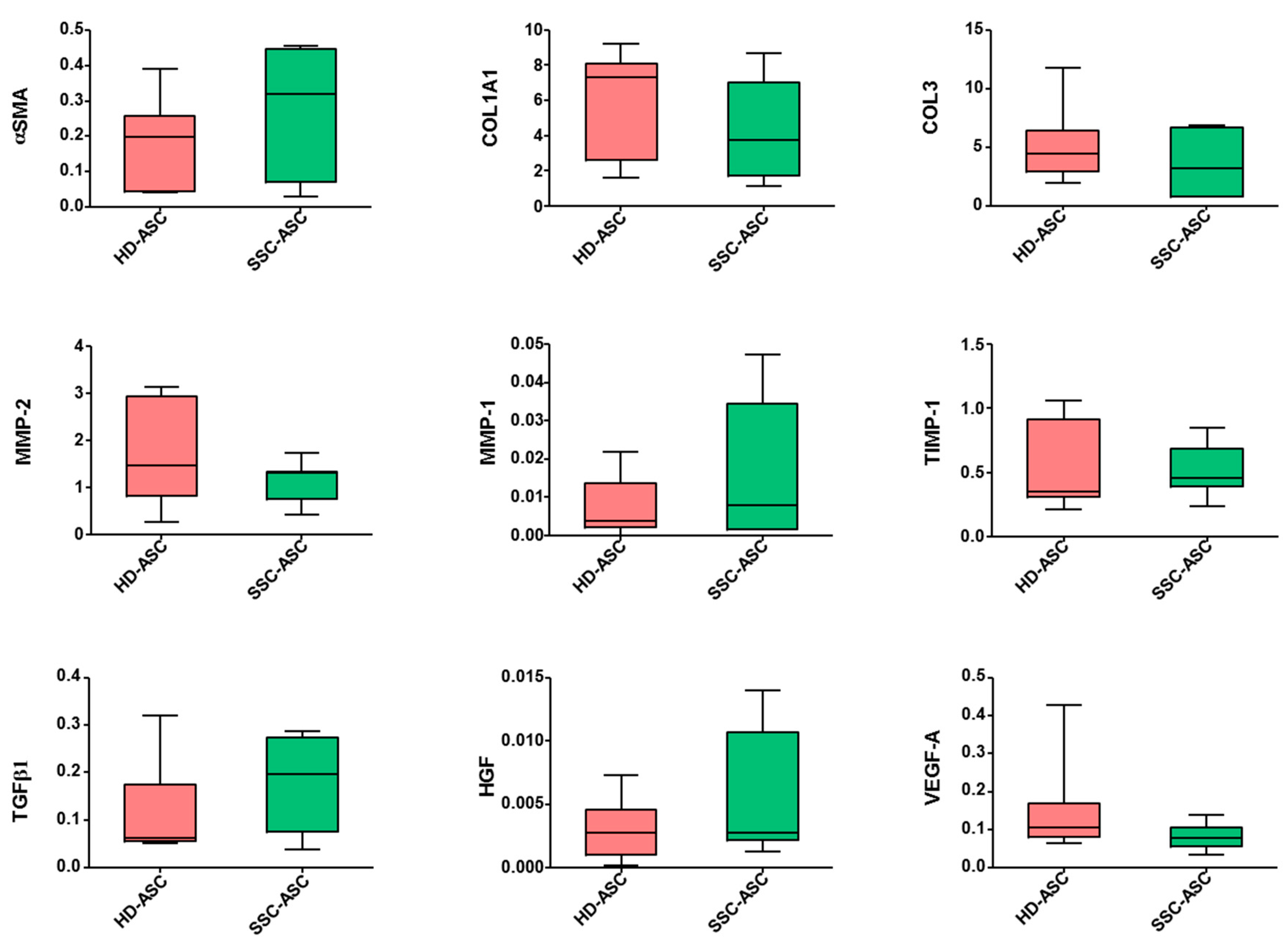
3.4. qRT-PCR and Secretome Analyses
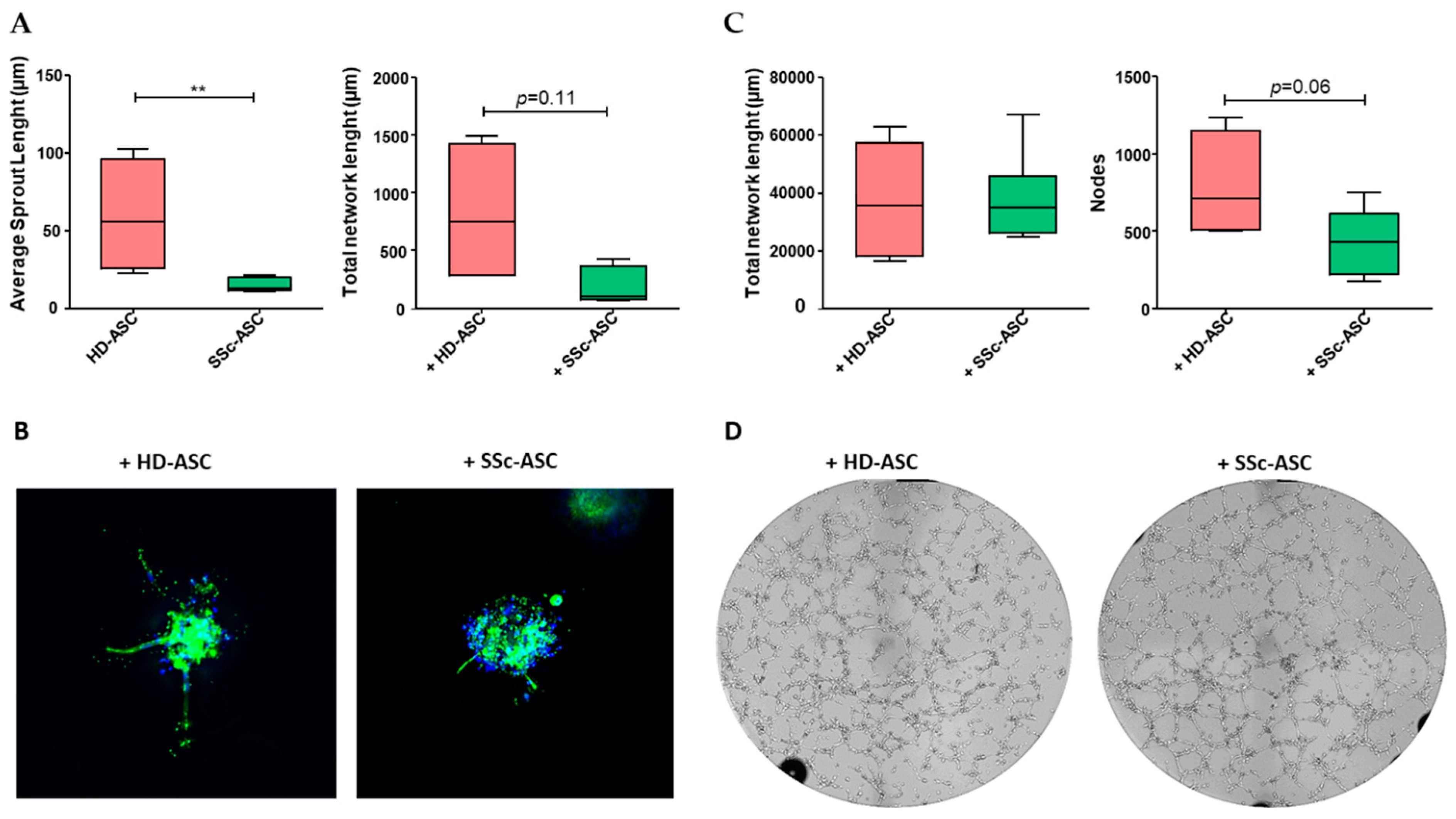
3.5. ASC Pro-Angiogenic Paracrine Activity
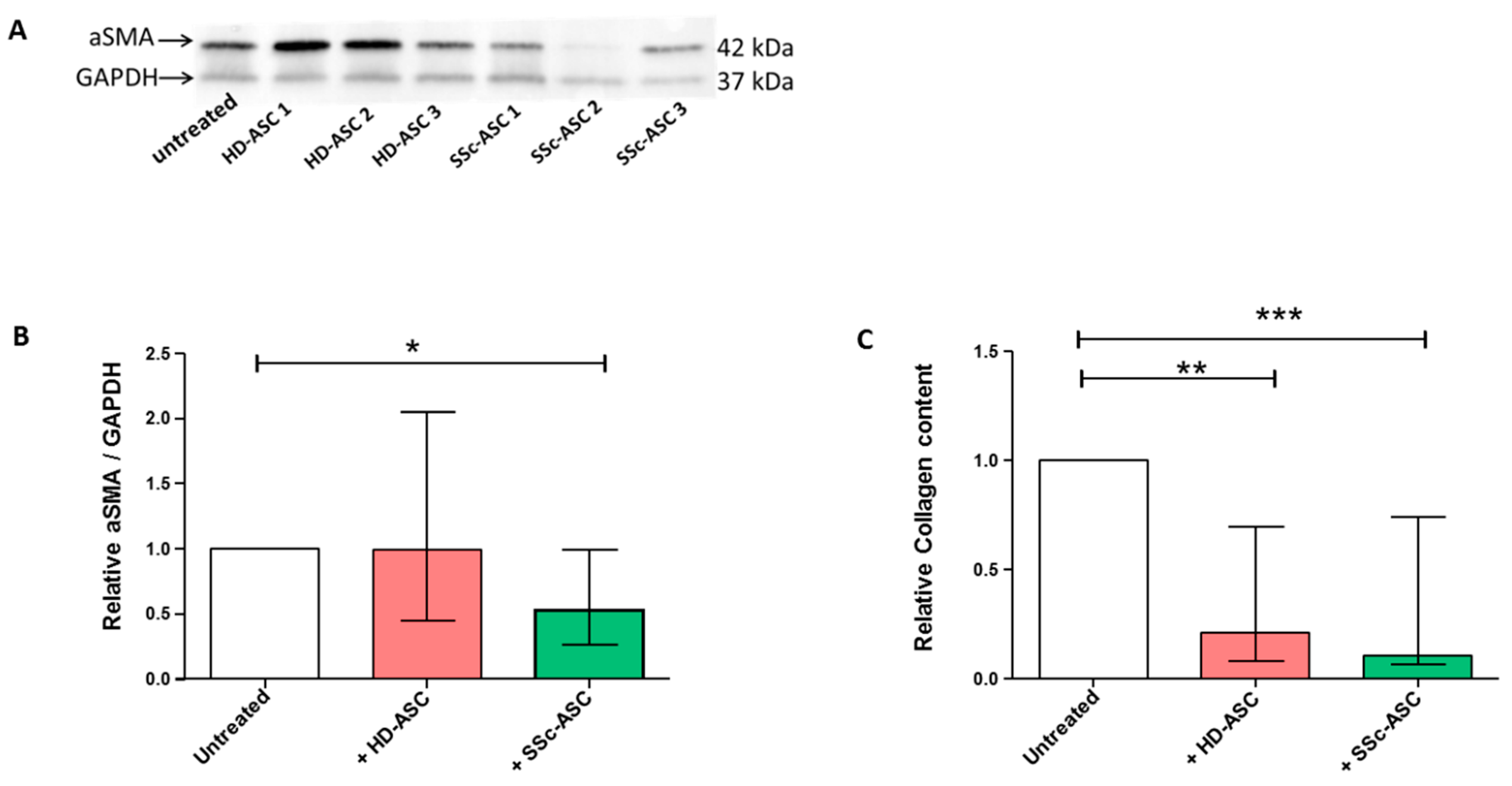
3.6. ASC Antifibrotic Paracrine Potential
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Del Papa, N.; Zaccara, E. From mechanisms of action to therapeutic application: A review on current therapeutic approaches and future directions in systemic sclerosis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 756–769. [Google Scholar] [CrossRef]
- Fransen, J.; Popa-Diaconu, D.; Hesselstrand, R.; Carreira, P.; Valentini, G.; Beretta, L.; Airo, P.; Inanc, M.; Ullman, S.; Balbir-Gurman, A.; et al. Clinical prediction of 5-year survival in systemic sclerosis: Validation of a simple prognostic model in EUSTAR centres. Ann. Rheum. Dis. 2011, 70, 1788–1792. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef]
- van Laar, J.M.; Farge, D.; Sont, J.K.; Naraghi, K.; Marjanovic, Z.; Larghero, J.; Schuerwegh, A.J.; Marijt, E.W.; Vonk, M.C.; Schattenberg, A.V.; et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: A randomized clinical trial. JAMA 2014, 311, 2490–2498. [Google Scholar] [CrossRef]
- Burt, R.K.; Farge, D. Systemic sclerosis: Autologous HSCT is efficacious, but can we make it safer? Nat. Rev. Rheumatol. 2018, 14, 189–191. [Google Scholar] [CrossRef]
- da Silva Meirelles, L. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Rozier, P.; Maria, A.; Goulabchand, R.; Jorgensen, C.; Guilpain, P.; Noël, D. Mesenchymal Stem Cells in Systemic Sclerosis: Allogenic or Autologous Approaches for Therapeutic Use? Front. Immunol. 2018, 9, 2938. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, S.; Enhe, J.; Ma, K.; Yang, S.; Sun, T.; Fu, X. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int. Wound J. 2014, 11, 701–710. [Google Scholar] [CrossRef]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, E.J.; Lee, S.Y.; Kim, J.H.; Shim, J.J.; Shin, C.; In, K.H.; Kang, K.H.; Uhm, C.S.; Kim, H.K.; et al. The effect of adipose stem cell therapy on pulmonary fibrosis induced by repetitive intratracheal bleomycin in mice. Exp. Lung Res. 2014, 40, 117–125. [Google Scholar] [CrossRef]
- Uji, M.; Kureya, Y.; Yamada, K.; Watanabe, T.; Konishi, K.; Asai, K.; Nakamura, T.; Hirata, K. Effect of intratracheal administration of adipose-derived stromal cells on bleomycin-induced lung injury in a rat model. Mech. Lung Inj. Repair 2015, 46, 81–91. [Google Scholar]
- Tashiro, J.; Elliot, S.J.; Gerth, D.J.; Xia, X.; Pereira-Simon, S.; Choi, R.; Catanuto, P.; Shahzeidi, S.; Toonkel, R.L.; Shah, R.H.; et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl. Res. 2015, 166, 554–567. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.C.; Petit, B.; Roger, P.; Batteux, F.; Le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis: MESENCHYMAL STEM CELLS IN MURINE SSc. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef]
- Scuderi, N.; Ceccarelli, S.; Onesti, M.G.; Fioramonti, P.; Guidi, C.; Romano, F.; Frati, L.; Angeloni, A.; Marchese, C. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013, 22, 779–795. [Google Scholar] [CrossRef]
- Onesti, M.G.; Fioramonti, P.; Carella, S.; Fino, P.; Marchese, C.; Scuderi, N. Improvement of Mouth Functional Disability in Systemic Sclerosis Patients over One Year in a Trial of Fat Transplantation versus Adipose-Derived Stromal Cells. Stem Cells Int. 2016, 2016, 2416192. [Google Scholar] [CrossRef]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.R.; Nguyen, P.S.; Chabannon, C.; Colavolpe, N.; Reynier, J.C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef]
- Daumas, A.; Magalon, J.; Jouve, E.; Truillet, R.; Casanova, D.; Giraudo, L.; Veran, J.; Benyamine, A.; Dignat-George, F.; Magalon, G.; et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Curr. Res. Transl. Med. 2017, 65, 40–43. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Cipriani, P.; Ruscitti, P.; Liakouli, V.; Giacomelli, R. Adipose stromal vascular fraction and regenerative therapy in SSc: Response to the article by Magalon et al. Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef]
- Magalon, J.; Velier, M.; Simoncini, S.; Dignat-George, F.; Granel, B.; Paul, P.; Sabatier, F. Response to: « Adipose stromal vascular fraction and regenerative therapy in SSc: Response to the article by Magalon et al » by De Benedetto et al. Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef]
- Manetti, M. Could autologous adipose-derived stromal vascular fraction turn out an unwanted source of profibrotic myofibroblasts in systemic sclerosis? Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef]
- Velier, M.; Magalon, J.; Simoncini, S.; Dignat-George, F.; Granel, B.; Paul, P.; Sabatier, F. Response to: « Could autologous adipose-derived stromal vascular fraction turn out an unwanted source of profibrotic myofibroblasts in systemic sclerosis? » by Manetti. Ann. Rheum. Dis. 2019. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Panzera, N.; Cipriani, P.; Mastroiaco, V.; Tessitore, A.; Liakouli, V.; Ruscitti, P.; Berardicurti, O.; Carubbi, F.; Guggino, G.; et al. Mesenchymal stem cells of Systemic Sclerosis patients, derived from different sources, show a profibrotic microRNA profiling. Sci. Rep. 2019, 9, 7144. [Google Scholar] [CrossRef]
- Lee, R.; Del Papa, N.; Introna, M.; Reese, C.F.; Zemskova, M.; Bonner, M.; Carmen-Lopez, G.; Helke, K.; Hoffman, S.; Tourkina, E. Adipose-derived mesenchymal stromal/stem cells in systemic sclerosis: Alterations in function and beneficial effect on lung fibrosis are regulated by caveolin-1. J. Scleroderma Relat. Disord. 2019, 4, 127–136. [Google Scholar] [CrossRef]
- Virzì, F.; Bianca, P.; Giammona, A.; Apuzzo, T.; Di Franco, S.; Mangiapane, L.R.; Colorito, M.L.; Catalano, D.; Scavo, E.; Nicotra, A.; et al. Combined platelet-rich plasma and lipofilling treatment provides great improvement in facial skin-induced lesion regeneration for scleroderma patients. Stem Cell Res. Ther. 2017, 8, 236. [Google Scholar] [CrossRef]
- Delorme, B.; Charbord, P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol. Med. 2007, 140, 67–81. [Google Scholar]
- Viau, S.; Chabrand, L.; Eap, S.; Lorant, J.; Rouger, K.; Goudaliez, F.; Sumian, C.; Delorme, B. Pathogen reduction through additive-free short-wave UV light irradiation retains the optimal efficacy of human platelet lysate for the expansion of human bone marrow mesenchymal stem cells. PLoS ONE 2017, 12, e0181406. [Google Scholar] [CrossRef][Green Version]
- Korff, T.; Kimmina, S.; Martiny-Baron, G.; Augustin, H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: Direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001, 15, 447–457. [Google Scholar] [CrossRef]
- Eglinger, J.; Karsjens, H.; Lammert, E. Quantitative assessment of angiogenesis and pericyte coverage in human cell-derived vascular sprouts. Inflamm. Regen. 2017, 37, 2. [Google Scholar] [CrossRef]
- Delorme, B.; Ringe, J.; Gallay, N.; Le Vern, Y.; Kerboeuf, D.; Jorgensen, C.; Rosset, P.; Sensebe, L.; Layrolle, P.; Häupl, T.; et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 2008, 111, 2631–2635. [Google Scholar] [CrossRef]
- Dominici, M.L.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Larghero, J.; Farge, D.; Braccini, A.; Lecourt, S.; Scherberich, A.; Fois, E.; Verrecchia, F.; Daikeler, T.; Gluckman, E.; Tyndall, A.; et al. Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann. Rheum. Dis. 2008, 67, 443–449. [Google Scholar] [CrossRef]
- Griffin, M.; Ryan, C.M.; Pathan, O.; Abraham, D.; Denton, C.P.; Butler, P.E. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: Implications for regenerative medicine. Stem Cell Res. Ther. 2017, 8, 23. [Google Scholar] [CrossRef]
- Capelli, C.; Zaccara, E.; Cipriani, P.; Di Benedetto, P.; Maglione, W.; Andracco, R.; Di Luca, G.; Pignataro, F.; Giacomelli, R.; Introna, M.; et al. Phenotypical and Functional Characteristics of In Vitro-Expanded Adipose-Derived Mesenchymal Stromal Cells From Patients With Systematic Sclerosis. Cell Transplant. 2017, 26, 841–854. [Google Scholar] [CrossRef]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. IJMS 2016, 17, 1164. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Liakouli, V.; Del Papa, B.; Di Padova, M.; Di Ianni, M.; Marrelli, A.; Alesse, E.; Giacomelli, R. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Treg s) with a functional phenotype: Implications for cellular-based therapy: SSc-MSC preserve immunomodulatory abilities. Clin. Exp. Immunol. 2013, 173, 195–206. [Google Scholar]
- Wyman, A.E.; Atamas, S.P. Sirtuins and Accelerated Aging in Scleroderma. Curr. Rheumatol. Rep. 2018, 20, 16. [Google Scholar] [CrossRef]
- Najar, M.; Bouhtit, F.; Melki, R.; Afif, H.; Hamal, A.; Fahmi, H.; Merimi, M.; Lagneaux, L. Mesenchymal Stromal Cell-Based Therapy: New Perspectives and Challenges. J. Clin. Med. 2019, 8, 626. [Google Scholar] [CrossRef]
- Magalon, J.; Velier, M.; Simoncini, S.; François, P.; Bertrand, B.; Daumas, A.; Benyamine, A.; Boissier, R.; Arnaud, L.; Lyonnet, L. Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 391–398. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Guggino, G.; Zazzeroni, F.; Alesse, E.; et al. Perivascular Cells in Diffuse Cutaneous Systemic Sclerosis Overexpress Activated ADAM12 and Are Involved in Myofibroblast Transdifferentiation and Development of Fibrosis. J. Rheumatol. 2016, 43, 1340–1349. [Google Scholar] [CrossRef]
- Nicolosi, P.A.; Tombetti, E.; Maugeri, N.; Rovere-Querini, P.; Brunelli, S.; Manfredi, A.A. Vascular Remodelling and Mesenchymal Transition in Systemic Sclerosis. Stem Cells Int. 2016, 2016, 4636859. [Google Scholar] [CrossRef] [PubMed]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Romano, E.; Rosa, I.; Fioretto, B.S.; Praino, E.; Guiducci, S.; Iannone, F.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Systemic Sclerosis Serum Steers the Differentiation of Adipose-Derived Stem Cells Toward Profibrotic Myofibroblasts: Pathophysiologic Implications. J. Clin. Med. 2019, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Nenasheva, T.; Nikolaev, A.; Diykanov, D.; Sukhanova, A.; Tcyganov, E.; Panteleev, A.; Bocharova, I.; Serdyuk, Y.; Nezlin, L.; Radaeva, T.; et al. The introduction of mesenchymal stromal cells induces different immunological responses in the lungs of healthy and M. tuberculosis infected mice. Olszewski MA, éditeur. PLoS ONE 2017, 12, e0178983. [Google Scholar] [CrossRef] [PubMed]

| Gene Abbreviation | Reference |
|---|---|
| αSMA | Hs00426835_g1 |
| COL1A1 | Hs00164004_m1 |
| COL3A1 | Hs00943809_m1 |
| MMP-2 | Hs01548727_m1 |
| MMP-1 | Hs00899658_m1 |
| TIMP-1 | Hs01092512_g1 |
| TGFβ1 | Hs00998133_m1 |
| HGF | Hs00300159_m1 |
| VEGF-A | Hs00900055_m1 |
| p16INK4 | Hs00233365_m1 |
| p21WAF | Hs00355782_m1 |
| p53 | Hs1034249_m1 |
| GAPDH | Hs02786624_g1 |
| SSc Patients | HD | |
|---|---|---|
| Gender F/M | 7/0 | 7/0 |
| Age (years, mean ± SD) | 45.8 ± 14.2 | 42.3 ± 13.1 |
| Body mass index (kg/m2, mean ± SD) | 22.5 ± 2.74 | 25.9 ± 3.11 |
| Tobacco (yes/no) | 1/6 | 3/4 |
| Arterial hypertension (yes/no) | 0/7 | 0/7 |
| SSc subclassification (Diffuse/Limited) | 4/3 | NA |
| % of Positive Cells ± SD | rMFI (Ratio of Mean Fluorescence Intensity) ± SD | Statistical Analysis | |||
|---|---|---|---|---|---|
| Membrane Marker | HD-ASC | SSc-ASC | HD-ASC | SSc-ASC | p-Value |
| CD40 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.97 ± 0.11 | 0.88 ± 0.01 | NS |
| CD34 | 0.35 ± 0.00 | 0.10 ± 0.00 | 0.97 ± 0.16 | 0.93 ± 0.02 | NS |
| CD45 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.94 ± 0.09 | 0.98 ± 0.03 | NS |
| HLA-DR | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.93 ± 0.05 | 0.95 ± 0.02 | NS |
| CD80 | 0.02 ± 0.00 | 0.02 ± 0.00 | 1.14 ± 0.10 | 1.00 ± 0.02 | NS |
| CD86 | 0.07 ± 0.00 | 0.02 ± 0.00 | 0.95 ± 0.09 | 1.01 ± 0.03 | NS |
| CD29 | 78.03 ± 0.14 | 60.07 ± 0.29 | 16.76 ± 6.22 | 15.23 ± 6.99 | NS |
| CD105 | 80.05 ± 0.13 | 72.58 ± 0.22 | 16.86 ± 6.95 | 22.38 ± 13.65 | NS |
| CD73 | 98.24 ± 0.00 | 97.32 ± 0.04 | 41.34 ± 1.73 | 46.71 ± 10.99 | NS |
| CD90 | 99.07 ± 0.01 | 97.52 ± 0.03 | 186.35 ± 31.57 | 171.04 ± 35.40 | NS |
| CD44 | 98.95 ± 0.01 | 99.79 ± 0.00 | 127.78 ± 14.44 | 161.73 ± 16.12 | NS |
| CD13 | 99.83 ± 0.00 | 99.92 ± 0.00 | 250.33 ± 106.47 | 352.41 ± 126.74 | NS |
| Molecule (pg/mL) | SSc-ASC | HD-ASC | p-Value |
|---|---|---|---|
| VEGF-A | 33.49 (±12.91) | 50.78 (±11.67) | 0.20 |
| MMP-2 | 28,635 (±1435) | 25,991 (±3883) | 0.70 |
| TIMP-1 | 39,805 (±48,657) | 51,625 (±41,909) | 0.51 |
| HGF | 10.90 (±1.721) | 11.70 (±4.676) | 0.82 |
| TGFβ1 | 667.1 (±72.94) | 620.3 (±64.06) | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VELIER, M.; SIMONCINI, S.; ABELLAN, M.; FRANCOIS, P.; EAP, S.; LAGRANGE, A.; BERTRAND, B.; DAUMAS, A.; GRANEL, B.; DELORME, B.; et al. Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro. J. Clin. Med. 2019, 8, 1979. https://doi.org/10.3390/jcm8111979
VELIER M, SIMONCINI S, ABELLAN M, FRANCOIS P, EAP S, LAGRANGE A, BERTRAND B, DAUMAS A, GRANEL B, DELORME B, et al. Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro. Journal of Clinical Medicine. 2019; 8(11):1979. https://doi.org/10.3390/jcm8111979
Chicago/Turabian StyleVELIER, Mélanie, Stéphanie SIMONCINI, Maxime ABELLAN, Pauline FRANCOIS, Sandy EAP, Anaïs LAGRANGE, Baptiste BERTRAND, Aurélie DAUMAS, Brigitte GRANEL, Bruno DELORME, and et al. 2019. "Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro" Journal of Clinical Medicine 8, no. 11: 1979. https://doi.org/10.3390/jcm8111979
APA StyleVELIER, M., SIMONCINI, S., ABELLAN, M., FRANCOIS, P., EAP, S., LAGRANGE, A., BERTRAND, B., DAUMAS, A., GRANEL, B., DELORME, B., DIGNAT GEORGE, F., MAGALON, J., & SABATIER, F. (2019). Adipose-Derived Stem Cells from Systemic Sclerosis Patients Maintain Pro-Angiogenic and Antifibrotic Paracrine Effects In Vitro. Journal of Clinical Medicine, 8(11), 1979. https://doi.org/10.3390/jcm8111979





