The Impact of Neighbourhood Deprivation on Embryonic Growth Trajectories: Rotterdam Periconception Cohort
Abstract
1. Introduction
2. Materials and Methods
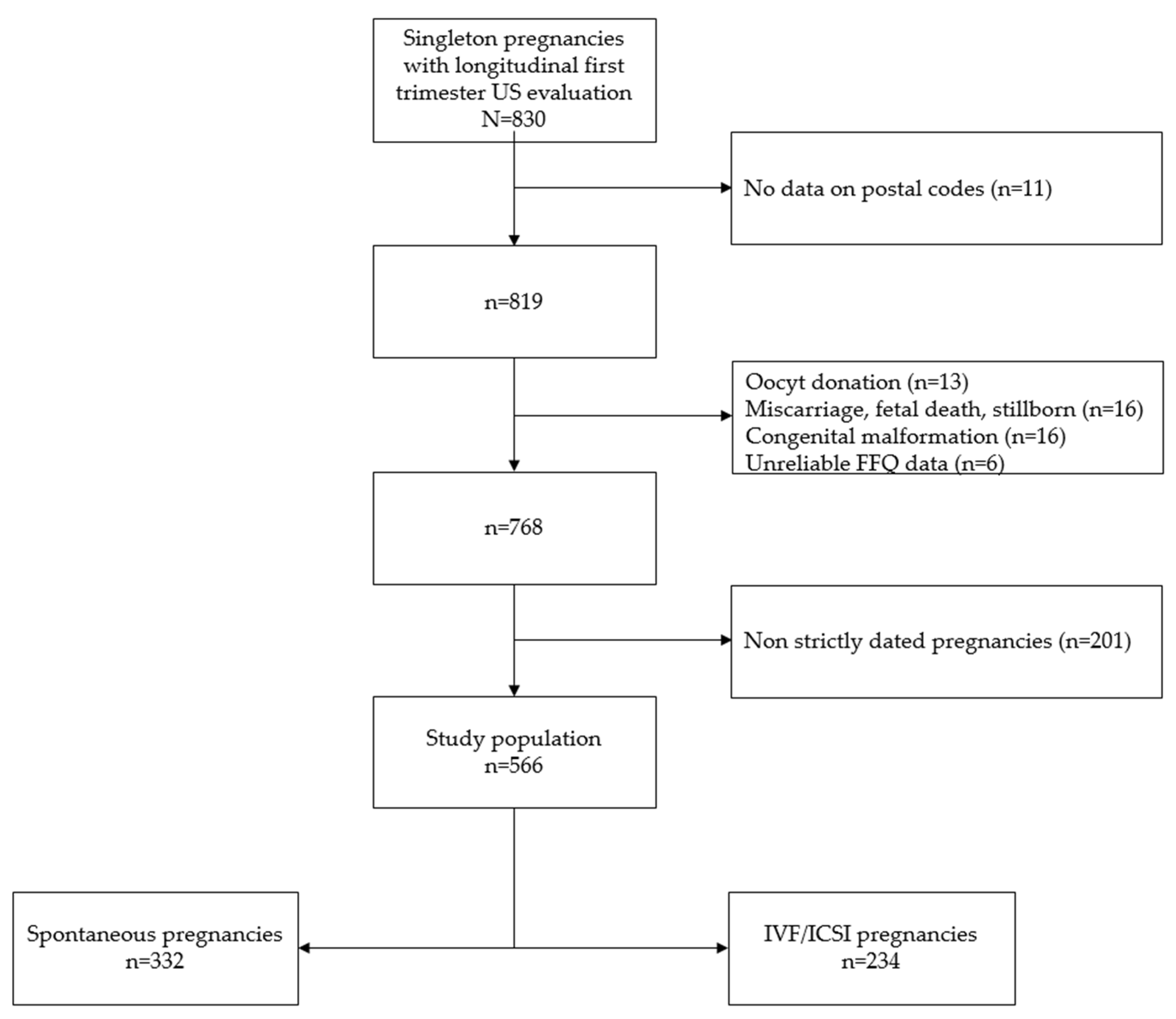
2.1. Cohort Selection
2.2. Data Collection
2.3. Pregnancy Dating
2.4. Exposure
2.5. Outcomes
2.6. Statistical Analysis
2.7. Ethics Approval
3. Results
3.1. Baseline Characteristics
3.2. Embryonic Growth Trajectories
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pickett, K.E.; Pearl, M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: A critical review. J. Epidemiol. Community Health 2001, 55, 111–122. [Google Scholar] [CrossRef]
- Jokela, M. Does neighbourhood deprivation cause poor health? Within-individual analysis of movers in a prospective cohort study. J. Epidemiol. Community Health 2015, 69, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Ribeiro, A.I.; Severo, M.; Barros, H.; Fraga, S. Neighbourhood socioeconomic deprivation and health-related quality of life: A multilevel analysis. PLoS ONE 2017, 12, e0188736. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.A.; Posthumus, A.G.; Bonsel, G.J.; Steegers, E.A.; Denktas, S. Deprived neighborhoods and adverse perinatal outcome: A systematic review and meta-analysis. Acta Obs. Gynecol. Scand. 2014, 93, 727–740. [Google Scholar] [CrossRef]
- Agyemang, C.; Vrijkotte, T.G.; Droomers, M.; van der Wal, M.F.; Bonsel, G.J.; Stronks, K. The effect of neighbourhood income and deprivation on pregnancy outcomes in Amsterdam, The Netherlands. J. Epidemiol. Community Health 2009, 63, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Bonsel, G.J.; Steegers-Theunissen, R.P.; Mackenbach, J.P.; Steyerberg, E.W.; Raat, H.; Verbrugh, H.A.; Tiemeier, H.W.; Hofman, A.; Birnie, E.; et al. Individual accumulation of heterogeneous risks explains perinatal inequalities within deprived neighbourhoods. Eur. J. Epidemiol. 2011, 26, 165–180. [Google Scholar] [CrossRef]
- De Graaf, J.P.; Ravelli, A.C.; de Haan, M.A.; Steegers, E.A.; Bonsel, G.J. Living in deprived urban districts increases perinatal health inequalities. J. Matern. Fetal. Neonatal Med. 2013, 26, 473–481. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef]
- Parisi, F.; Rousian, M.; Huijgen, N.A.; Koning, A.H.; Willemsen, S.P.; de Vries, J.H.; Cetin, I.; Steegers, E.A.; Steegers-Theunissen, R.P. Periconceptional maternal high fish and olive oil, low meat dietary pattern is associated with increased embryonic growth: The Rotterdam periconceptional cohort (predict study). Ultrasound Obs. Gynecol. 2017, 50, 709–716. [Google Scholar] [CrossRef]
- Parisi, F.; Rousian, M.; Steegers-Theunissen, R.P.M.; Koning, A.H.J.; Willemsen, S.P.; de Vries, J.H.M.; Cetin, I.; Steegers, E.A.P. Early first trimester maternal high fish and olive oil and low meat dietary pattern is associated with accelerated human embryonic development. Eur. J. Clin. Nutr. 2018, 72, 1655–1662. [Google Scholar] [CrossRef]
- Van Dijk, M.R.; Borggreven, N.V.; Willemsen, S.P.; Koning, A.H.J.; Steegers-Theunissen, R.P.M.; Koster, M.P.H. Maternal lifestyle impairs embryonic growth: The Rotterdam periconception cohort. Reprod. Sci. 2017, 25, 916–922. [Google Scholar] [CrossRef]
- Mook-Kanamori, D.O.; Steegers, E.A.; Eilers, P.H.; Raat, H.; Hofman, A.; Jaddoe, V.W. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 2010, 303, 527–534. [Google Scholar] [CrossRef]
- van Uitert, E.M.; van der Elst-Otte, N.; Wilbers, J.J.; Exalto, N.; Willemsen, S.P.; Eilers, P.H.; Koning, A.H.; Steegers, E.A.; Steegers-Theunissen, R.P. Periconception maternal characteristics and embryonic growth trajectories: The Rotterdam predict study. Hum. Reprod. 2013, 28, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Verwoerd-Dikkeboom, C.M.; Koning, A.H.; Hop, W.C.; Rousian, M.; Van Der Spek, P.J.; Exalto, N.; Steegers, E.A. Reliability of three-dimensional sonographic measurements in early pregnancy using virtual reality. Ultrasound Obs. Gynecol. 2008, 32, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Rousian, M.; Koning, A.H.; van Oppenraaij, R.H.; Hop, W.C.; Verwoerd-Dikkeboom, C.M.; van der Spek, P.J.; Exalto, N.; Steegers, E.A. An innovative virtual reality technique for automated human embryonic volume measurements. Hum. Reprod. 2010, 25, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Rousian, M.; Verwoerd-Dikkeboom, C.M.; Koning, A.H.; Hop, W.C.; van der Spek, P.J.; Exalto, N.; Steegers, E.A. Early pregnancy volume measurements: Validation of ultrasound techniques and new perspectives. BJOG 2009, 116, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Verwoerd-Dikkeboom, C.M.; Koning, A.H.; Hop, W.C.; van der Spek, P.J.; Exalto, N.; Steegers, E.A. Innovative virtual reality measurements for embryonic growth and development. Hum. Reprod. 2010, 25, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.R.; Huijgen, N.A.; Willemsen, S.P.; Laven, J.S.; Steegers, E.A.; Steegers-Theunissen, R.P. Impact of an mhealth platform for pregnancy on nutrition and lifestyle of the reproductive population: A survey. JMIR Mhealth Uhealth 2016, 4, e53. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Verheijden-Paulissen, J.J.; van Uitert, E.M.; Wildhagen, M.F.; Exalto, N.; Koning, A.H.; Eggink, A.J.; Duvekot, J.J.; Laven, J.S.; Tibboel, D.; et al. Cohort profile: The Rotterdam periconceptional cohort (predict study). Int. J. Epidemiol. 2016, 45, 374–381. [Google Scholar] [CrossRef]
- Siebelink, E.; Geelen, A.; de Vries, J.H. Self-reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br. J. Nutr. 2011, 106, 274–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention of neural tube defects. In Standards for Maternal and Neonatal Care; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- The Netherlands Nutrition Centre. Hoe Eet ik Gezond Tijdens Mijn Zwangerschap? Available online: https://www.voedingscentrum.nl/nl/mijn-kind-en-ik/zwanger/gezond-eten-tijdens-de-zwangerschap.aspx (accessed on 5 November 2019).
- Van Dijk, M.R.; Koster, M.P.H.; Willemsen, S.P.; Huijgen, N.A.; Laven, J.S.E.; Steegers-Theunissen, R.P.M. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod. Biomed. Online 2017, 35, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6 (Suppl. 2), 125–140. [Google Scholar] [CrossRef] [PubMed]
- Slama, R.; Khoshnood, B.; Kaminski, M. How to control for gestational age in studies involving environmental effects on fetal growth. Environ. Health Perspect. 2008, 116, A284–A285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sociaal en Cultureel Planbureau SCP. Verwijzing naar de data van: Statusscores—Rangorde naar sociale status van postcodegebieden in Nederland. DANS 2006. Available online: https://easy.dans.knaw.nl/ui/datasets/id/easy-dataset:38019 (accessed on 5 November 2019). [CrossRef]
- Helmerhorst, F.M.; Perquin, D.A.; Donker, D.; Keirse, M.J. Perinatal outcome of singletons and twins after assisted conception: A systematic review of controlled studies. BMJ 2004, 328, 261. [Google Scholar] [CrossRef]
- Jackson, R.A.; Gibson, K.A.; Wu, Y.W.; Croughan, M.S. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obs. Gynecol. 2004, 103, 551–563. [Google Scholar] [CrossRef]
- Wisborg, K.; Ingerslev, H.J.; Henriksen, T.B. In vitro fertilization and preterm delivery, low birth weight, and admission to the neonatal intensive care unit: A prospective follow-up study. Fertil. Steril. 2010, 94, 2102–2106. [Google Scholar] [CrossRef]
- De Graaf, J.P.; Schutte, J.M.; Poeran, J.J.; van Roosmalen, J.; Bonsel, G.J.; Steegers, E.A. Regional differences in dutch maternal mortality. BJOG 2012, 119, 582–588. [Google Scholar] [CrossRef]
- Nieuwenhuis, J.; Hooimeijer, P. The association between neighbourhoods and educational achievement, a systematic review and meta-analysis. J. Hous. Built Environ. 2016, 31, 321–347. [Google Scholar] [CrossRef]
- De Graaf, J.P.; Steegers, E.A.; Bonsel, G.J. Inequalities in perinatal and maternal health. Curr. Opin. Obs. Gynecol. 2013, 25, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, F.B.; Clark, M.A.; Rogers, M.L.; Linkletter, C.D.; Phipps, M.G.; Padbury, J.F.; Vivier, P.M. Pregnant and moving: Understanding residential mobility during pregnancy and in the first year of life using a prospective birth cohort. Matern. Child. Health J. 2013, 17, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Dodds, L.; King, W.D. Residential mobility during pregnancy. Paediatr. Perinat. Epidemiol. 2004, 18, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Chastang, J.F.; Leclerc, A.; Zins, M.; Bonenfant, S.; Bugel, I.; Kaniewski, N.; Schmaus, A.; Niedhammer, I.; Piciotti, M.; et al. Socioeconomic, demographic, occupational, and health factors associated with participation in a long-term epidemiologic survey: A prospective study of the french gazel cohort and its target population. Am. J. Epidemiol. 2001, 154, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Van Uitert, E.M.; Exalto, N.; Burton, G.J.; Willemsen, S.P.; Koning, A.H.; Eilers, P.H.; Laven, J.S.; Steegers, E.A.; Steegers-Theunissen, R.P. Human embryonic growth trajectories and associations with fetal growth and birthweight. Hum. Reprod. 2013, 28, 1753–1761. [Google Scholar] [CrossRef]
- Assibey-Mensah, V.; Fabio, A.; Mendez, D.D.; Lee, P.C.; Roberts, J.M.; Catov, J.M. Neighbourhood assets and early pregnancy cardiometabolic risk factors. Paediatr. Perinat. Epidemiol. 2019, 33, 79–87. [Google Scholar] [CrossRef]
- Hesselman, S.; Wikstrom, A.K.; Skalkidou, A.; Sundstrom-Poromaa, I.; Wikman, A. Neighborhood deprivation and adverse perinatal outcomes in sweden: A population-based register study. Acta Obs. Gynecol. Scand. 2019, 98, 1004–1013. [Google Scholar] [CrossRef]
- Waelput, A.J.M.; Sijpkens, M.K.; Lagendijk, J.; van Minde, M.R.C.; Raat, H.; Ernst-Smelt, H.E.; de Kroon, M.L.A.; Rosman, A.N.; Been, J.V.; Bertens, L.C.M.; et al. Geographical differences in perinatal health and child welfare in the netherlands: Rationale for the healthy pregnancy 4 all-2 program. BMC Pregnancy Childbirth 2017, 17, 254. [Google Scholar] [CrossRef]
- Lagendijk, J.; Vos, A.A.; Bertens, L.C.M.; Denktas, S.; Bonsel, G.J.; Steyerberg, E.W.; Been, J.V.; Steegers, E.A.P. Antenatal non-medical risk assessment and care pathways to improve pregnancy outcomes: A cluster randomised controlled trial. Eur. J. Epidemiol 2018, 33, 579–589. [Google Scholar] [CrossRef]
- Bottomley, C.; Daemen, A.; Mukri, F.; Papageorghiou, A.T.; Kirk, E.; Pexsters, A.; De Moor, B.; Timmerman, D.; Bourne, T. Assessing first trimester growth: The influence of ethnic background and maternal age. Hum. Reprod. 2009, 24, 284–290. [Google Scholar] [CrossRef]
- Simic, M.; Wikstrom, A.K.; Stephansson, O. Accelerated fetal growth in early pregnancy and risk of severe large-for-gestational-age and macrosomic infant: A cohort study in a low-risk population. Acta Obs. Gynecol. Scand. 2017, 96, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Hackmon, R.; Le Scale, K.B.; Horani, J.; Ferber, A.; Divon, M.Y. Is severe macrosomia manifested at 11–14 weeks of gestation? Ultrasound Obs. Gynecol. 2008, 32, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Tarantal, A.F.; Berglund, L. Obesity and lifespan health—Importance of the fetal environment. Nutrients 2014, 6, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.E.; Farrell, W.E.; Emes, R.D.; Ismail, K.M.; Carroll, W.D.; Hubball, E.; Rooney, A.; Yates, A.M.; Mein, C.; Fryer, A.A. Methylation of the FGFR2 gene is associated with high birth weight centile in humans. Epigenomics 2014, 6, 477–491. [Google Scholar] [CrossRef]
- Martin, R.J.; Hausman, G.J.; Hausman, D.B. Regulation of adipose cell development in utero. Proc. Soc. Exp. Biol. Med. 1998, 219, 200–210. [Google Scholar] [CrossRef]
- Algren, M.H.; Ekholm, O.; Nielsen, L.; Ersbøll, A.K.; Bak, C.K.; Andersen, P.T. Associations between perceived stress, socioeconomic status, and health-risk behaviour in deprived neighbourhoods in denmark: A cross-sectional study. BMC Public Health 2018, 18, 250. [Google Scholar] [CrossRef]
- Zannas, A.S.; Chrousos, G.P. Epigenetic programming by stress and glucocorticoids along the human lifespan. Mol. Psychiatry 2017, 22, 640. [Google Scholar] [CrossRef]
- Ouni, M.; Castell, A.L.; Linglart, A.; Bougneres, P. Genetic and epigenetic modulation of growth hormone sensitivity studied with the igf-1 generation test. J. Clin. Endocrinol. Metab. 2015, 100, E919–E925. [Google Scholar] [CrossRef]
- Zhang, Y.; Kutateladze, T.G. Diet and the epigenome. Nat. Commun. 2018, 9, 3375. [Google Scholar] [CrossRef]
- Enuka, Y.; Feldman, M.E.; Chowdhury, A.; Srivastava, S.; Lindzen, M.; Sas-Chen, A.; Massart, R.; Cheishvili, D.; Suderman, M.J.; Zaltsman, Y.; et al. Epigenetic mechanisms underlie the crosstalk between growth factors and a steroid hormone. Nucleic Acids Res. 2017, 45, 12681–12699. [Google Scholar] [CrossRef]
- Slieker, R.C.; Roost, M.S.; van Iperen, L.; Suchiman, H.E.D.; Tobi, E.W.; Carlotti, F.; de Koning, E.J.P.; Slagboom, P.E.; Heijmans, B.T.; Chuva de Sousa Lopes, S.M. DNA methylation landscapes of human fetal development. PLoS Genet. 2015, 11, e1005583. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, R.; Josefson, J. The relationship of insulin-like growth factor 2 to fetal growth and adiposity. Horm. Res. Paediatr. 2016, 85, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nawathe, A.R.; Christian, M.; Kim, S.H.; Johnson, M.; Savvidou, M.D.; Terzidou, V. Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin. Epigenet. 2016, 8, 11. [Google Scholar] [CrossRef] [PubMed]
| Study Population n = 566 | Low NSS n = 170 | Intermediate NSS n = 280 | High NSS n = 116 | Missing n (%) | |
|---|---|---|---|---|---|
| Neighbourhood status score, median (IQR) | 0.02 (−1.13–0.77) | −1.63 (−2.55– −1.14) | 0.19 (−0.26–0.57) | 1.47 (1.21–1.68) | - |
| Age (years), median (IQR) | 32 (29–36) | 33 (30–36) | 32 (29–35) | 33 (30–36) | 14 (2.5) |
| Nulliparous n (%) | 244 (46.3) | 72 (46.2) | 126 (47.7) | 46 (43.0) | 39 (6.9) |
| Geographical origin (Western), n (%) | 475 (87.0) | 120 (73.6) | 251 (92.6) | 104 (92.9) | 20 (3.5) |
| Educational level, n (%) | 20 (3.5) | ||||
| High | 317 (58.1) | 96 (58.9) | 134 (49.4) | 87 (77.7) | |
| Intermediate | 188 (34.4) | 52 (31.9) | 116 (42.8) | 20 (17.9) | |
| Low | 41 (7.5) | 15 (9.2) | 21 (7.7) | 5 (4.5) | |
| BMI (kg/m2), median (IQR) | 24.4 (21.9–28.2) | 25.3 (22.7–29.7) | 24.4 (21.9–28.3) | 23.3 (21.1–26.2) | 42 (7.4) |
| Mode of conception (spontaneous), n (%) | 332 (58.7) | 116 (68.2) | 148 (52.9) | 68 (58.6) | - |
| Folic acid supplement use (adequate), n (%) | 447 (82.6) | 124 (77.0) | 225 (84.0) | 98 (87.5) | 25 (4.4) |
| Fruit intake (adequate), n (%) | 292 (55.2) | 87 (56.9) | 146 (54.7) | 59 (54.1) | 37 (6.5) |
| Vegetable intake (adequate), n (%) | 184 (34.8) | 64 (41.8) | 84 (31.5) | 36 (33.0) | 437 (6.5) |
| Alcohol consumption (no), n (%) | 369 (68.0) | 55 (34.2) | 86 (31.9) | 33 (29.5) | 23 (4.1) |
| Smoking (no), n (%) | 467 (85.8) | 136 (84.0) | 238 (88.1) | 93 (83.0) | 22 (3.9) |
| Total Study Population (n = 569) | β (95% CI) | p-Value |
|---|---|---|
| Age (years) | 0.01 (−0.002, 0.03) | 0.09 |
| BMI (kg/m2) | −0.05 (−0.06, −0.04) | <0.001 *** |
| Parity (multiparous vs. nulliparous) | −0.06 (−0.20, 0.07) | 0.34 |
| Geographical origin (non-Western vs. Western) | −1.30 (−1.49, −1.10) | <0.001 *** |
| Educational level (low vs. intermediate) | −0.22 (−0.47, 0.04) | 0.10 |
| (high vs. intermediate) | 0.29 (0.15, 0.43) | <0.001 *** |
| Mode of conception (spontaneous vs. IVF/ICSI) | 0.37 (0.25, 0.50) | <0.001 *** |
| Folic acid supplement use (inadequate vs. adequate) | −0.47 (−0.64, −0.29) | <0.001 *** |
| Fruit intake (inadequate vs. adequate) | −0.001 (−0.13, 0.13) | 0.99 |
| Vegetable intake (inadequate vs. adequate) | 0.08 (−0.06, 0.21) | 0.28 |
| Alcohol consumption (yes vs. no) | 0.06 (−0.07, 0.20) | 0.38 |
| Smoking (yes vs. no) | 0.10 (−0.08, 0.28) | 0.29 |
| Total risk score | 0.003 (−0.01, 0.02) | 0.72 |
| Total Study Population n = 566 | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| CRL (√mm) | −0.015 (−0.034, 0.003) | 0.11 | −0.025 (−0.046, −0.003) | 0.03 * |
| EV (3√cm3) | −0.008 (−0.018, 0.001) | 0.10 | −0.015 (−0.026, −0.003) | 0.01 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gootjes, D.V.; Koster, M.P.H.; Willemsen, S.P.; Koning, A.H.J.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. The Impact of Neighbourhood Deprivation on Embryonic Growth Trajectories: Rotterdam Periconception Cohort. J. Clin. Med. 2019, 8, 1913. https://doi.org/10.3390/jcm8111913
Gootjes DV, Koster MPH, Willemsen SP, Koning AHJ, Steegers EAP, Steegers-Theunissen RPM. The Impact of Neighbourhood Deprivation on Embryonic Growth Trajectories: Rotterdam Periconception Cohort. Journal of Clinical Medicine. 2019; 8(11):1913. https://doi.org/10.3390/jcm8111913
Chicago/Turabian StyleGootjes, Dionne V., Maria P. H. Koster, Sten P. Willemsen, Anton H. J. Koning, Eric A. P. Steegers, and Régine P. M. Steegers-Theunissen. 2019. "The Impact of Neighbourhood Deprivation on Embryonic Growth Trajectories: Rotterdam Periconception Cohort" Journal of Clinical Medicine 8, no. 11: 1913. https://doi.org/10.3390/jcm8111913
APA StyleGootjes, D. V., Koster, M. P. H., Willemsen, S. P., Koning, A. H. J., Steegers, E. A. P., & Steegers-Theunissen, R. P. M. (2019). The Impact of Neighbourhood Deprivation on Embryonic Growth Trajectories: Rotterdam Periconception Cohort. Journal of Clinical Medicine, 8(11), 1913. https://doi.org/10.3390/jcm8111913







