A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis
Abstract
:1. Introduction
2. Method
2.1. Search Strategy
- Anticoagulants OR Heparin OR LMWH OR UFH OR Danaparoid OR Protease Inhibitors OR Gabexate OR Nafamostat OR Thrombomodulin OR Tissue-factor-pathway Inhibitor OR Antithrombin OR Protein C.
- Sepsis OR Systemic Inflammatory Response Syndrome OR Hemorrhagic Disorders OR Blood Coagulation Disorders OR Thrombophilia OR Disseminated Intravascular Coagulation.
- Systematic Review OR Meta-analysis.
2.2. Study Selection and Inclusion Criteria
- Study types: Systematic reviews and meta-analyses of randomized controlled trials.
- Population: Sepsis patients regardless of the causes and age.
- Intervention: Any anticoagulants such as antithrombin, rTM, unfractionated heparin and low-molecular weight heparin, recombinant human activated protein C, and tissue pathway factor inhibitor.
- Control: No restriction on controls.
- Outcomes: All-cause mortality and bleeding complications.
2.3. Data Extraction
2.4. Quality Assessment and Data Synthesis
3. Results
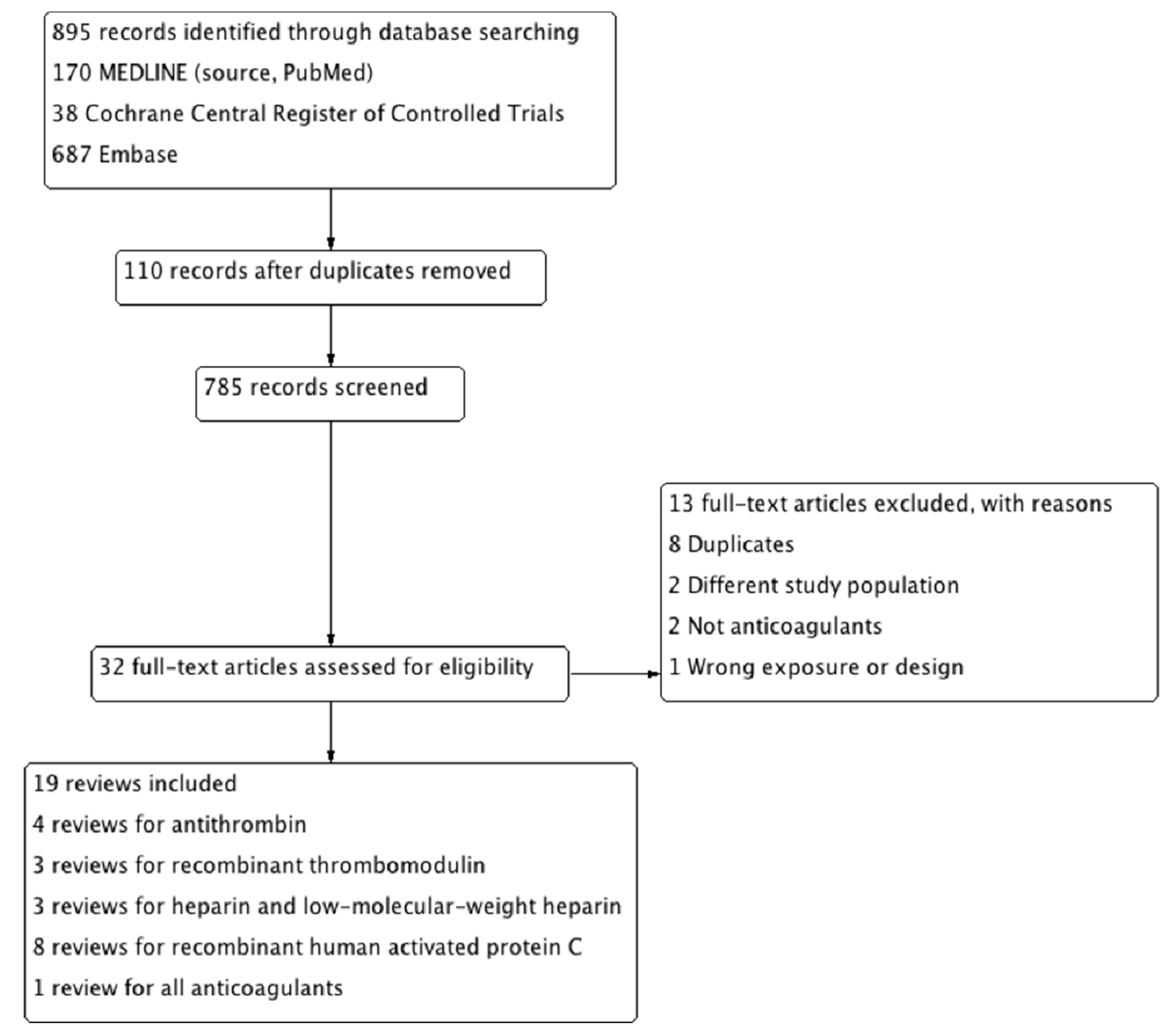
3.1. Literature Search
3.2. Included Studies
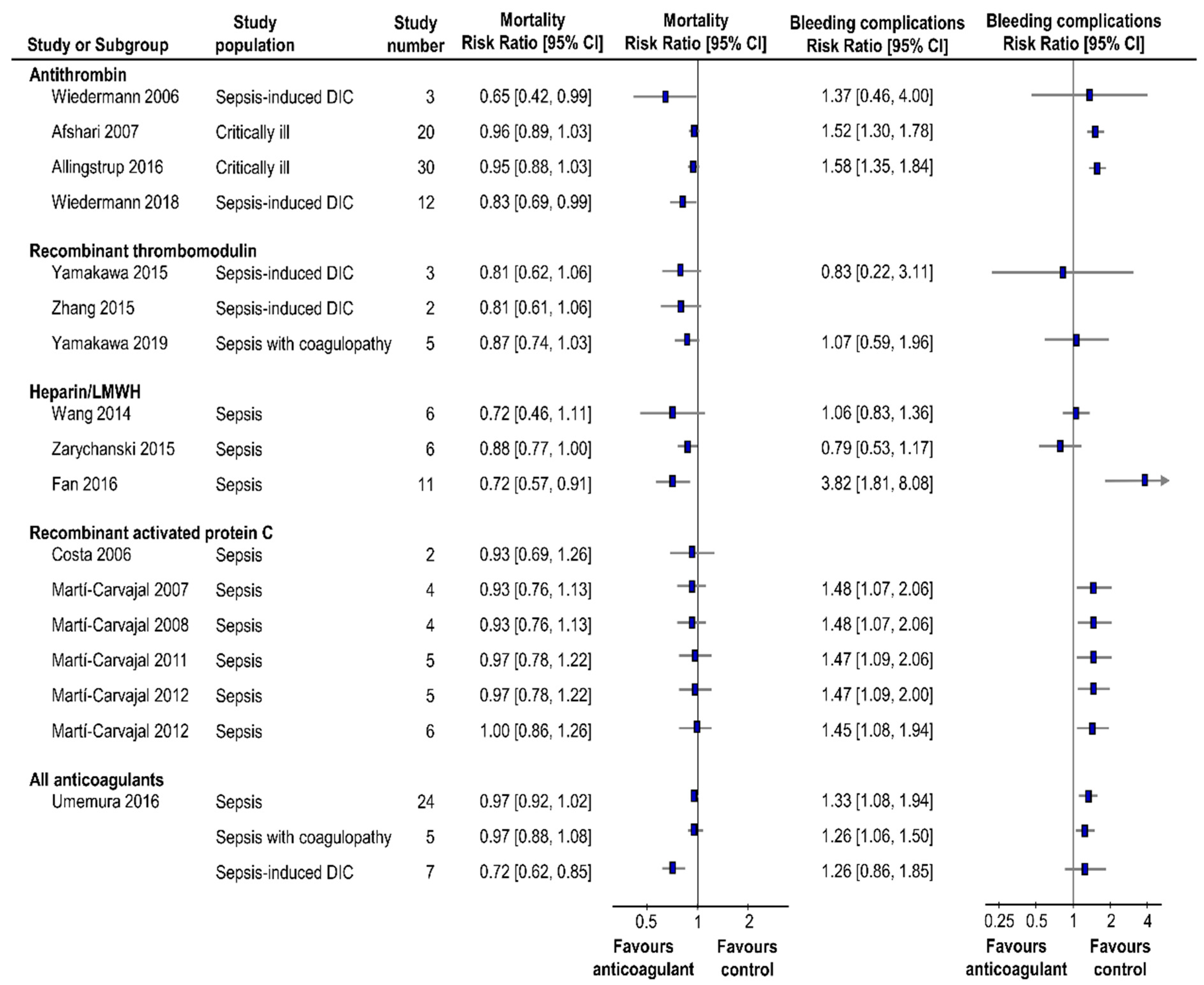
4. Summary of Systematic Reviews
4.1. Antithrombin
4.2. Recombinant Thrombomodulin
4.3. Unfractionnated Heparin and Low-Molucular-Weight Heparin
4.4. Recombinant Activated Protein C
4.5. All Anticoagulant Therapy Classified to Specific Population
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gando, S.; Kamene, T.; Nanzaki, S.; Nakanishi, Y. Disseminated intravascular coagulation is a frequent complication of systemic inflammatory response syndrome. Thromb. Haemost. 1996, 75, 224–228. [Google Scholar] [CrossRef]
- Vervloet, M.G.; Thijs, L.G.; Hack, C.E. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin. Thromb. Hemost. 1998, 24, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Saitoh, D.; Ogura, H.; Fujishima, S.; Mayumi, T.; Araki, T.; Ikeda, H.; Kotani, J.; Kushimoto, S.; Miki, Y.; et al. Japanese Association for Acute Medicine Sepsis Registry Study Group. A multicenter, prospective validation study of the Japanese Association for Acute Medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit. Care 2013, 17, R111. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.L.; Eid, A.; Singer, P.; Pillay, S.S.; Carl, P.; Novak, I.; Chalupa, P.; Atherstone, A.; Pénzes, I.; Kübler, A.; et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: A randomized controlled trial. JAMA 2001, 286, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Ramesh, M.K.; Ernest, D.; LaRosa, S.P.; Pachl, J.; Aikawa, N.; Hoste, E.; Levy, H.; Hirman, J.; Levi, M.; et al. A randomized, double- blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit. Care Med. 2013, 41, 2069–2079. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef]
- Woolf, S.; Schünemann, H.J.; Eccles, M.P.; Grimshaw, J.M.; Shekelle, P. Developing clinical practice guidelines: Types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement. Sci. 2012, 7, 61. [Google Scholar] [CrossRef]
- Mulrow, C.D. Systematic reviews: Rationale for systematic reviews. BMJ 1994, 309, 597–599. [Google Scholar] [CrossRef]
- Yamakawa, K.; Murao, S.; Aihara, M. Recombinant human soluble thrombomodulin in sepsis-induced coagulopathy: An updated systematic review and meta-analysis. Thromb. Haemost. 2019, 119, 56–65. [Google Scholar] [CrossRef]
- Allingstrup, M.; Wetterslev, J.; Ravn, F.B.; Møller, A.M.; Afshari, A. Antithrombin III for critically ill patients: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016, 42, 505–520. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Antithrombin concentrate use in disseminated intravascular coagulation of sepsis: Meta-analyses revisited. J. Thromb. Haemost. 2018, 16, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Kaneider, N.C. A systematic review of antithrombin concentrate use in patients with disseminated intravascular coagulation of severe sepsis. Blood Coagul. Fibrinolysis 2006, 17, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Aihara, M.; Ogura, H.; Yuhara, H.; Hamasaki, T.; Shimazu, T. Recombinant human soluble thrombomodulin in severe sepsis: A systematic review and meta-analysis. J. Thromb. Haemost. 2015, 13, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Yang, H.; Tong, Z. Recombinant human soluble thrombomodulin and short-term mortality of infection patients with DIC: Meta-analysis. Am. J. Emerg. Med. 2016, 34, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chi, C.; Guo, L.; Wang, X.; Guo, L.; Sun, J.; Sun, B.; Liu, S.; Chang, X.; Li, E. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: A systematic review and meta-analysis. Crit. Care 2014, 18, 563. [Google Scholar] [CrossRef]
- Zarychanski, R.; Abou-Setta, A.M.; Kanji, S.; Turgeon, A.F.; Kumar, A.; Houston, D.S.; Rimmer, E.; Houston, B.L.; McIntyre, L.; Fox-Robichaud, A.E.; et al. The efficacy and safety of heparin in patients with sepsis: A systematic review and metaanalysis. Crit. Care Med. 2015, 43, 511–518. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, M.; Gong, D.; Zou, C. Efficacy and safety of low-molecular-weight heparin in patients with sepsis: A meta-analysis of randomized controlled trials. Sci. Rep. 2016, 6, 25984. [Google Scholar] [CrossRef]
- Kylat, R.I.; Ohlsson, A. Recombinant human activated protein C for severe sepsis in neonates. Cochrane Database Syst. Rev. 2006, 2, CD005385. [Google Scholar]
- Costa, V.; Broophy, J.M. Drotrecogin alfa (activated) in severe sepsis: A systematic review and new-cost-effectiveness analysis. BMC Anesthesiol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.; Salanti, G.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2007, 3, CD004388. [Google Scholar]
- Martí-Carvajal, A.J.; Salanti, G.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2008, 1, CD004388. [Google Scholar]
- Kylat, R.I.; Ohlsson, A. Recombinant human activated protein C for severe sepsis in neonates. Cochrane Database Syst. Rev. 2012, 4, CD005385. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2011, 4, CD004388. [Google Scholar]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Cardona, A.F. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst. Rev. 2012, 3, CD00438. [Google Scholar]
- Martí-Carvajal, A.J.; Solà, I.; Gluud, C.; Lathyris, D.; Anand, V. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst. Rev. 2012, 12, CD00438. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamakawa, K.; Ogura, H.; Yuhara, H.; Fujimi, S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: A meta-analysis of randomized controlled trials. J. Thromb. Haemost. 2016, 14, 518–530. [Google Scholar] [CrossRef]
- Afshari, A.; Wetterslev, J.; Brok, J.; Møller, A. Antithrombin III for critically ill patients. Cochrane Database Syst. Rev. 2008, 3, CD005370. [Google Scholar]
- Kienast, J.; Juers, M.; Wiedermann, C.J.; Hoffmann, J.N.; Ostermann, H.; Strauss, R.; Keinecke, H.O.; Warren, B.L.; Opal, S.M.; KyberSept investigators. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. J. Thromb. Haemost. 2006, 4, 90–97. [Google Scholar] [CrossRef]
- Nishida, O.; Ogura, H.; Egi, M.; Hayashi, Y.; Iba, T.; Imaizumi, H.; Inoue, S.; Kakihana, Y.; Kotani, J.; Kushimoto, S.; et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 (J-SSCG 2016). J. Intensiv. Care 2018, 6, 7. [Google Scholar] [CrossRef]
- Asahi Kasei Pharma Corporation. Preliminary Results of Overseas Phase III Clinical Study for ART-123. Available online: https://www.asahi-kasei.co.jp/asahi/en/news/2018/e180802.html (accessed on 2 August 2018).
- Bernard, G.R.; Vincent, J.L.; Laterre, P.F.; LaRosa, S.P.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Laterre, P.F.; Garg, R.; Levy, H.; Talwar, D.; Trzaskoma, B.L.; François, B.; Guy, J.S.; Brückmann, M.; Rea-Neto, A.; et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 2005, 353, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L. The coming era of precision medicine for intensive care. Crit. Care 2017, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Seymour, C.W.; Kennedy, J.N.; Wang, S.; Chang, C.C.H.; Elliott, C.F.; Xu, Z.; Berry, S.; Clermont, G.; Cooper, G.; Gomez, H.; et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019, 321, 2003–2017. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef]
- Gando, S.; Otomo, Y. Local hemostasis, immunothrombosis, and systemic disseminated intravascular coagulation in trauma and traumatic shock. Crit. Care 2015, 19, 72. [Google Scholar] [CrossRef]
| Type of Anticoagulant | Author | Population | Number of Trials | Number of Patients | AMSTAR |
|---|---|---|---|---|---|
| Antithrombin | Wiedermann, 2006 [13] | Sepsis-induced DIC | 3 | 364 | 10 |
| Afshari, 2008 [28] | Critically ill | 20 | 3458 | 11 | |
| Allingstrup, 2016 [10] | Critically ill | 30 | 3933 | 11 | |
| Wiedermann, 2018 [11] | Sepsis-induced DIC | 12 | 766 | 7 | |
| Thrombomodulin | Yamakawa, 2015 [14] | Sepsis-induced DIC | 3 | 838 | 10 |
| Zhang, 2016 [15] | Sepsis-induced DIC | 2 | 821 | 9 | |
| Yamakawa, 2019 [9] | Sepsis with coagulopathy | 5 | 1762 | 10 | |
| Heparin/ | Wang, 2014 [16] | Sepsis | 6 | 604 | 9 |
| Zarychanski, 2015 [17] | Sepsis | 6 | 2477 | 9 | |
| Low-molecular-weight heparin | Fan, 2016 [18] | Sepsis | 11 | 594 | 8 |
| Activated protein C | Kylat, 2006 [19] | Neonate with sepsis | 0 | NA | 11 |
| Costa, 2007 [20] | Severe sepsis | 2 | 4330 | 7 | |
| Martí-Carvajal, 2007 [21] | Severe sepsis | 4 | 4911 | 11 | |
| Martí-Carvajal, 2008 [22] | Severe sepsis | 4 | 4911 | 11 | |
| Martí-Carvajal, 2011 [24] | Severe sepsis | 5 | 5101 | 11 | |
| Kylat, 2012 [23] | Neonates with sepsis | 0 | NA | 11 | |
| Martí-Carvajal, 2012 [25] | Severe sepsis | 5 | 5101 | 11 | |
| Martí-Carvajal, 2012 [26] | Adult and pediatric sepsis | 6 | 6781 | 11 | |
| All anticoagulants | Umemura, 2016 [27] | Sepsis, Sepsis with coagulopathy, Sepsis-induced DIC | 24 | 14767 | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murao, S.; Yamakawa, K. A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis. J. Clin. Med. 2019, 8, 1869. https://doi.org/10.3390/jcm8111869
Murao S, Yamakawa K. A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis. Journal of Clinical Medicine. 2019; 8(11):1869. https://doi.org/10.3390/jcm8111869
Chicago/Turabian StyleMurao, Shuhei, and Kazuma Yamakawa. 2019. "A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis" Journal of Clinical Medicine 8, no. 11: 1869. https://doi.org/10.3390/jcm8111869
APA StyleMurao, S., & Yamakawa, K. (2019). A Systematic Summary of Systematic Reviews on Anticoagulant Therapy in Sepsis. Journal of Clinical Medicine, 8(11), 1869. https://doi.org/10.3390/jcm8111869






