Incidence and Risk Factors for Tractional Macular Detachment after Anti-Vascular Endothelial Growth Factor Agent Pretreatment before Vitrectomy for Complicated Proliferative Diabetic Retinopathy
Abstract
1. Introduction
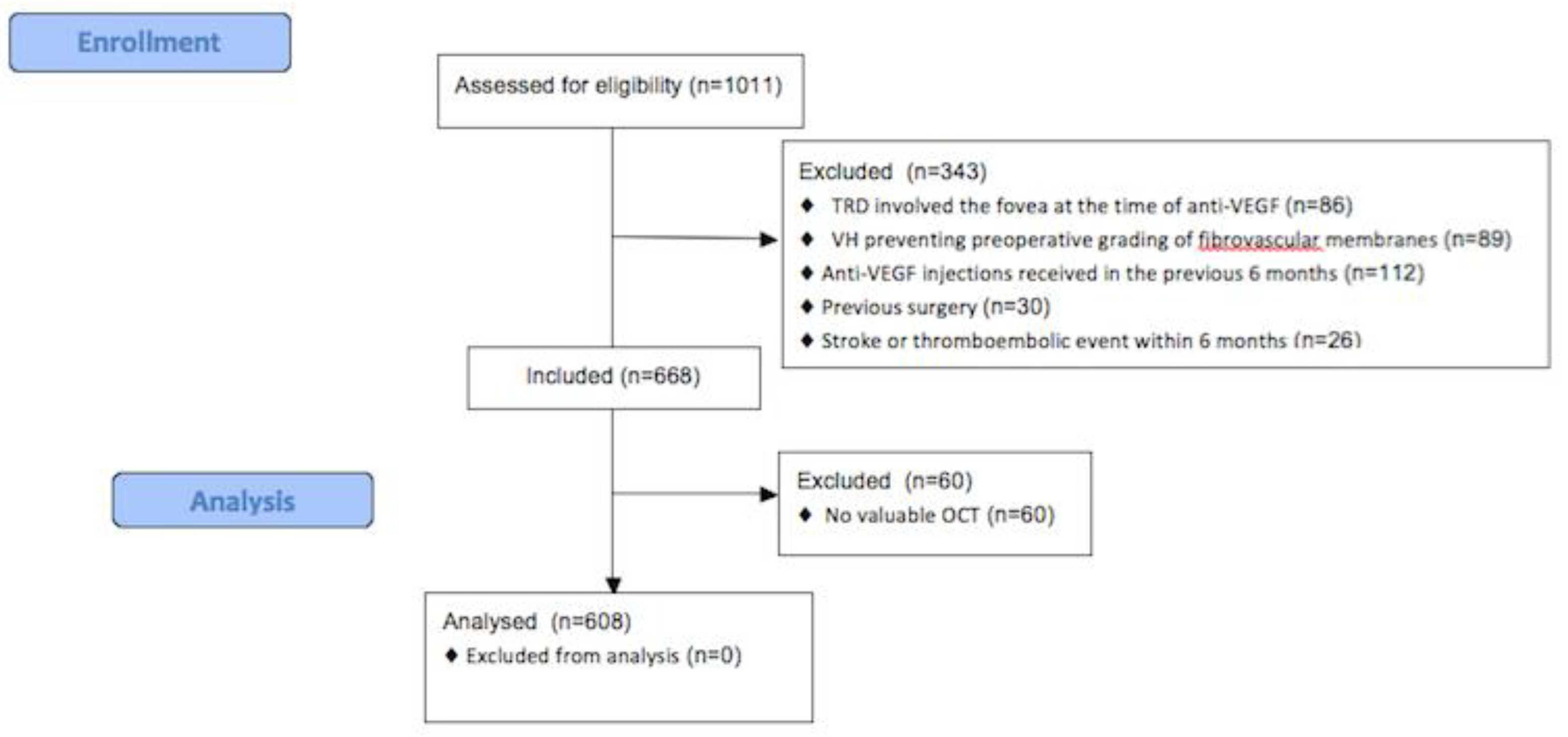
2. Materials and Methods
Statistical Analysis
3. Results
3.1. TMD Incidence
3.2. Univariate Analysis
3.3. Multivariable Analysis
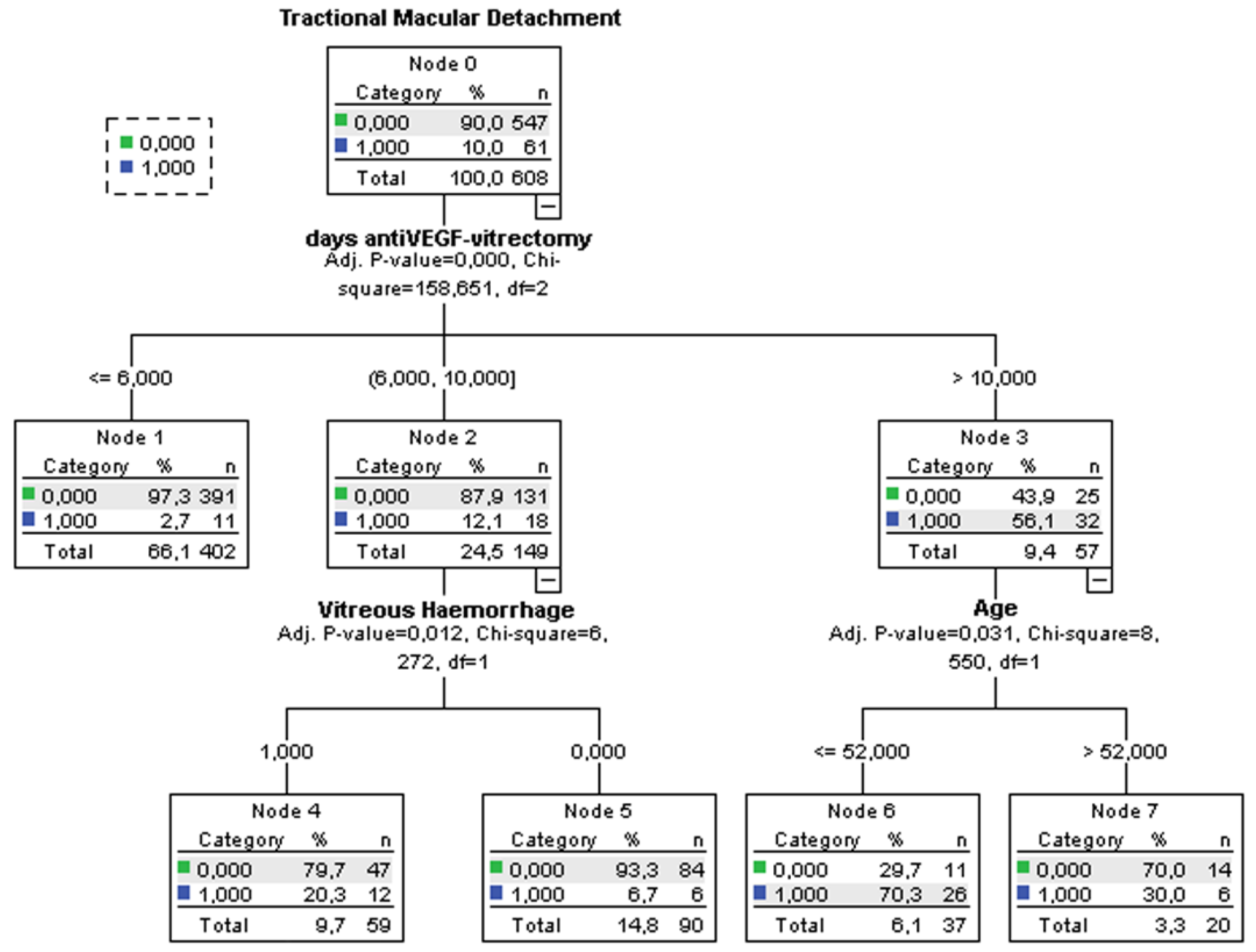
3.4. Decision-Tree Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliott, D.; Lee, M.S.; Abrams, G.W. Proliferative diabetic retinopathy: Principles and techniques of surgical treatment. In Retina, 4th ed.; Ryan, S.J., Ed.; Elsevier Mosby: St Louis, MO, USA, 2006; pp. 2413–2449. [Google Scholar]
- Segato, T.; Midena, E.; Grigoletto, F.; Zucchetto, M.; Fedele, D.; Piermarocchi, S.; Crepaldi, G. Veneto Group for Diabetic Retinopathy. The epidemiology and prevalence of diabetic retinopathy in the Veneto region of North East Italy. Diabet. Med. 1991, 8, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Tomalino, M.G.; Santoro, F.; Ghigo, L.D.; Cairo, M.; Aimone, M.; Pietragalla, G.B.; Passera, P.; Montanaro, M.; Molinatti, G.M. Diabetic retinopathy as a cause of blindness in the province of Turin, North-west Italy, in 1967–1991. Diabet. Med. 1995, 12, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.A.; Michels, R.G.; Rice, E.F. Vitrectomy for Diabetic Traction Retinal Detachment Involving the Macula. Am. J. Ophthalmol. 1983, 95, 22–33. [Google Scholar] [CrossRef]
- Schrey, S.; Krepler, K.; Wedrich, A. Incidence of rhegmatogenous retinal detachment after vitrectomy in eyes of diabetic patients. Retina 2006, 26, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, V.S.; Augsburger, J.J.; Hutchins, R.K.; Raymond, L.A.; Krug, S. Fibrovascular ingrowth at sclerotomy sites in vitrectomized diabetic eyes with recurrent vitreous hemorrhage: Ultrasound biomicroscopy findings. Ophthalmology 2004, 111, 1215–1221. [Google Scholar] [CrossRef]
- Shi, L.; Huang, Y.-F. Postvitrectomy diabetic vitreous hemorrhage in proliferative diabetic retinopathy. J. Res. Med. Sci. 2012, 17, 865–871. [Google Scholar]
- Blum, A.; Socea, D.; Ben-Shushan, R.S.; Keinan-Boker, L.; Naftali, M.; Segol, G.; Tamir, S. A decrease in VEGF and inflammatory markers is associated with diabetic proliferative retinopathy. Eur. Cytokine Netw. 2012, 23, 158–162. [Google Scholar] [CrossRef]
- Morera, Y.; González, R.; Lamdan, H.; Pérez, L.; González, Y.; Agüero, J.; Castro, J.; Romero, J.C.; Etchegoyen, A.Y.; Ayala, M.; et al. Vaccination with a mutated variant of human Vascular Endothelial Growth Factor (VEGF) blocks VEGF-induced retinal neovascularization in a rabbit experimental model. Exp. Eye Res. 2014, 122, 102–109. [Google Scholar] [CrossRef]
- Wisniewska-Kruk, J.; Klaassen, I.; Vogels, I.M.; Magno, A.L.; Lai, C.M.; Van Noorden, C.J.; Schlingemann, R.O.; Rakoczy, E.P. Molecular analysis of blood-retinal barrier loss in the Akimba mouse, a model of advanced diabetic retinopathy. Exp. Eye Res. 2014, 122, 123–131. [Google Scholar] [CrossRef]
- Smith, J.M.; Steel, D.H.W. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2015, 2015, CD008214. [Google Scholar] [CrossRef]
- Pakzad-Vaezi, K.; Albiani, D.A.; Kirker, A.W.; Merkur, A.B.; Kertes, P.J.; Eng, K.T.; Fallah, N.; Forooghian, F. A Randomized Study Comparing the Efficacy of Bevacizumab and Ranibizumab as Pre-treatment for Pars Plana Vitrectomy in Proliferative Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.P.; Maberley, D.A. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: A systematic review and meta-analysis. Retina 2015, 35, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Liu, H.-Y.; Mota, S.E.H.-D.; Romano, M.R.; Falavarjani, K.G.; Ahmadieh, H.; Xu, X.; Liu, K. Vitrectomy With or Without Preoperative Intravitreal Bevacizumab for Proliferative Diabetic Retinopathy: A Meta-Analysis of Randomized Controlled Trials. Am. J. Ophthalmol. 2013, 156, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Q.; Zhu, H.; Zhao, P.-Q.; Hu, Y.-Q. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br. J. Ophthalmol. 2011, 95, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ren, X.; Wei, H.; Zhao, L.; Zhang, X.; Liu, J.; Su, C.; Tan, L.; Li, X. Intravitreal Conbercept (Kh902) for Surgical Treatment of Severe Proliferative Diabetic Retinopathy. Retina 2016, 36, 938–943. [Google Scholar] [CrossRef]
- Hernández-Da Mota, S.E.; Nuñez-Solorio, S.M. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2010, 20, 1047–1052. [Google Scholar] [CrossRef]
- Comyn, O.; Wickham, L.; Charteris, D.G.; Sullivan, P.M.; Ezra, E.; Gregor, Z.; Aylward, G.W.; da Cruz, L.; Fabinyi, D.; Peto, T.; et al. Ranibizumab pretreatment in diabetic vitrectomy: A pilot randomised controlled trial (the RaDiVit study). Eye 2017, 31, 1253–1258. [Google Scholar] [CrossRef]
- Farahvash, M.S.; Majidi, A.R.; Roohipoor, R.; Ghassemi, F. Preoperative injection of intravitreal bevacizumab in dense diabetic vitreous hemorrhage. Retina 2011, 31, 1254–1260. [Google Scholar] [CrossRef]
- Ahn, J.; Woo, S.J.; Chung, H.; Park, K.H. The Effect of Adjunctive Intravitreal Bevacizumab for Preventing Postvitrectomy Hemorrhage in Proliferative Diabetic Retinopathy. Ophthalmology 2011, 118, 2218–2226. [Google Scholar] [CrossRef]
- Castillo, J.; Aleman, I.; Rush, S.W.; Rush, R.B. Preoperative Bevacizumab Administration in Proliferative Diabetic Retinopathy Patients Undergoing Vitrectomy: A Randomized and Controlled Trial Comparing Interval Variation. Am. J. Ophthalmol. 2017, 183, 1–10. [Google Scholar] [CrossRef]
- Reibaldi, M.; Longo, A.; Pulvirenti, A.; Avitabile, T.; Russo, A.; Cillino, S.; Mariotti, C.; Casuccio, A. Geo-Epidemiology of Age-Related Macular Degeneration: New Clues Into the Pathogenesis. Am. J. Ophthalmol. 2016, 161, 78–93.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Oyakawa, R.T.; Schachat, A.P.; Michels, R.G.; Rice, T.A. Complications of vitreous surgery for diabetic retinopathy. (I. Postoperative complications). Ophthalmology 1983, 90, 517–521. [Google Scholar] [CrossRef]
- Schachat, A.P.; Oyakawa, R.T.; Michels, R.G.; Rice, T.A. Complications of vitreous surgery for diabetic retinopathy (II. Postoperative complications). Ophthalmology 1983, 90, 522–530. [Google Scholar] [CrossRef]
- Tolentino, F.I.; Cajita, V.N.; Gancayco, T.; Skates, S. Vitreous hemorrhage after closed vitrectomy for proliferative diabetic retinopathy. Ophthalmology 1989, 96, 1495–1500. [Google Scholar] [CrossRef]
- Novak, M.A.; Rice, T.A.; Michels, R.G.; Auer, C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology 1984, 91, 1485–1489. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fisher, Y.L. Intravitreal bevacizumab treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006, 26, 275–278. [Google Scholar] [CrossRef]
- Avery, R.L.; Pearlman, J.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J.; Wendel, R.; Patel, A. Intravitreal bevacizumab in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006, 113, 1695–1705. [Google Scholar] [CrossRef]
- Chen, E.; Park, C.H. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006, 26, 699–700. [Google Scholar] [CrossRef]
- El-Sabagh, H.A.; Abdelghaffar, W.; Labib, A.M.; Mateo, C.; Hashem, T.M.; Al-Tamimi, D.M.; Selim, A.A. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: Histopathologic findings and clinical implications. Ophthalmology 2011, 118, 636–641. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Maia, M.; Flynn HWJr Saravia, M.; Avery, R.L.; Wu, L.; Eid Farah, M.; Pieramici, D.J.; Berrocal, M.H.; Sanchez, J.G. Tractional retinal detachment following intravitreal bevacizumab in patients with severe proliferative diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 213–216. [Google Scholar] [CrossRef]
- Jorge, R.; Costa, R.A.; Calucci, D.; Cintra, L.P.; Scott, I.U. Intravitreal bevacizumab for persistent new vessels in diabetic retinopathy. Retina 2006, 26, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Van Geest, R.J.; Lesnik-Oberstein, S.Y.; Tan, H.S.; Mura, M.; Goldschmeding, R.; Van Noorden, C.J.; Klaassen, I.; Schlingemann, R.O. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012, 96, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, T.; Jiang, R.; Chang, Q.; Zhang, Y.; Huang, X.; Gao, X.; Jin, H.; Xu, G. Vitreous Fibronectin and Fibrinogen Expression Increased in Eyes With Proliferative Diabetic Retinopathy After Intravitreal Anti-VEGF Therapy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5783–5791. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Shoeibi, N.; Entezari, M.; Monshizadeh, R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: A randomized clinical trial. Ophthalmology 2009, 116, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Honda, S.; Tsukahara, Y.; Negi, A. Preferable use of intravitreal bevacizumab as a pretreatment of vitrectomy for severe proliferative diabetic retinopathy. Eye (Lond) 2009, 23, 108–111. [Google Scholar] [CrossRef]
- Oshima, Y.; Shima, C.; Wakabayashi, T.; Kusaka, S.; Shiraga, F.; Ohji, M.; Tano, Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009, 116, 927–938. [Google Scholar] [CrossRef]
- Reibaldi, M.; Longo, A.; Romano, M.R.; Cennamo, G.; Mariotti, C.; Boscia, F.; Bonfiglio, V.; Avitabile, T. Delayed suprachoroidal hemorrhage after pars plana vitrectomy: 5-year results of a retrospective multicenter cohort study. Am. J. Ophthalmol. 2015, 160, 1235–1242. [Google Scholar] [CrossRef]
- Piper, M.E.; Loh, W.Y.; Smith, S.S.; Japuntich, S.J.; Baker, T.B. Using decision tree analysis to identify risk factors for relapse to smoking. Subst. Use Misuse 2011, 46, 492–510. [Google Scholar] [CrossRef]
| Variables | Eyes with TMD after anti-VEGF n = 61 | Eyes without TMD after anti-VEGF n = 547 | p Value | OR (95% CI) |
|---|---|---|---|---|
| Age, mean ± SD (years) | 48.7 ± 10.8 | 54.8 ± 10.9 | <0.001 a | 0.95 (0.93–0.97) |
| Males, n (%) | 33 (54.1) | 279 (51.0) | 0.686 | 1.13 (0.67–1.92) |
| Diabetes type 1, n (%) | 4 (6.6) | 26 (4.8) | 0.758 | 0.71 (0.24–2.11) |
| HbA1c, (%) | 7.9 ± 0.7 | 7.7 ± 0.4 | 0.004 a | 2.12 (1.27–3.53) |
| Length of diabetes, (years) | 16.3 ± 4.9 | 16.3 ± 3.4 | 0.969 | 0.99 (0.94–1.06) |
| Pseudophakic/aphakic, n (%) | 18 (33.8) | 194 (35.0) | 0.397 | 0.76 (0.43–1.36) |
| Vitreous hemorrhage | 44 (72.1) | 301 (55.0) | 0.014 a | 2.12 (1.18–3.80) |
| Localized photocoagulation, n (%) | 51 (86.6) | 385 (70.4) | 0.035 a | 2.14 (1.06–4.33) |
| Extensive photocoagulation, n (%) | 10 (16.4) | 162 (29.6) | 0.035 a | 0.47 (0.23–0.94) |
| Days between anti-VEGF and vitrectomy | 11.8 ± 6.1 | 5.7 ± 2.7 | <0.001 a | 1.40 (1.30–1.52) |
| Variables | OR | (95% CI) | p Value |
|---|---|---|---|
| Days between anti-VEGF and vitrectomy | 0.71 | 0.65–0.76 | <0.001 |
| Vitreous Hemorrhage | 0.23 | 0.11–0.49 | <0.001 |
| Age | 1.05 | 1.02–1.08 | 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, A.; Longo, A.; Avitabile, T.; Bonfiglio, V.; Fallico, M.; Boscia, F.; Furino, C.; Cillino, S.; Toro, M.; Rejdak, R.; et al. Incidence and Risk Factors for Tractional Macular Detachment after Anti-Vascular Endothelial Growth Factor Agent Pretreatment before Vitrectomy for Complicated Proliferative Diabetic Retinopathy. J. Clin. Med. 2019, 8, 1960. https://doi.org/10.3390/jcm8111960
Russo A, Longo A, Avitabile T, Bonfiglio V, Fallico M, Boscia F, Furino C, Cillino S, Toro M, Rejdak R, et al. Incidence and Risk Factors for Tractional Macular Detachment after Anti-Vascular Endothelial Growth Factor Agent Pretreatment before Vitrectomy for Complicated Proliferative Diabetic Retinopathy. Journal of Clinical Medicine. 2019; 8(11):1960. https://doi.org/10.3390/jcm8111960
Chicago/Turabian StyleRusso, Andrea, Antonio Longo, Teresio Avitabile, Vincenza Bonfiglio, Matteo Fallico, Francesco Boscia, Claudio Furino, Salvatore Cillino, Mario Toro, Robert Rejdak, and et al. 2019. "Incidence and Risk Factors for Tractional Macular Detachment after Anti-Vascular Endothelial Growth Factor Agent Pretreatment before Vitrectomy for Complicated Proliferative Diabetic Retinopathy" Journal of Clinical Medicine 8, no. 11: 1960. https://doi.org/10.3390/jcm8111960
APA StyleRusso, A., Longo, A., Avitabile, T., Bonfiglio, V., Fallico, M., Boscia, F., Furino, C., Cillino, S., Toro, M., Rejdak, R., Nowomiejska, K., & Reibaldi, M. (2019). Incidence and Risk Factors for Tractional Macular Detachment after Anti-Vascular Endothelial Growth Factor Agent Pretreatment before Vitrectomy for Complicated Proliferative Diabetic Retinopathy. Journal of Clinical Medicine, 8(11), 1960. https://doi.org/10.3390/jcm8111960









