Relationship between Lower Urinary Tract Symptoms and Prostatic Urethral Stiffness Using Strain Elastography: Initial Experiences
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Selection and Medical Ethics
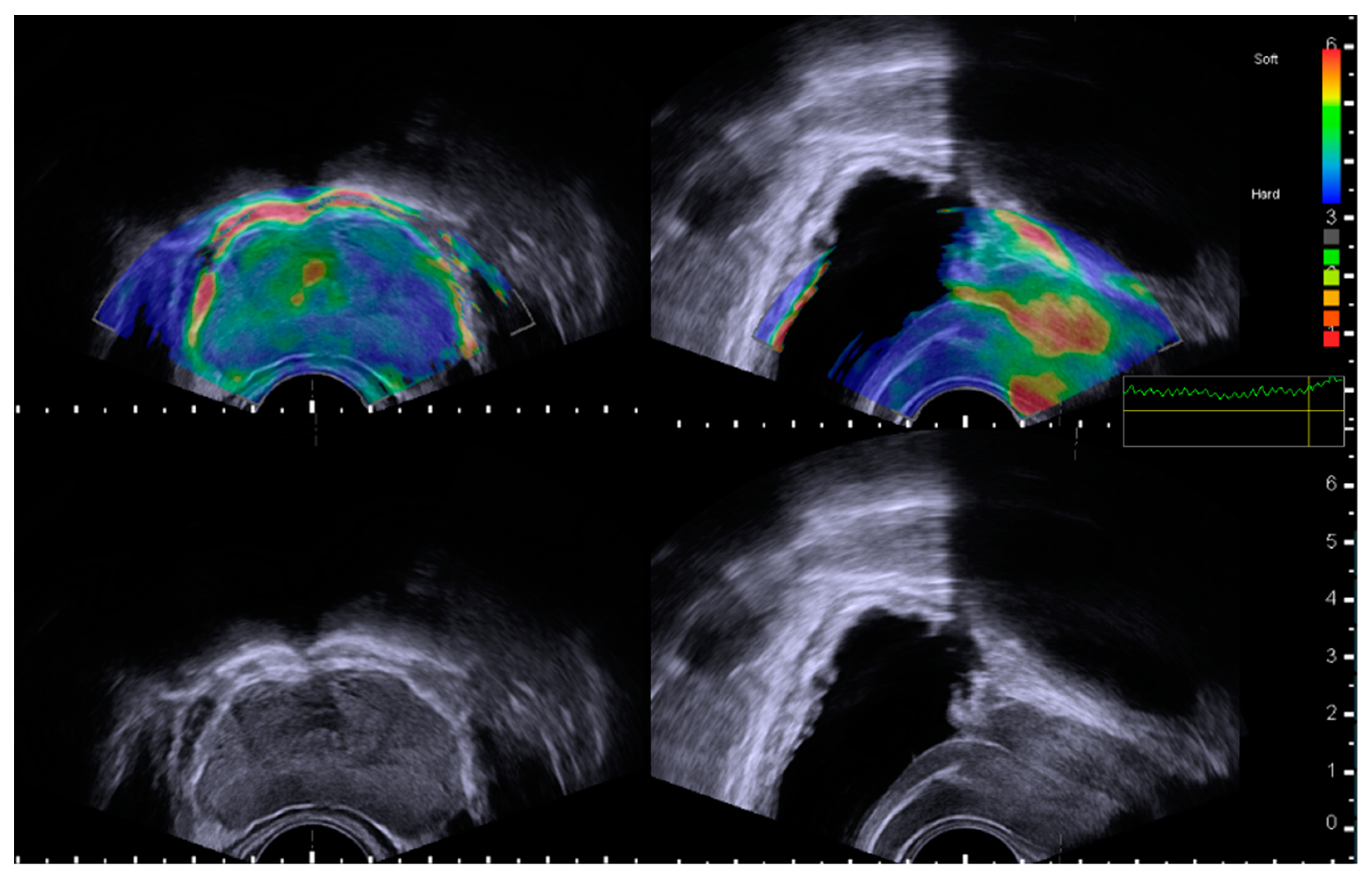
2.2. Assessment of Prostatic Anatomical Factors Using TRUS and Elastography
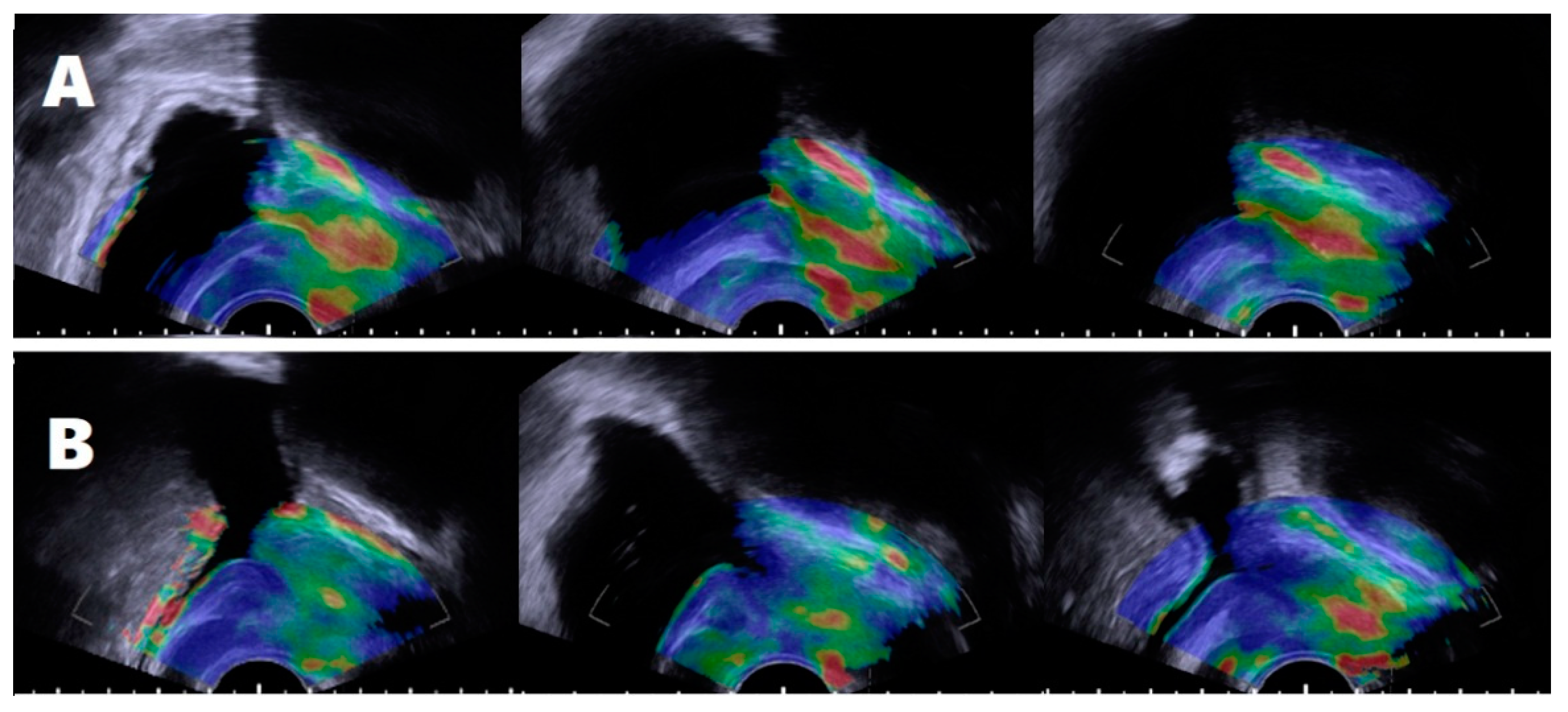
2.3. Defining the Subject’s Groups According to Periurethral Elastography
2.4. Statistical Analysis
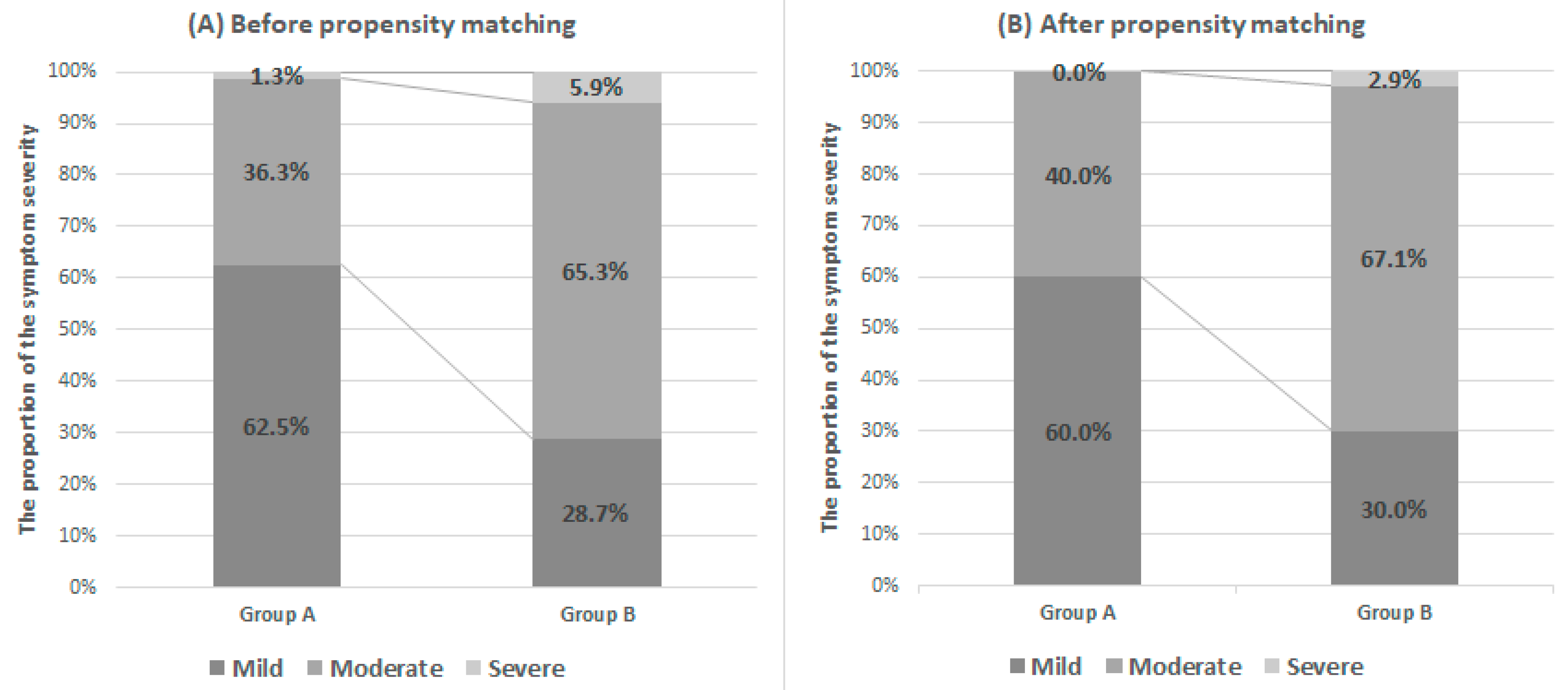
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Te, A.E.; Pressler, L.B.; Olsson, C.A. Transition zone index as a method of assessing benign prostatic hyperplasia: Correlation with symptoms, urine flow and detrusor pressure. J. Urol. 1995, 154, 1764–1769. [Google Scholar] [CrossRef]
- Kurita, Y.; Ushiyama, T.; Suzuki, K.; Fujita, K.; Kawabe, K. Transition zone ratio and prostate-specific antigen density: The index of response of benign prostatic hypertrophy to an alpha blocker. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 1996, 3, 361–366. [Google Scholar] [CrossRef]
- Kurita, Y.; Masuda, H.; Terada, H.; Suzuki, K.; Fujita, K. Transition zone index as a risk factor for acute urinary retention in benign prostatic hyperplasia. Urology 1998, 51, 595–600. [Google Scholar] [CrossRef]
- Herbison, A.E.; Fraundorfer, M.R.; Walton, J.K. Association between symptomatology and uroflowmetry in benign prostatic hypertrophy. Br. J. Urol. 1988, 62, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Girman, C.J.; Jacobsen, S.J.; Guess, H.A.; Oesterling, J.E.; Chute, C.G.; Panser, L.A.; Lieber, M.M. Natural history of prostatism: Relationship among symptoms, prostate volume and peak urinary flow rate. J. Urol. 1995, 153, 1510–1515. [Google Scholar] [CrossRef]
- Anderson, J.B.; Roehrborn, C.G.; Schalken, J.A.; Emberton, M. The progression of benign prostatic hyperplasia: Examining the evidence and determining the risk. Eur. Urol. 2001, 39, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Slawin, K.M.; Kattan, M.W. The Use of Nomograms for Selecting BPH Candidates for Dutasteride Therapy. Rev. Urol. 2004, 6, S40–S45. [Google Scholar]
- Watanabe, H. New concept of BPH: PCAR theory. Prostate 1998, 37, 116–125. [Google Scholar] [CrossRef]
- Keqin, Z.; Zhishun, X.; Jing, Z.; Haixin, W.; Dongqing, Z.; Benkang, S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology 2007, 70, 1096–1099. [Google Scholar] [CrossRef]
- Doo, C.K.; Uh, H.S. Anatomic configuration of prostate obtained by noninvasive ultrasonography can predict clinical voiding parameters for determining BOO in men with LUTS. Urology 2009, 73, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Bang, W.J.; Kim, H.W.; Lee, J.Y.; Lee, D.H.; Hah, Y.S.; Lee, H.H.; Koo, K.C.; Yu, H.S.; Ham, W.S.; Cho, K.S. Prostatic urethral angulation associated with urinary flow rate and urinary symptom scores in men with lower urinary tract symptoms. Urology 2012, 80, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.K.; Han, J.H.; Choi, H.C.; Kang, D.H.; Lee, J.Y.; Kim, J.H.; Oh, C.K.; Choi, Y.D.; Cho, K.S. Clinical significance of peripheral zone thickness in men with lower urinary tract symptoms/benign prostatic hyperplasia. BJU Int. 2016, 117, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cantiello, F.; Cicione, A.; Salonia, A.; Autorino, R.; Tucci, L.; Madeo, I.; Damiano, R. Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: A prospective cohort study. Urology 2013, 81, 1018–1023. [Google Scholar] [CrossRef]
- Fujisaki, A.; Shigeta, M.; Shimoinaba, M.; Yoshimura, Y. Influence of adequate pelvic floor muscle contraction on the movement of the coccyx during pelvic floor muscle training. J. Phys. Ther. Sci. 2018, 30, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; Rodriguez-Nieves, J.A.; Mehra, R.; Vezina, C.A.; Sarma, A.V.; Macoska, J.A. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate 2013, 73, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gharaee-Kermani, M.; Kunju, L.; Hollingsworth, J.M.; Adler, J.; Arruda, E.M.; Macoska, J.A. Prostatic fibrosis is associated with lower urinary tract symptoms. J. Urol. 2012, 188, 1375–1381. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, J.K.; Lee, J.Y.; Kang, D.H.; Choi, H.C.; Lee, J.S.; Cho, K.S. Is periurethral calcification associated with urinary flow rate and symptom severity in men with lower urinary tract symptoms-benign prostatic hyperplasia? A retrospective review. Urology 2015, 85, 1156–1161. [Google Scholar] [CrossRef]
- Garra, B.S. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007, 23, 255–268. [Google Scholar] [CrossRef]
- Palmeri, M.L.; Nightingale, K.R. Acoustic radiation force-based elasticity imaging methods. Interface Focus 2011, 1, 553–564. [Google Scholar] [CrossRef]
- Zhai, L.; Madden, J.; Foo, W.C.; Mouraviev, V.; Polascik, T.J.; Palmeri, M.L.; Nightingale, K.R. Characterizing stiffness of human prostates using acoustic radiation force. Ultrason. Imaging 2010, 32, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Madden, J.; Foo, W.C.; Palmeri, M.L.; Mouraviev, V.; Polascik, T.J.; Nightingale, K.R. Acoustic radiation force impulse imaging of human prostates ex vivo. Ultrasound Med. Biol. 2010, 36, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, S.; Zhang, Y.; Tang, J.; Zhou, Y. Elastic modulus of the prostate: A new non-invasive feature to diagnose bladder outlet obstruction in patients with benign prostatic hyperplasia. Ultrasound Med. Biol. 2014, 40, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nieves, J.A.; Macoska, J.A. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat. Rev. Urol. 2013, 10, 546–550. [Google Scholar] [CrossRef]
- McNeal, J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol. Clin. N. Am. 1990, 17, 477–486. [Google Scholar]
- Treece, G.; Lindop, J.; Chen, L.; Housden, J.; Prager, R.; Gee, A. Real-time quasi-static ultrasound elastography. Interface Focus 2011, 1, 540–552. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Li, Y.M.; Fei, X.; Lv, F.Q.; He, E.H.; Li, Q.Y.; Shi, H.Y. Differentiation of prostate cancer from benign lesions using strain index of transrectal real-time tissue elastography. Eur. J. Radiol. 2012, 81, 857–862. [Google Scholar] [CrossRef]
- Barry, M.J.; Fowler, F.J.J.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Costa, W.S.; de Carvalho, A.M.; Babinski, M.A.; Chagas, M.A.; Sampaio, F.J. Volumetric density of elastic and reticular fibers in transition zone of controls and patients with benign prostatic hyperplasia. Urology 2004, 64, 693–697. [Google Scholar] [CrossRef]
- Shapiro, E.; Becich, M.J.; Hartanto, V.; Lepor, H. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J. Urol. 1992, 147, 1293–1297. [Google Scholar] [CrossRef]
- Alan, B.; Utangac, M.; Goya, C.; Daggulli, M. Role of Acoustic Radiation Force Impulse (ARFI) Elastography in Determination of Severity of Benign Prostate Hyperplasia. Med. Sci. Monit. 2016, 22, 4523–4528. [Google Scholar] [CrossRef] [PubMed]

| Midline Cyst or Periurethral Degenerative Cystic Nodule | 4 |
|---|---|
| Prostate calcification including periurethral calcification | 24 |
| Inappropriate quality-scale bar | 14 |
| Lacking echotexture of bladder neck and proximal urethral course in B-mode | 27 |
| Before Matching | Group A (N = 80) | Group B (N = 101) | p-Value |
| Age (y) | 51.5 (47.0–56.0) | 54.0 (50.0–59.0) | 0.022 |
| PSA (ng/mL) | 0.949 (0.60–1.10) | 1.004 (0.60–1.20) | 0.735 |
| TPV (mL) | 25.54 ± 6.49 | 27.27 ± 7.08 | 0.092 |
| TZV (mL) | 6.76 ± 2.91 | 9.44 ± 6.42 | 0.001 |
| TZI | 0.26 ± 0.06 | 0.35 ± 0.37 | 0.033 |
| IPSS | |||
| Total | 7.38 ±4.42 | 11.12 ± 4.85 | <0.001 |
| IPSS-V | 3.18 ± 2.63 | 5.62 ± 2.98 | <0.001 |
| IPSS-S | 3.01 ± 2.17 | 4.12 ± 2.18 | <0.001 |
| Post-micturition | 1.19 ± 0.91 | 1.38 ± 1.87 | 0.074 |
| QoL | 1.81 ± 1.10 | 2.31 ± 1.01 | 0.002 |
| After Matching | Group A (N = 70) | Group B (N = 70) | p-Value |
| Age (y) | 52.0 (48.5–58.0) | 52.5 (48.0–57.0) | 0.660 |
| PSA (ng/mL) | 0.89 (0.60–1.10) | 0.96 (0.60–1.20) | 0.869 |
| TPV (mL) | 25.46 ± 5.76 | 24.97 ± 54.04 | 0.596 |
| TZV (mL) | 6.99 ± 2.90 | 7.01 ± 2.74 | 0.952 |
| TZI | 0.27 ± 0.62 | 0.28 ± 0.60 | 0.509 |
| IPSS | |||
| Total | 7.66 ± 4.36 | 10.51 ± 4.68 | <0.001 |
| IPSS-V | 3.24 ± 2.20 | 5.31 ± 2.73 | <0.001 |
| IPSS-S | 3.14 ± 2.17 | 3.61 ± 2.04 | 0.187 |
| Post-micturition | 1.27 ± 0.95 | 1.59 ± 1.21 | 0.089 |
| QoL | 1.84 ± 1.14 | 2.24 ± 1.04 | 0.032 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.K.; Kim, D.K.; Lee, J.Y.; Kim, J.W.; Cho, K.S. Relationship between Lower Urinary Tract Symptoms and Prostatic Urethral Stiffness Using Strain Elastography: Initial Experiences. J. Clin. Med. 2019, 8, 1929. https://doi.org/10.3390/jcm8111929
Kwon JK, Kim DK, Lee JY, Kim JW, Cho KS. Relationship between Lower Urinary Tract Symptoms and Prostatic Urethral Stiffness Using Strain Elastography: Initial Experiences. Journal of Clinical Medicine. 2019; 8(11):1929. https://doi.org/10.3390/jcm8111929
Chicago/Turabian StyleKwon, Jong Kyou, Do Kyung Kim, Joo Yong Lee, Jong Won Kim, and Kang Su Cho. 2019. "Relationship between Lower Urinary Tract Symptoms and Prostatic Urethral Stiffness Using Strain Elastography: Initial Experiences" Journal of Clinical Medicine 8, no. 11: 1929. https://doi.org/10.3390/jcm8111929
APA StyleKwon, J. K., Kim, D. K., Lee, J. Y., Kim, J. W., & Cho, K. S. (2019). Relationship between Lower Urinary Tract Symptoms and Prostatic Urethral Stiffness Using Strain Elastography: Initial Experiences. Journal of Clinical Medicine, 8(11), 1929. https://doi.org/10.3390/jcm8111929






