Identification of Risk of QT Prolongation by Pharmacists When Conducting Medication Reviews in Residential Aged Care Settings: A Missed Opportunity?
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Li, Y.; Li, P.; Wang, X.; Karmakar, C.; Liu, C.; Liu, C. Short-term QT interval variability in patients with coronary artery disease and congestive heart failure: A comparison with healthy control subjects. Med. Biol. Eng. Comput. 2019, 57, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Moss, A.J.; Wojciech, Z. QT Interval: How to measure it and what is “normal”. J. Cardiovasc. Electrophysiol. 2006, 17, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Dessertenne, F. La tachycardie ventriculaire a deux foyers opposes variables. Arch. Mal. Coeur. Vaiss 1966, 59, 263–272. [Google Scholar] [PubMed]
- Turner, J.R.; Rodriguez, I.; Mantovani, E.; Gintant, G.; Kowey, P.R.; Klotzbaugh, R.J.; Prasad, K.; Sager, P.T.; Stockbridge, N.; Strnadova, C. On behalf of the Cardiac Safety Research Consortium. Drug-induced proarrhythmia and Torsade de Pointes: A primer for students and practitioners of medicine and pharmacy. J. Clin. Pharmacol. 2018, 58, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Link, M.G.; Yan, G.-X.; Kowey, P.R. Evaluation of toxicity for heart failure therapeutics: Studying effects on the QT interval. Circ. Heart Fail. 2010, 3, 547–555. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, P.A.; Gaviria-Mendoza, A.; Cañón, M.M.; Machado-Alba, J.E. High prevalence of risk factors in elderly patients using drugs associated with acquired torsades de pointes chronically in Colombia. Br. J. Clin. Pharmacol. 2016, 82, 504–511. [Google Scholar] [CrossRef]
- Trinkley, K.E.; Page, R.L.; Lien, H.; Yamanouye, K.; Tisdale, J.E. QT interval prolongation and the risk of torsades de pointes: Essentials for clinicians. Curr. Med. Res. Opin. 2013, 29, 1719–1726. [Google Scholar] [CrossRef]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk factors for QTc-prolongation: Systematic review of the evidence. Int. J. Clin. Pharm. 2017, 39, 16–25. [Google Scholar] [CrossRef]
- Vlachos, K.; Georgopoulos, S.; Efremidis, M.; Sideris, A.; Letsas, K.P. An update on risk factors for drug-induced arrhythmias. Exp. Rev. Clin. Pharmacol. 2016, 9, 117–127. [Google Scholar] [CrossRef]
- Heemskerk, C.P.M.; Pereboom, M.; van Stralen, K.; Berger, F.A.; van den Bemt, P.M.L.A.; Kuijper, A.F.M.; van der Hoeven, R.T.M.; Mantel-Teeuwisse, A.K.; Becker, M.L. Risk factors for QTc interval prolongation. Eur. J. Clin. Pharmacol. 2018, 74, 183–191. [Google Scholar] [CrossRef]
- Sluggett, J.K.; Ilomäki, J.; Seaman, K.L.; Corlis, M.; Bell, J.S. Medication management policy, practice and research in Australian residential aged care: Current and future directions. Pharmacol. Res. 2017, 116, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Koria, L.G.; Zaidi, T.S.; Peterson, G.; Nishtala, P.; Hannah, P.J.; Castelino, R. Impact of medication reviews on inappropriate prescribing in aged care. Curr. Med. Res. Opin. 2018, 34, 833–838. [Google Scholar] [CrossRef] [PubMed]
- The Pharmacy Guild of Australia. Residential Medication Management Review Programs (RMMR) and Quality use of Medicines Program (QUM) Program Rules. 2015. Available online: file:///C:/Users/s429825/Downloads/6CPA_RMMR-QUM-Program-Rules_Jul2017.pdf (accessed on 3 May 2019).
- Buss, V.H.; Lee, K.; Naunton, M.; Peterson, G.M.; Kosari, S. Identification of patients at-risk of QT interval prolongation during medication reviews: A missed opportunity? J. Clin. Med. 2018, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Woosley, R.L.; Heise, C.W.; Romero, K.A. QT Drugs List [Internet]. Arizona: AZCERT. Available online: www.Crediblemeds.org (accessed on 9 July 2019).
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Spriet, I.; Willems, R.; Foulon, V. Development of a risk score for QTc-prolongation: The RISQ-PATH study. Int. J. Clin. Pharm. 2017, 39, 424–432. [Google Scholar] [CrossRef]
- Cortejoso, L.; Dietz, R.A.; Hofmann, G.; Gosch, M.; Sattler, A. Impact of pharmacist interventions in older patients: A prospective study in a tertiary hospital in Germany. Clin. Interv. Aging 2016, 11, 1343–1350. [Google Scholar] [CrossRef]
- Sawan, M.; Chen, T.F.; Myint, P.K.; Jeon, YH.; Hilmer, S.N. Now is the time to address the Culture of Residential Aged Care Facilities to support pharmacists in reducing psychotropic prescribing. Int. J. Pharm. Pract. 2019, 27, 404–405. [Google Scholar] [CrossRef]
- Wang, K.N.; Bell, J.S.; Tan, E.C.K.; Gilmartin-Thomas, J.F.M.; Dooley, M.J.; Ilomäki, J. Proton Pump Inhibitors and Infection-Related Hospitalizations Among Residents of Long-Term Care Facilities: A Case-Control Study. Drugs Aging 2019. [Google Scholar] [CrossRef]
- Kosari, S.; McDerby, N.; Thomas, J.; Naunton, M. Quality use of medicines in aged care facilities: A need for new models of care. J. Clin. Pharm. Ther. 2018, 43, 591–593. [Google Scholar] [CrossRef]
- Grindrod, K.A. Simplifying QT prolongation for busy clinicians. Can. Fam. Physician 2019, 65, 268–270. [Google Scholar]
- Vandael, E.; De Wulf, I.; Foulon, V. Drug-drug interactions with risk of QT-prolongation. An epidemiological study in Belgian community pharmacies. J. Pharm. Belg. 2016, 4, 14–23. [Google Scholar]
- Dhanani, T.C.; Mantovani, E.H.; Turner, J.R. Clinical pharmacists’ opportunities to reduce inappropriate prescription of QT-prolonging medications: Calls to action. Int. J. Pharm. Pract. 2017, 25, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Allen LaPointe, N.M.; Kramer, J.M.; Chen, A.Y.; Hammill, B.G.; Delong, L.; Califf, R.M. A survey of health care practitioners’ knowledge of the QT interval. J. Gen. Int. Med. 2005, 20, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E. Drug-induced QT interval prolongation and torsades de pointes: Role of the pharmacist in risk assessment, prevention and management. Can. Pharm. J. 2016, 149, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R.; Karnad, D.R.; Cabell, C.H.; Kothari, S. Recent developments in the science of proarrhythmic cardiac safety of new drugs. Eur. Heart J. Cardiovasc. Pharm. 2017, 3, 118–124. [Google Scholar] [CrossRef]
- Thind, M.; Ignacio, R.; Kosari, S.; Turner, J.R. How to prescribe drugs with an identified proarrhythmic liability. J. Clin. Pharm. 2019, in press. [Google Scholar]
- Klotzbaugh, R.J.; Martin, A.; Turner, J.R. Drug-induced proarrhythmia: Discussion and considerations for clinical practice. JAANP JAAPA 2019, in press. [Google Scholar]
- Turner, J.R. Proposed proarrhythmic cardiac safety education in medical, pharmacy, and nursing schools: An interprofessional model. Ther. Innov. Regul. Sci. 2018, 52, 529–530. [Google Scholar] [CrossRef]
| Risk Factor | Points Allocated |
|---|---|
| Age ≥65 years | 3 |
| Female gender | 3 |
| Smoking | 3 |
| Body mass index ≥30 kg/m2 | 1 |
| Ischaemic cardiomyopathy | 3 |
| Hypertension | 3 |
| Arrhythmia | 3 |
| Prolonged QT interval on baseline ECG | 6 |
| Thyroid disturbance | 3 |
| Liver failure | 1 |
| Neurological disorder (stroke, trauma, infection, tumour) | 0.5 |
| Diabetes | 0.5 |
| Hypokalaemia (potassium ≤3.5 mmol/L) | 6 |
| Hypocalcaemia (calcium <2.15 mmol/L) | 3 |
| CRP >5mg/L (inflammation) | 1 |
| Renal impairment (eGFR ≤30 mL/min/1.73 m2) | 0.5 |
| For each known risk category 1 drug | 3 |
| For each possible risk category 2 drug | 0.5 |
| For each conditional risk category 3 drug | 0.25 |
| Total RISQ-PATH score | Max 40.5 + sum QT drugs |
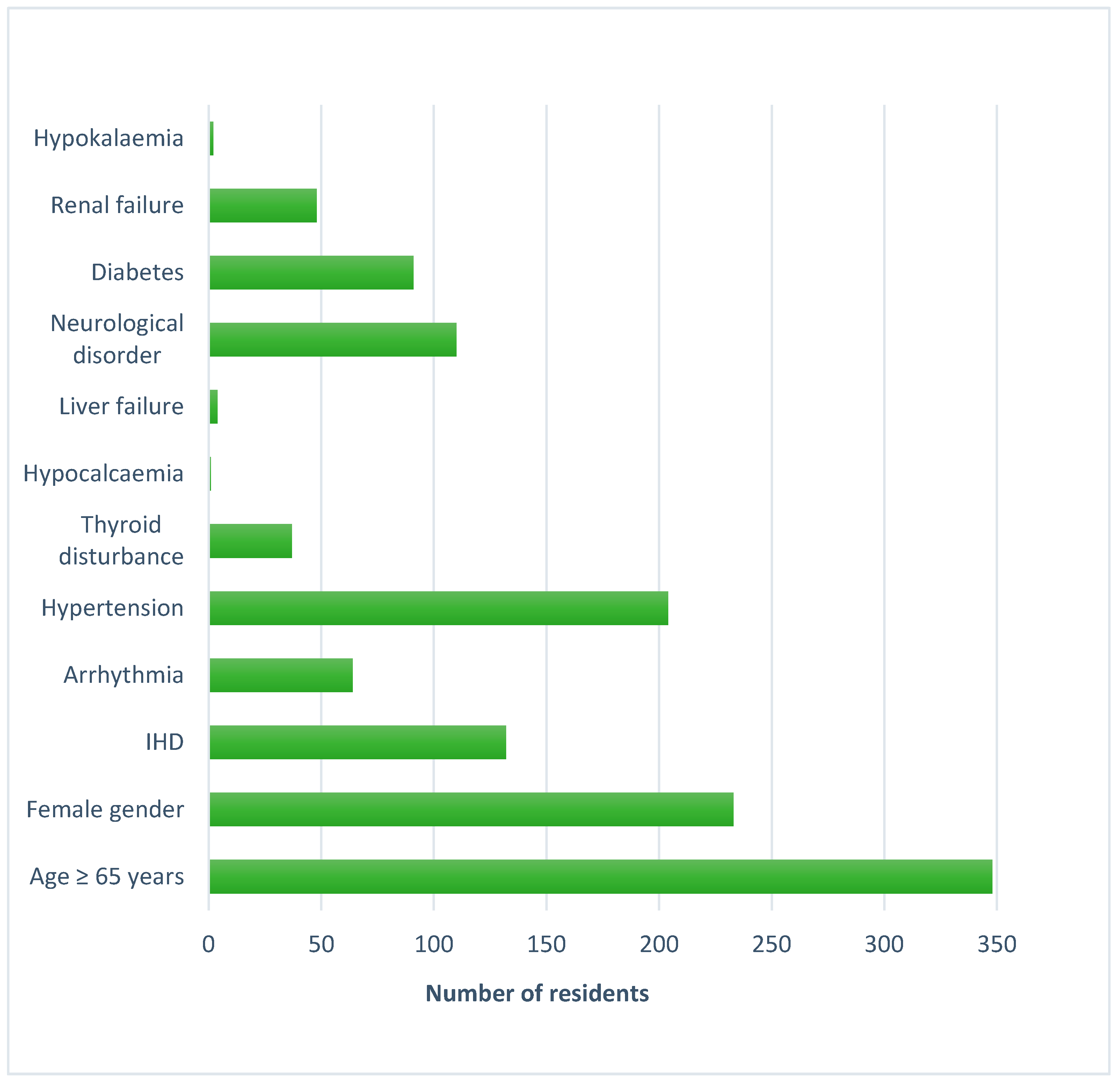
| Characteristics | Mean ± SD (Range) or n (%) |
|---|---|
| Number of residents | 400 |
| Age, years | 79 ± 13 (37–101) |
| Female | 233 (58%) |
| Number of medications per resident Number of medications with known risk of QT prolongation per resident Number of medications with possible risk of QT prolongation per resident Number of medications with conditional risk of QT prolongation per resident | 12 ± 4 (0–26) 0.2 ± 0.5 (0–2) 1 ± 0.8 (0–4) 1 ± 1.1 (0–5) |
| Number of medical conditions per resident | 10 ± 4 (1–26) |
| Mean RISQ-PATH score per resident | 9.5 ± 4 (0–25.75) |
| “Known Risk” | Number of Residents | % of Total Number of Medications | “Possible Risk” | Number of Residents | % of Total Number of Medications | “Conditional Risk” | Number of Residents | % of Total Number of Medications |
|---|---|---|---|---|---|---|---|---|
| escitalopram | 21 | 2.2 | risperidone | 73 | 7.7 | furosemide | 108 | 11.4 |
| citalopram | 21 | 2.2 | mirtazapine | 65 | 6.9 | metoclopramide | 92 | 9.7 |
| donepezil | 19 | 2.0 | buprenorphine | 46 | 4.9 | quetiapine | 71 | 7.5 |
| haloperidol | 18 | 1.9 | memantine | 25 | 2.6 | esomeprazole | 61 | 6.5 |
| amiodarone | 6 | 0.6 | tramadol | 23 | 2.4 | olanzapine | 56 | 5.9 |
| domperidone | 4 | 0.4 | venlafaxine | 16 | 1.7 | pantoprazole | 34 | 3.6 |
| ondansetron | 2 | 0.2 | aripiprazole | 12 | 1.3 | sertraline | 30 | 3.2 |
| sotalol | 2 | 0.2 | lithium | 11 | 1.2 | omeprazole | 26 | 2.8 |
| fingolimod | 1 | 0.1 | clozapine | 8 | 0.8 | loperamide | 22 | 2.3 |
| flecainide | 1 | 0.1 | promethazine | 4 | 0.4 | paroxetine | 11 | 1.2 |
| ketoconazole | 1 | 0.1 | imipramine | 1 | 0.1 | galantamine | 11 | 1.2 |
| TOTAL | 96 | 10 | paliperidone | 1 | 0.1 | hydrochlorothiazide | 10 | 1.1 |
| tamoxifen | 1 | 0.1 | amitriptyline | 9 | 1.0 | |||
| tetrabenazine | 1 | 0.1 | indapamide | 7 | 0.7 | |||
| tolterodine | 1 | 0.1 | amisulpride | 4 | 0.4 | |||
| TOTAL | 288 | 30 | solifenacin | 3 | 0.3 | |||
| amantidine | 2 | 0.2 | ||||||
| fluoxetine | 1 | 0.1 | ||||||
| hydroxychloroquine | 1 | 0.1 | ||||||
| metronidazole | 1 | 0.1 | ||||||
| lansoprazole | 1 | 0.1 | ||||||
| TOTAL | 561 | 59 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, L.; Turner, J.R.; Peterson, G.M.; Naunton, M.; Thomas, J.; Yee, K.C.; Kosari, S. Identification of Risk of QT Prolongation by Pharmacists When Conducting Medication Reviews in Residential Aged Care Settings: A Missed Opportunity? J. Clin. Med. 2019, 8, 1866. https://doi.org/10.3390/jcm8111866
Christensen L, Turner JR, Peterson GM, Naunton M, Thomas J, Yee KC, Kosari S. Identification of Risk of QT Prolongation by Pharmacists When Conducting Medication Reviews in Residential Aged Care Settings: A Missed Opportunity? Journal of Clinical Medicine. 2019; 8(11):1866. https://doi.org/10.3390/jcm8111866
Chicago/Turabian StyleChristensen, Louise, J. Rick Turner, Gregory M. Peterson, Mark Naunton, Jackson Thomas, Kwang Choon Yee, and Sam Kosari. 2019. "Identification of Risk of QT Prolongation by Pharmacists When Conducting Medication Reviews in Residential Aged Care Settings: A Missed Opportunity?" Journal of Clinical Medicine 8, no. 11: 1866. https://doi.org/10.3390/jcm8111866
APA StyleChristensen, L., Turner, J. R., Peterson, G. M., Naunton, M., Thomas, J., Yee, K. C., & Kosari, S. (2019). Identification of Risk of QT Prolongation by Pharmacists When Conducting Medication Reviews in Residential Aged Care Settings: A Missed Opportunity? Journal of Clinical Medicine, 8(11), 1866. https://doi.org/10.3390/jcm8111866








