Management of Dehydration in Patients Suffering Swallowing Difficulties
Abstract
1. Introduction
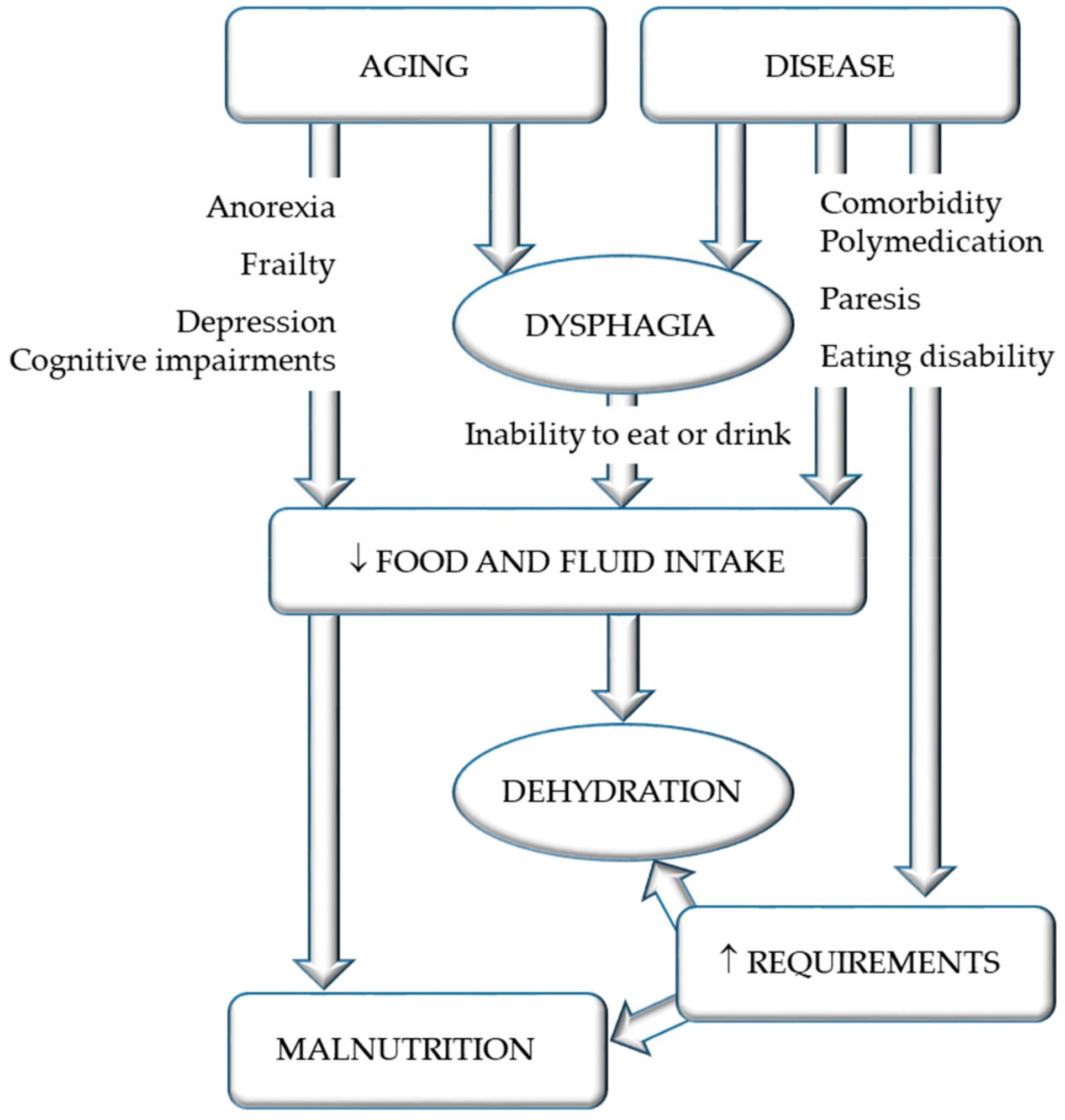
2. Dysphagia
3. Dehydration
3.1. Adequate Intake
3.2. Prevalence
3.3. Impact and Risk Factors
4. Pathophysiological Considerations
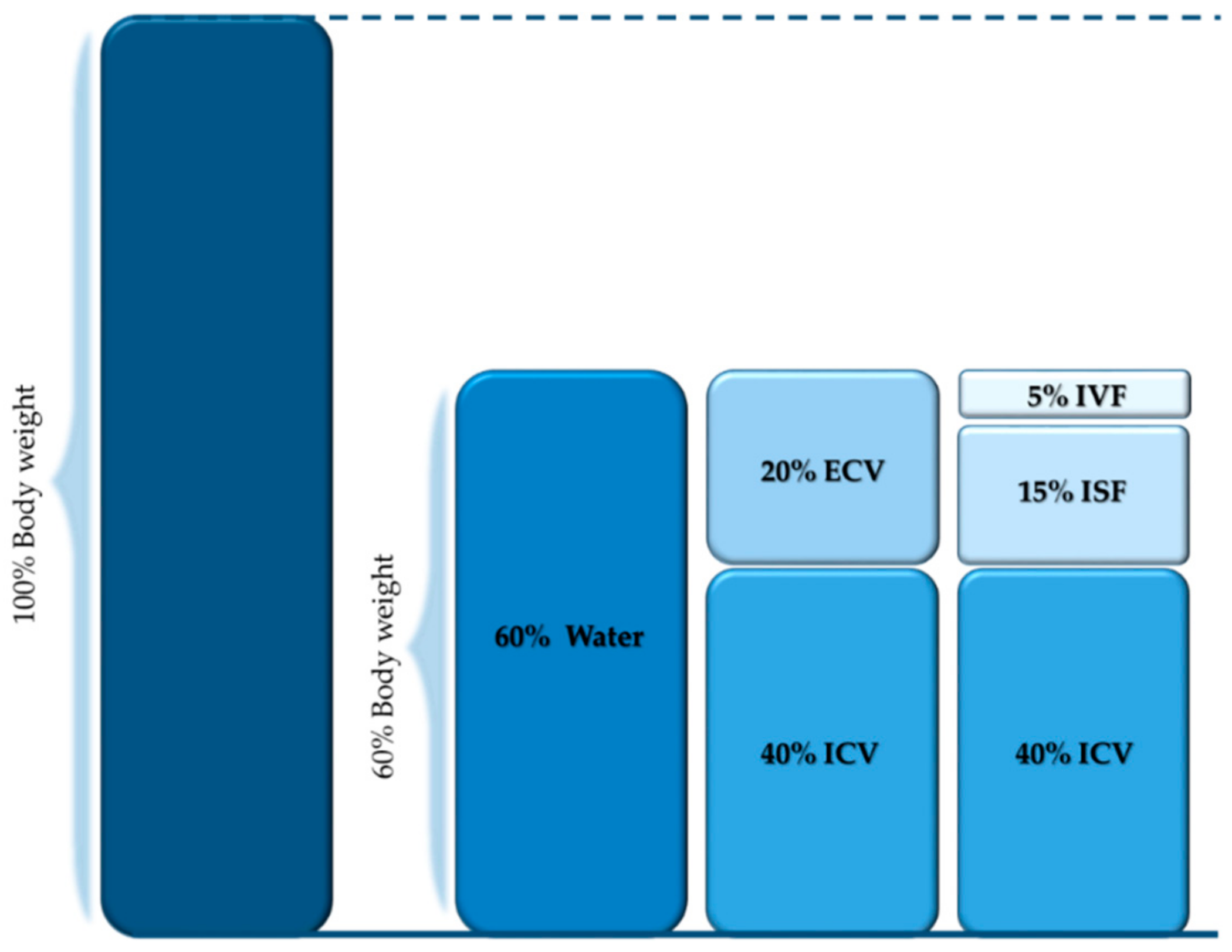
4.1. Body Compartments
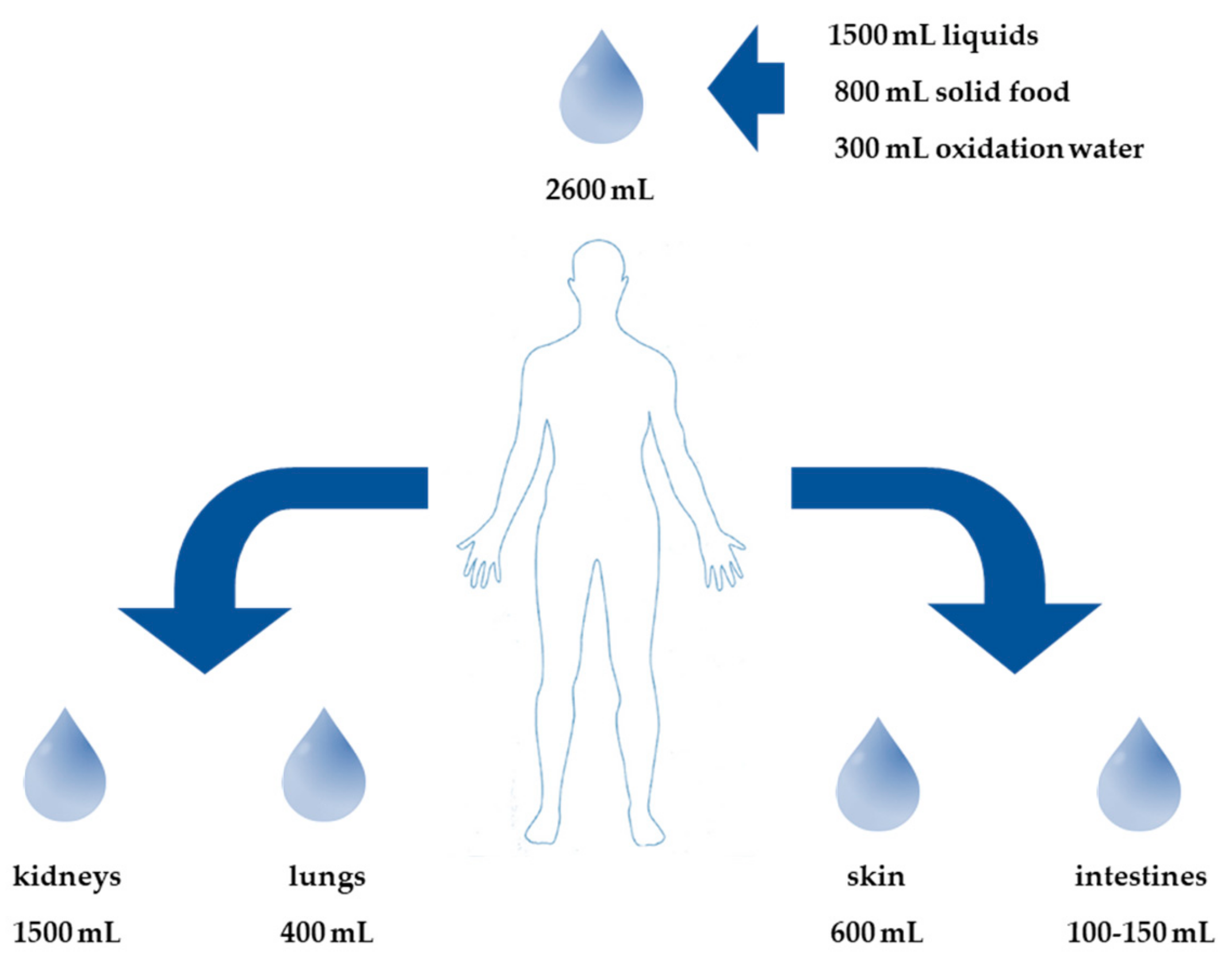
4.2. External Fluid Balance
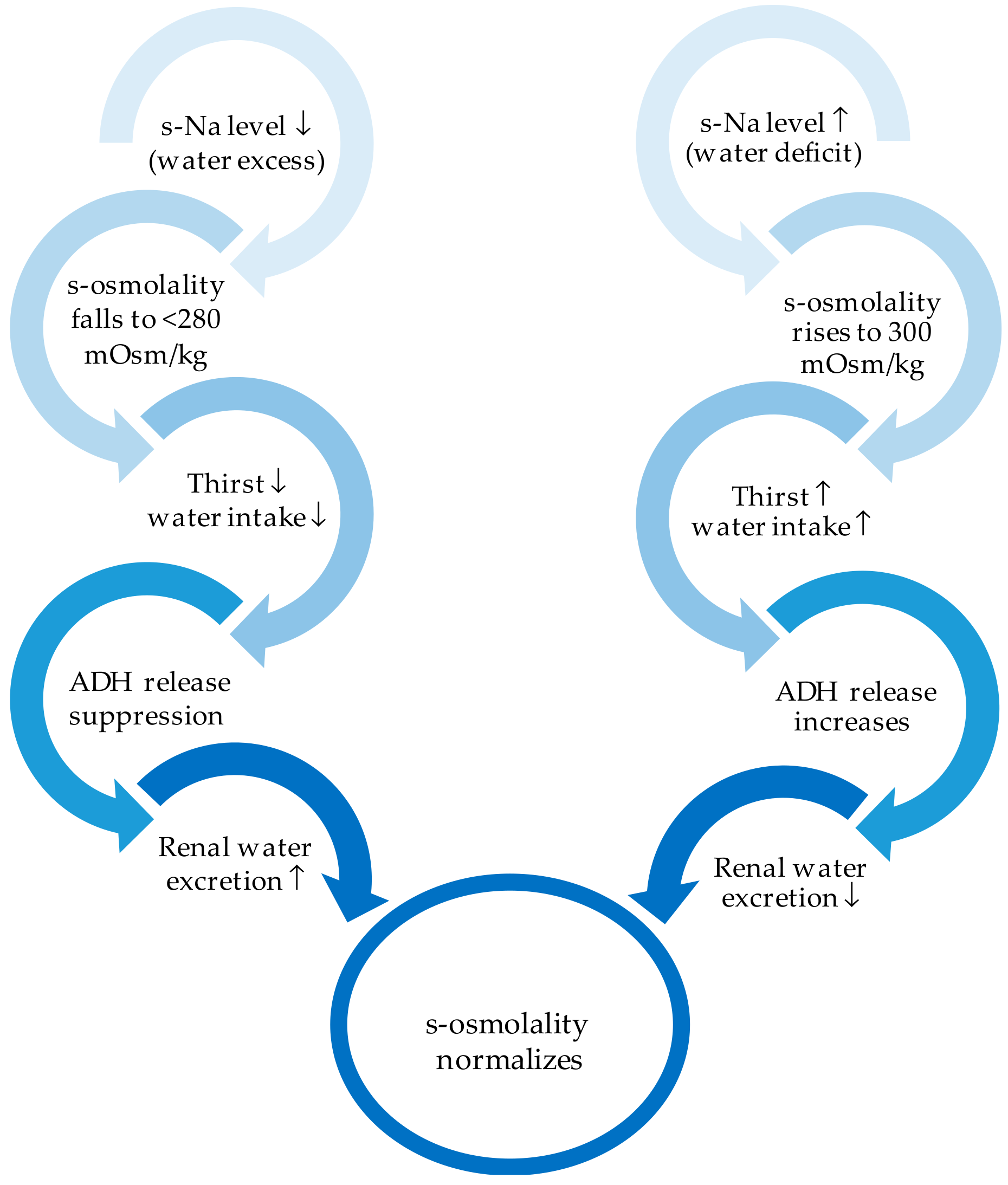
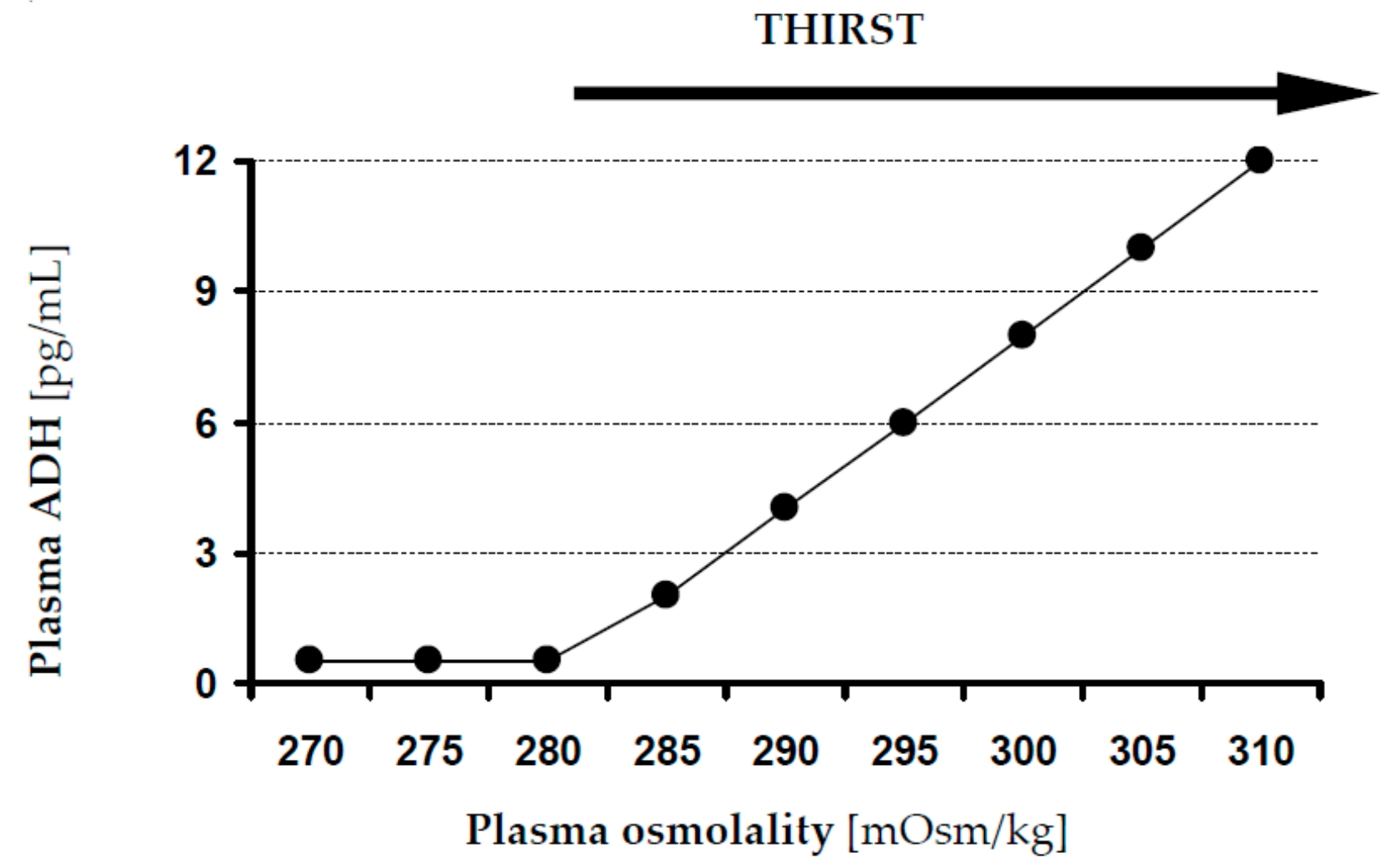
4.3. Internal Fluid Balance
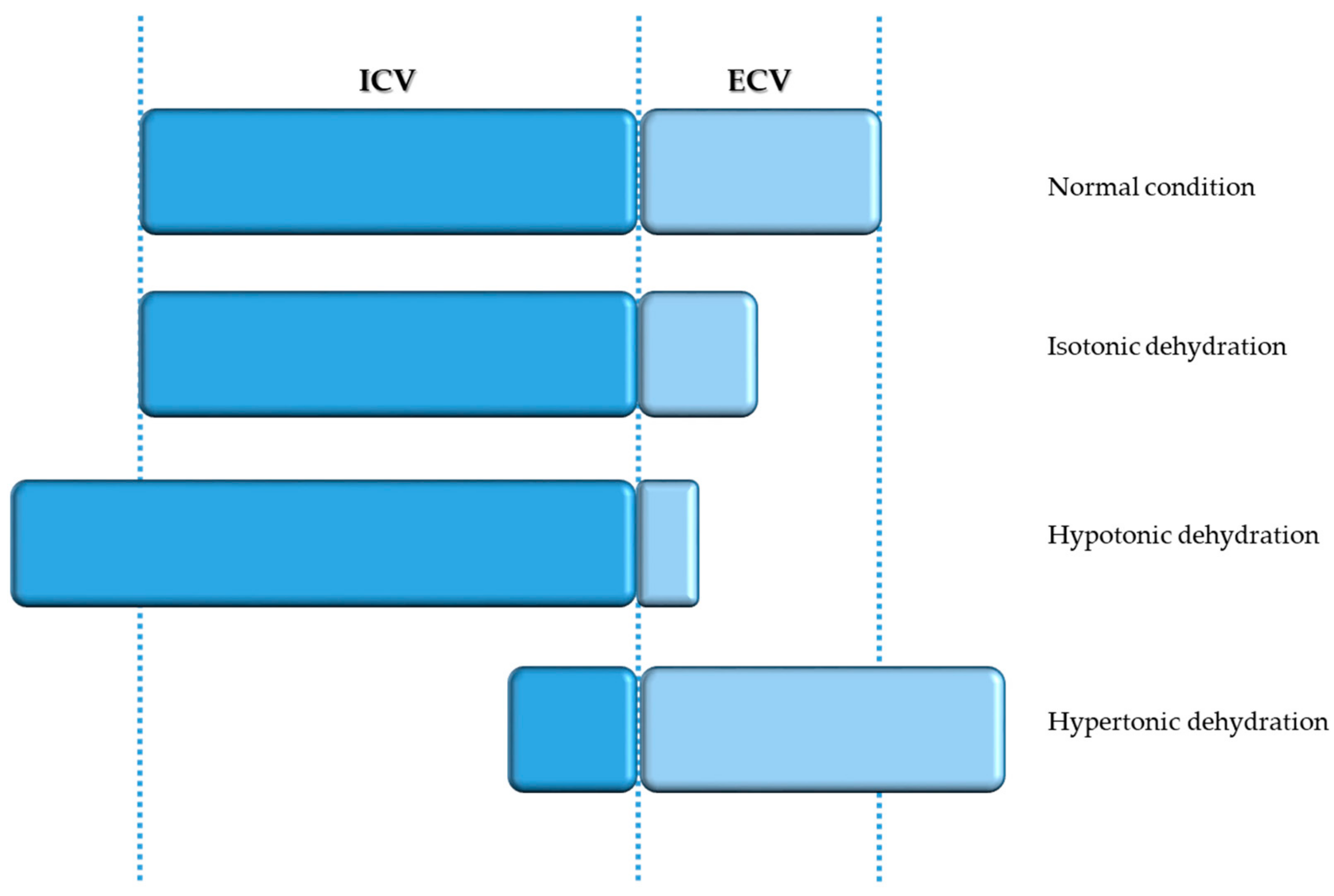
5. Disorders of Fluid Balance
5.1. Isotonic and Hypotonic Dehydration
5.2. Hypertonic Dehydration
6. Clinical and Biochemical Diagnostic Features
7. Management of Dehydration
7.1. General Recommendations
7.2. Oral/Enteral Replacement
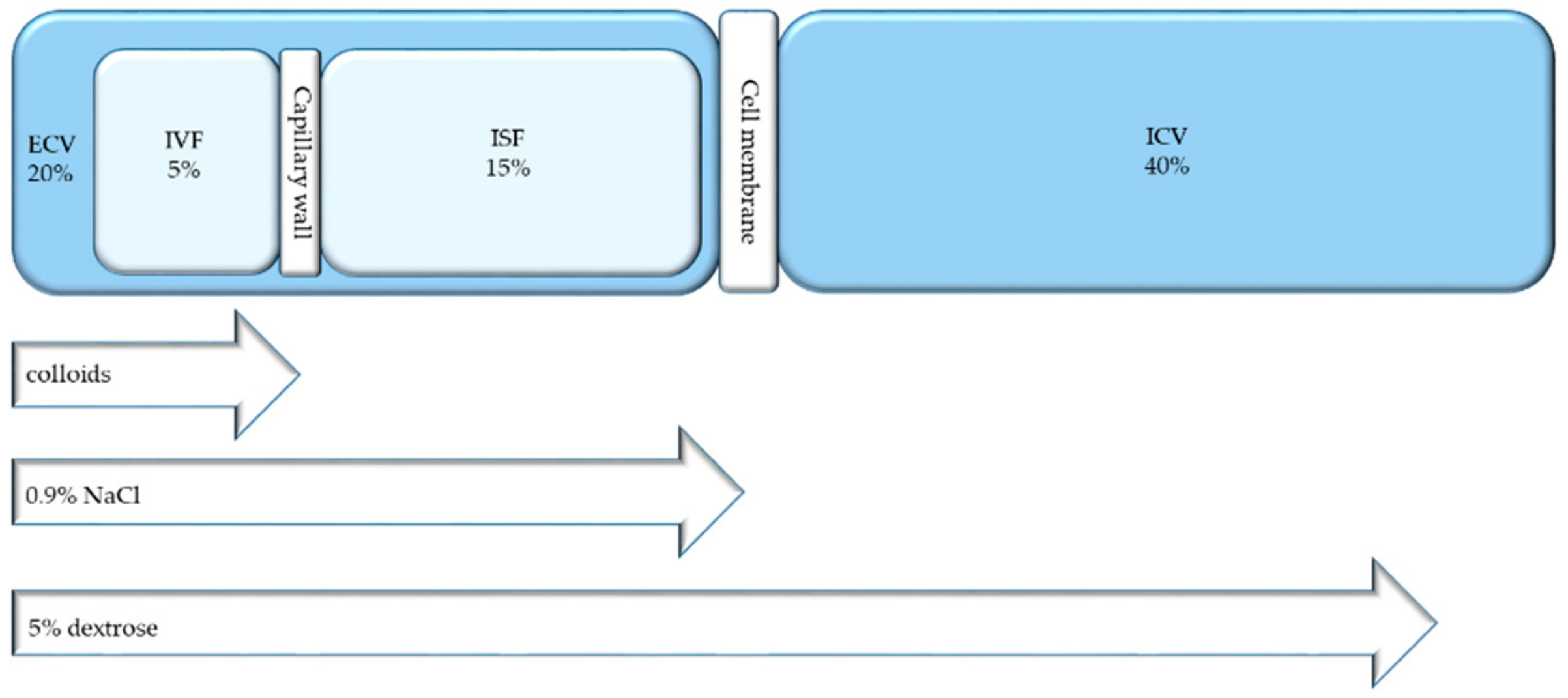
7.3. Parenteral Replacement
8. Hypodermoclysis
Colloids and (Balanced) Crystalloids
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burgos, R.; Breton, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jesus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef] [PubMed]
- Geeganage, C.; Beavan, J.; Ellender, S.; Bath, P.M. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane. Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, A.; LaGorio, L.A.; Crary, M.A.; Dahl, W.J.; Carnaby, G.D. Prevalence of and Risk Factors for Dysphagia in the Community Dwelling Elderly: A Systematic Review. J. Nutr. Health Aging 2016, 20, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.P.; Campbell, K.L.; Suter, M.S.; Hannan-Jones, M.T.; Hulcombe, J.A. Contribution of thickened drinks, food and enteral and parenteral fluids to fluid intake in hospitalised patients with dysphagia. J. Hum. Nutr. Diet 2009, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.; Lewington, A.; Allison, S. Basic concepts of fluid and electrolyte therapy. Clin. Med. 2013. [Google Scholar] [CrossRef]
- Bartolome, G.; Schröter-Morasch, H. Schluckstörungen: Diagnostik und Rehabilitation; Elsevier, Urban & Fischer: Munich, Germany, 2013. [Google Scholar]
- Prosiegel, M.; Weber, S. Dysphagie: Diagnostik und Therapie: Ein Wegweiser für Kompetentes Handeln; Springer: Berlin, Germany, 2018. [Google Scholar]
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clave, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.; Leischker, A.H.; Martino, R.; Pluschinski, P.; et al. Oropharyngeal dysphagia in older persons—From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208. [Google Scholar] [CrossRef]
- Amella, E.J. Feeding and hydration issues for older adults with dementia. Nurs. Clin. North Am. 2004, 39, 607–623. [Google Scholar] [CrossRef]
- Kolb, C. Nahrungsverweigerung bei Demenzkranken: PEG-Sonde-Ja oder Nein? Mabuse-Verlag: Frankfurt am Main, Germany, 2004. [Google Scholar]
- Schreier, M.M.; Bartholomeyczik, S. Mangelernährung bei Alten und Pflegebedürftigen Menschen: Ursachen und Prävention aus Pflegerischer Perspektive-Review/Literaturanalyse; Schlütersche: Hannover, Germany, 2004. [Google Scholar]
- Baijens, L.W.; Clave, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; Mateos-Nozal, J.; Ortega, O.; et al. European society for swallowing disorders—European union geriatric medicine society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Lauretani, F.; Pela, G.; Meschi, T.; Maggio, M. The risk of dysphagia is associated with malnutrition and poor functional outcomes in a large population of outpatient older individuals. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Logemann, J.A.; Veis, S.; Colangelo, L. A screening procedure for oropharyngeal dysphagia. Dysphagia 1999, 14, 44–51. [Google Scholar] [CrossRef]
- Sonies, B.C. Swallowing disorders and rehabilitation techniques. J. Pediatr. Gastroenterol. Nutr. 1997, 25, S32–S33. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, G.; Hofmayer, A.; Lindner-Pfleghar, B.; Pluschinski, P.; Riecker, A.; Schelling, A.; Prosiegel, M. Standardisierung des Untersuchungsablaufs bei neurogener oropharyngealer Dysphagie (NOD). Neurol. Rehabil. 2009, 15, 290–300. [Google Scholar]
- Wu, M.-C.; Chang, Y.-C.; Wang, T.-G.; Lin, L.-C. Evaluating swallowing dysfunction using a 100-mL water swallowing test. Dysphagia 2004, 19, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, P. Critical Review: Effectiveness of FEES in Comparison to VFSS at Identifying Aspiration. Available online: https://www.uwo.ca/fhs/lwm/teaching/EBP/2006_07/Marcotte.pdf (accessed on 7 November 2007).
- Yamamura, K.; Kurose, M.; Okamoto, K. Guide to Enhancing Swallowing Initiation: Insights from Findings in Healthy Subjects and Dysphagic Patients. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Lewis, S.; Cranswick, G.; Forbes, J. FOOD: A multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol. Assess. 2006, 10, 1–120. [Google Scholar] [CrossRef]
- Foley, N.; Finestone, H.; Woodbury, M.G.; Teasell, R.; Greene Finestone, L. Energy and protein intakes of acute stroke patients. J. Nutr. Health Aging 2006, 10, 171–175. [Google Scholar]
- Whelan, K. Inadequate fluid intakes in dysphagic acute stroke. Clin. Nutr. 2001, 20, 423–428. [Google Scholar] [CrossRef]
- Cichero, J.A. Thickening agents used for dysphagia management: Effect on bioavailability of water, medication and feelings of satiety. Nutr. J. 2013. [Google Scholar] [CrossRef]
- Clave, P.; de Kraa, M.; Arreola, V.; Girvent, M.; Farre, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.; Chen, J.; Cichero, J.A.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef]
- Cichero, J.A.; Lam, P.; Steele, C.M.; Hanson, B.; Chen, J.; Dantas, R.O.; Duivestein, J.; Kayashita, J.; Lecko, C.; Murray, J.; et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia 2017, 32, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Marcason, W. What is the international dysphagia diet standardisation initiative? J. Acad. Nutr. Diet 2017. [Google Scholar] [CrossRef] [PubMed]
- Cichero, J.A.; Steele, C.; Duivestein, J.; Clave, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P.; et al. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: Foundations of a global initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- International Dysphagia Diet Standardisation Committee. Complete International Dysphagia Diet Standardisation Initiative (IDDSI) Framework: Detailed Definitions. Available online: https://iddsi.org/Documents/IDDSIFramework-CompleteFramework.pdf (accessed on 29 July 2019).
- Lippert, W.C.; Chadha, R.; Sweigart, J.R. Things We Do for No Reason: The Use of Thickened Liquids in Treating Hospitalized Adult Patients with Dysphagia. J. Hosp. Med. 2019, 14, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mange, K.; Matsuura, D.; Cizman, B.; Soto, H.; Ziyadeh, F.N.; Goldfarb, S.; Neilson, E.G. Language guiding therapy: The case of dehydration versus volume depletion. Ann. Intern. Med. 1997, 127, 848–853. [Google Scholar] [CrossRef]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.Z.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E. Understanding clinical dehydration and its treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Kavouras, S.A.; Walsh, N.P.; Roberts, W.O. Diagnosing dehydration? Blend evidence with clinical observations. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 434–438. [Google Scholar] [CrossRef]
- Weinberg, A.D.; Minaker, K.L. Dehydration. Evaluation and management in older adults. Council on Scientific Affairs, American Medical Association. JAMA 1995, 274, 1552–1556. [Google Scholar] [CrossRef]
- Stanga, Z.; Aubry, E. Dehydration in Dysphagia. In Dysphagia: Diagnosis and Treatment; Ekberg, O., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 859–871. [Google Scholar]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Institute of Medicine; Food and Nutrition Board; Panel on Dietary Reference Intakes for Electrolytes and Water; Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI, Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- EFSA Panel on Dietetic Products; Nutrition; Allergies (NDA). Scientific opinion on dietary reference values for water. EFSA J. 2010. [Google Scholar] [CrossRef]
- Stookey, J.D.; Pieper, C.F.; Cohen, H.J. Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+years. Public Health Nutr. 2005, 8, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Thomas, V.; Riegel, B. Unrecognized chronic dehydration in older adults: Examining prevalence rate and risk factors. J. Gerontol. Nurs. 2004, 30, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Barber, J.; Campbell, E.S. Economic burden of dehydration among hospitalized elderly patients. Am. J. Health Syst. Pharm. 2004, 61, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Larsen, P.D. Dehydration in the elderly surgical patient. AORN J. 1994, 60, 666–671. [Google Scholar] [CrossRef]
- Warren, J.L.; Bacon, W.E.; Harris, T.; McBean, A.M.; Foley, D.J.; Phillips, C. The burden and outcomes associated with dehydration among US elderly, 1991. Am. J. Public Health 1994, 84, 1265–1269. [Google Scholar] [CrossRef]
- Murray, J.; Doeltgen, S.; Miller, M.; Scholten, I. A Descriptive Study of the Fluid Intake, Hydration, and Health Status of Rehabilitation Inpatients without Dysphagia Following Stroke. J. Nutr. Gerontol. Geriatr. 2015, 34, 292–304. [Google Scholar] [CrossRef]
- Leibovitz, A.; Baumoehl, Y.; Lubart, E.; Yaina, A.; Platinovitz, N.; Segal, R. Dehydration among long-term care elderly patients with oropharyngeal dysphagia. Gerontology 2007, 53, 179–183. [Google Scholar] [CrossRef]
- Thomas, D.R.; Tariq, S.H.; Makhdomm, S.; Haddad, R.; Moinuddin, A. Physician misdiagnosis of dehydration in older adults. J. Am. Med. Dir. Assoc. 2003, 4, 251–254. [Google Scholar] [CrossRef]
- Phillips, P.A.; Johnston, C.I.; Gray, L. Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people. Age Ageing 1993, 22, S26–S33. [Google Scholar] [CrossRef]
- Luckey, A.E.; Parsa, C.J. Fluid and electrolytes in the aged. Arch. Surg. 2003, 138, 1055–1060. [Google Scholar] [CrossRef]
- Mentes, J. Oral hydration in older adults: Greater awareness is needed in preventing, recognizing, and treating dehydration. Am. J. Nurs. 2006, 106, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kayser-Jones, J.; Schell, E.S.; Porter, C.; Barbaccia, J.C.; Shaw, H. Factors contributing to dehydration in nursing homes: Inadequate staffing and lack of professional supervision. J. Am. Geriatr. Soc. 1999, 47, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lavizzo-Mourey, R.; Johnson, J.; Stolley, P. Risk factors for dehydration among elderly nursing home residents. J. Am. Geriatr. Soc. 1988, 36, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Lubos, S.; Simon, A.; Zeno, S. Water and electrolytes in health and disease. In Basics in Clinical Nutrition, 5th ed.; Luboš, S., Ed.; Galen: Belfast, Ireland, 2019. [Google Scholar]
- Heilig, C.W.; Stromski, M.E.; Blumenfeld, J.D.; Lee, J.P.; Gullans, S.R. Characterization of the major brain osmolytes that accumulate in salt-loaded rats. Am. J. Physiol. 1989, 257, F1108–F1116. [Google Scholar] [CrossRef]
- Lien, Y.H.; Shapiro, J.I.; Chan, L. Effects of hypernatremia on organic brain osmoles. J. Clin. Invest. 1990, 85, 1427–1435. [Google Scholar] [CrossRef]
- Robertson, G.L. Physiology of ADH secretion. Kidney Int. Suppl. 1987, 21, S20–S26. [Google Scholar]
- Robertson, G.L.; Aycinena, P.; Zerbe, R.L. Neurogenic disorders of osmoregulation. Am. J. Med. 1982, 72, 339–353. [Google Scholar] [CrossRef]
- McGee, S.; Abernethy, W.B., 3rd; Simel, D.L. The rational clinical examination. Is this patient hypovolemic? JAMA 1999, 281, 1022–1029. [Google Scholar] [CrossRef]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane. Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Eaton, D.; Bannister, P.; Mulley, G.P.; Connolly, M.J. Axillary sweating in clinical assessment of dehydration in ill elderly patients. BMJ 1994. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R. Hydration and cognition: A critical review and recommendations for future research. J. Am. Coll. Nutr. 2007, 26, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Challenges of linking chronic dehydration and fluid consumption to health outcomes. Nutr. Rev. 2012, 70, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Ganio, M.S.; Casa, D.J.; Lee, E.C.; McDermott, B.P.; Klau, J.F.; Jimenez, L.; Le Bellego, L.; Chevillotte, E.; Lieberman, H.R. Mild dehydration affects mood in healthy young women. J. Nutr. 2012, 142, 382–388. [Google Scholar] [CrossRef]
- Cowen, L.E.; Hodak, S.P.; Verbalis, J.G. Age-associated abnormalities of water homeostasis. Endocrinol. Metab. Clin. North Am. 2013, 42, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Kenefick, R.W.; Charkoudian, N.; Sawka, M.N. Physiologic basis for understanding quantitative dehydration assessment. Am. J. Clin. Nutr. 2013, 97, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sollanek, K.J.; Kenefick, R.W.; Walsh, N.P.; Fortes, M.B.; Esmaeelpour, M.; Cheuvront, S.N. Assessment of thermal dehydration using the human eye: What is the potential? J. Therm. Biol. 2012, 37, 111–117. [Google Scholar] [CrossRef]
- Clark, W.F.; Sontrop, J.M.; Huang, S.H.; Moist, L.; Bouby, N.; Bankir, L. Hydration and Chronic Kidney Disease Progression: A Critical Review of the Evidence. Am. J. Nephrol. 2016, 43, 281–292. [Google Scholar] [CrossRef]
- Sontrop, J.M.; Dixon, S.N.; Garg, A.X.; Buendia-Jimenez, I.; Dohein, O.; Huang, S.H.; Clark, W.F. Association between water intake, chronic kidney disease, and cardiovascular disease: A cross-sectional analysis of NHANES data. Am. J. Nephrol. 2013, 37, 434–442. [Google Scholar] [CrossRef]
- Bunn, D.; Jimoh, F.; Wilsher, S.H.; Hooper, L. Increasing fluid intake and reducing dehydration risk in older people living in long-term care: A systematic review. J. Am. Med. Dir. Assoc. 2015, 16, 101–113. [Google Scholar] [CrossRef]
- National Clinical Guideline Centre (UK). Intravenous Fluid Therapy: Intravenous Fluid Therapy in Adults in Hospital. Available online: https://www.nice.org.uk/guidance/cg174 (accessed on 10 December 2013).
- Chidester, J.C.; Spangler, A.A. Fluid intake in the institutionalized elderly. J. Am. Diet Assoc. 1997, 97, 23–28. [Google Scholar] [CrossRef]
- Beck, A.M.; Kjaersgaard, A.; Hansen, T.; Poulsen, I. Systematic review and evidence based recommendations on texture modified foods and thickened liquids for adults (above 17 years) with oropharyngeal dysphagia—An updated clinical guideline. Clin. Nutr. 2018, 37, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Bardis, C.N.; Kavouras, S.A.; Kosti, L.; Markousi, M.; Sidossis, L.S. Mild hypohydration decreases cycling performance in the heat. Med. Sci. Sports Exerc. 2013, 45, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Whale, A.; Mears, S.A.; Reyner, L.A.; Maughan, R.J. Mild hypohydration increases the frequency of driver errors during a prolonged, monotonous driving task. Physiol. Behav. 2015, 147, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Cassano, A.M. Subcutaneous dextrose for rehydration of elderly patients--An evidence-based review. BMC Geriatr. 2004. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Binns, M.A.; Rochon, P.A. Subcutaneous fluid infusion in a long-term care setting. J. Am. Geriatr. Soc. 2000, 48, 795–799. [Google Scholar] [CrossRef]
- Remington, R.; Hultman, T. Hypodermoclysis to treat dehydration: A review of the evidence. J. Am. Geriatr. Soc. 2007, 55, 2051–2055. [Google Scholar] [CrossRef]
- Slesak, G.; Schnurle, J.W.; Kinzel, E.; Jakob, J.; Dietz, P.K. Comparison of subcutaneous and intravenous rehydration in geriatric patients: A randomized trial. J. Am. Geriatr. Soc. 2003, 51, 155–160. [Google Scholar] [CrossRef]
- Jain, S.; Mansfield, B.; Wilcox, M.H. Subcutaneous fluid administration--Better than the intravenous approach? J. Hosp. Infect. 1999, 41, 269–272. [Google Scholar] [CrossRef]
- Sasson, M.; Shvartzman, P. Hypodermoclysis: An alternative infusion technique. Am. Fam. Physician. 2001, 64, 1575–1578. [Google Scholar]
- Walsh, G. Hypodermoclysis: An alternate method for rehydration in long-term care. J. Infus. Nurs. 2005, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, S.; Campbell, A.J.; Roberts, M.S.; Wanwimolruk, S.; McQueen, E.G.; McQueen, M.; Firth, L.A. Subcutaneous fluid administration in elderly subjects: Validation of an under-used technique. J. Am. Geriatr. Soc. 1991, 39, 6–9. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, S.T.; Lavan, J.N. Subcutaneous fluids in elderly hospital patients with cognitive impairment. Gerontology 1996, 42, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Pershad, J. A systematic data review of the cost of rehydration therapy. Appl. Health Econ. Health Policy 2010, 8, 203–214. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Wanderer, J.P.; Wang, L.; Byrne, D.W.; Collins, S.P.; Slovis, C.M.; Lindsell, C.J.; Ehrenfeld, J.M.; Siew, E.D.; et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N. Engl. J. Med. 2018, 378, 819–828. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
| Electrolyte | Plasma (mmol/L) | Extracellular Volume (mmol/L) | Intracellular Volume (mmol/L) |
|---|---|---|---|
| Sodium | 135–145 | 142–155 | 10–18 |
| Potassium | 3.5–5.3 | 4.0–5.5 | 120–145 |
| Calcium | 2.2–2.6 | 2.2–2.5 | 1.5 |
| Chloride | 95–105 | 98–108 | 2–6 |
| Magnesium | 0.8–1.2 | 0.7–1.2 | 15–25 |
| Phosphate | 0.81–1.45 | 0.7–1.3 | 8–20 |
| Bicarbonate | 22–30 | 22–30 | 10 |
| Assessment of Hydration Status | Feasibility of Test | Scientific Value |
|---|---|---|
| Signs and symptoms | ||
| Seated systolic blood pressure ≤100 mmHg | H | H |
| Blood pressure change supine/standing ≥20 mmHg | H | H |
| Thirst sensation | H | M |
| Dark urine colour | H | M |
| Laboratory tests | ||
| Urine specific gravity ≥1.025 | H | H |
| Blood urea nitrogen/creatinine ratio ≥20 | M | H |
| Blood osmolality calculated ≥300 mmol/kg | M | H |
| Haematocrit/haemoglobin ratio | M | M |
| Mean corpuscular volume | M | M |
| Serum sodium concentration >150 mmol/L | M | M |
| Additional measurements (mainly for scientific purposes) | ||
| Total body water (isotope dilution) | L | M |
| Total body water (bioelectrical impedance analysis) | H | M |
| Fluid volumes and ionic content (neutron activation analysis) | L | M |
| Blood osmolality (measured) | M | H |
| Urine osmolality | H | H |
| Salivary osmolality | H | H |
| Tear osmolality | M | M |
| Intraocular pressure (measured) | M | L |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reber, E.; Gomes, F.; Dähn, I.A.; Vasiloglou, M.F.; Stanga, Z. Management of Dehydration in Patients Suffering Swallowing Difficulties. J. Clin. Med. 2019, 8, 1923. https://doi.org/10.3390/jcm8111923
Reber E, Gomes F, Dähn IA, Vasiloglou MF, Stanga Z. Management of Dehydration in Patients Suffering Swallowing Difficulties. Journal of Clinical Medicine. 2019; 8(11):1923. https://doi.org/10.3390/jcm8111923
Chicago/Turabian StyleReber, Emilie, Filomena Gomes, Ilka A. Dähn, Maria F. Vasiloglou, and Zeno Stanga. 2019. "Management of Dehydration in Patients Suffering Swallowing Difficulties" Journal of Clinical Medicine 8, no. 11: 1923. https://doi.org/10.3390/jcm8111923
APA StyleReber, E., Gomes, F., Dähn, I. A., Vasiloglou, M. F., & Stanga, Z. (2019). Management of Dehydration in Patients Suffering Swallowing Difficulties. Journal of Clinical Medicine, 8(11), 1923. https://doi.org/10.3390/jcm8111923





