Symbolic Recurrence Analysis of RR Interval to Detect Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
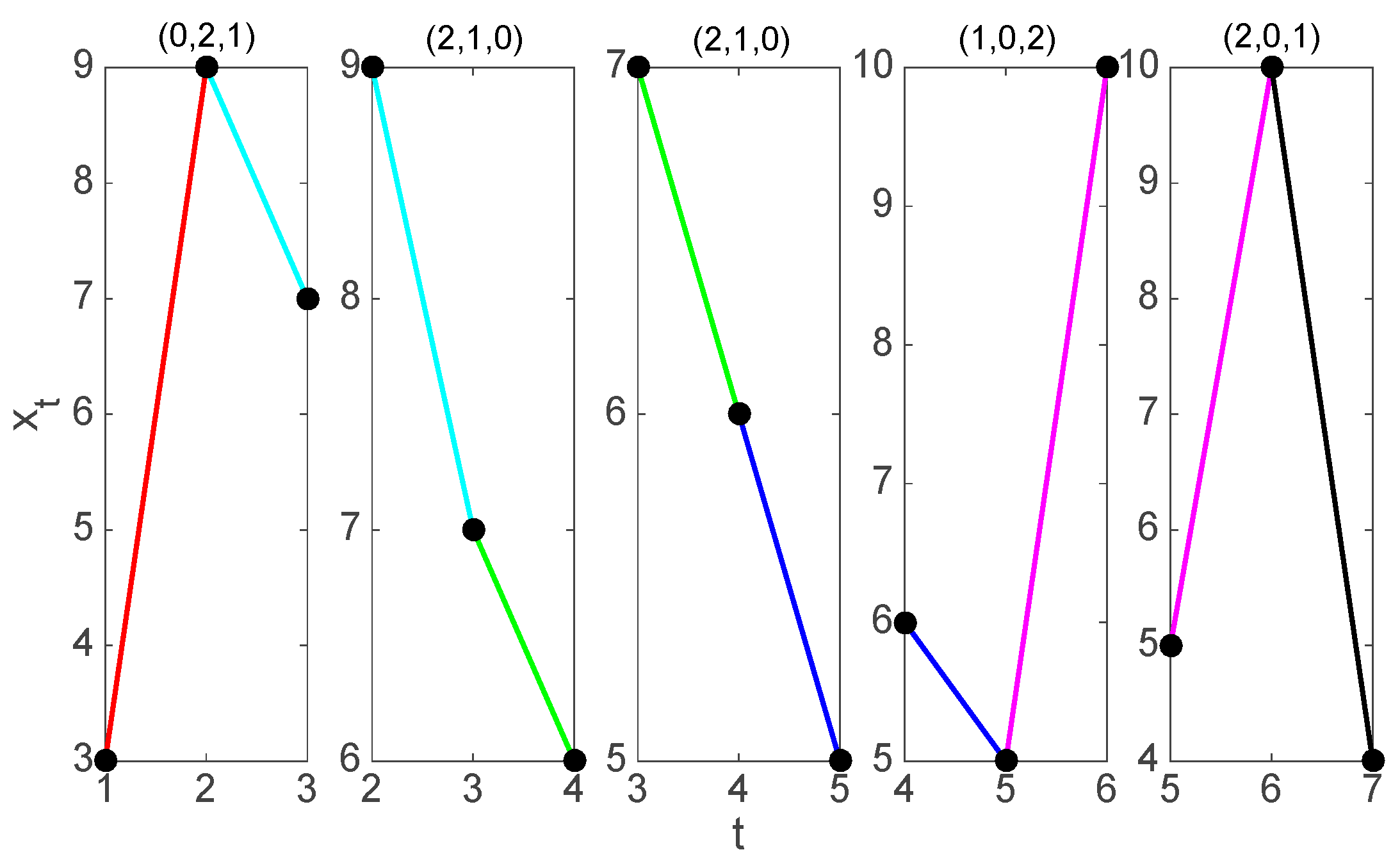
2.1. Symbolization
2.2. Symbolic Recurrence Analysis
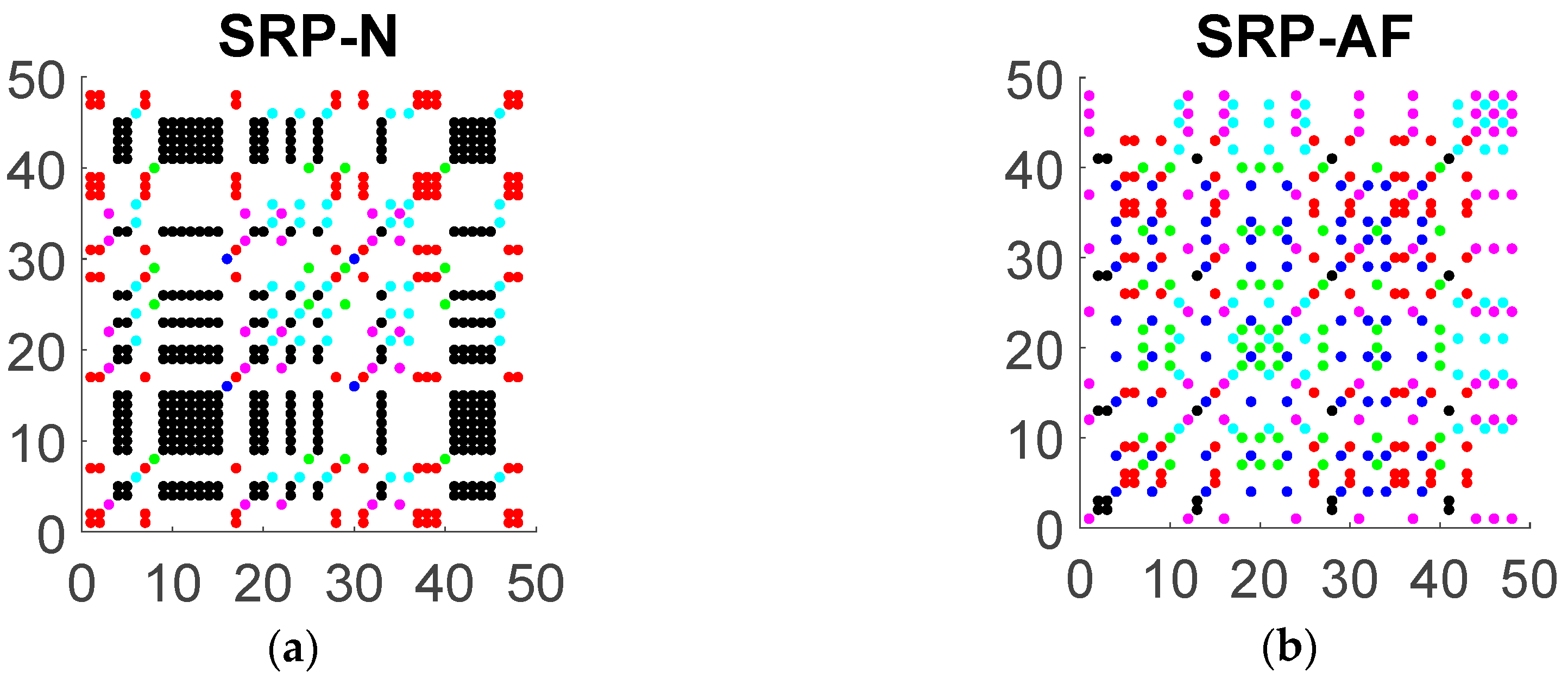
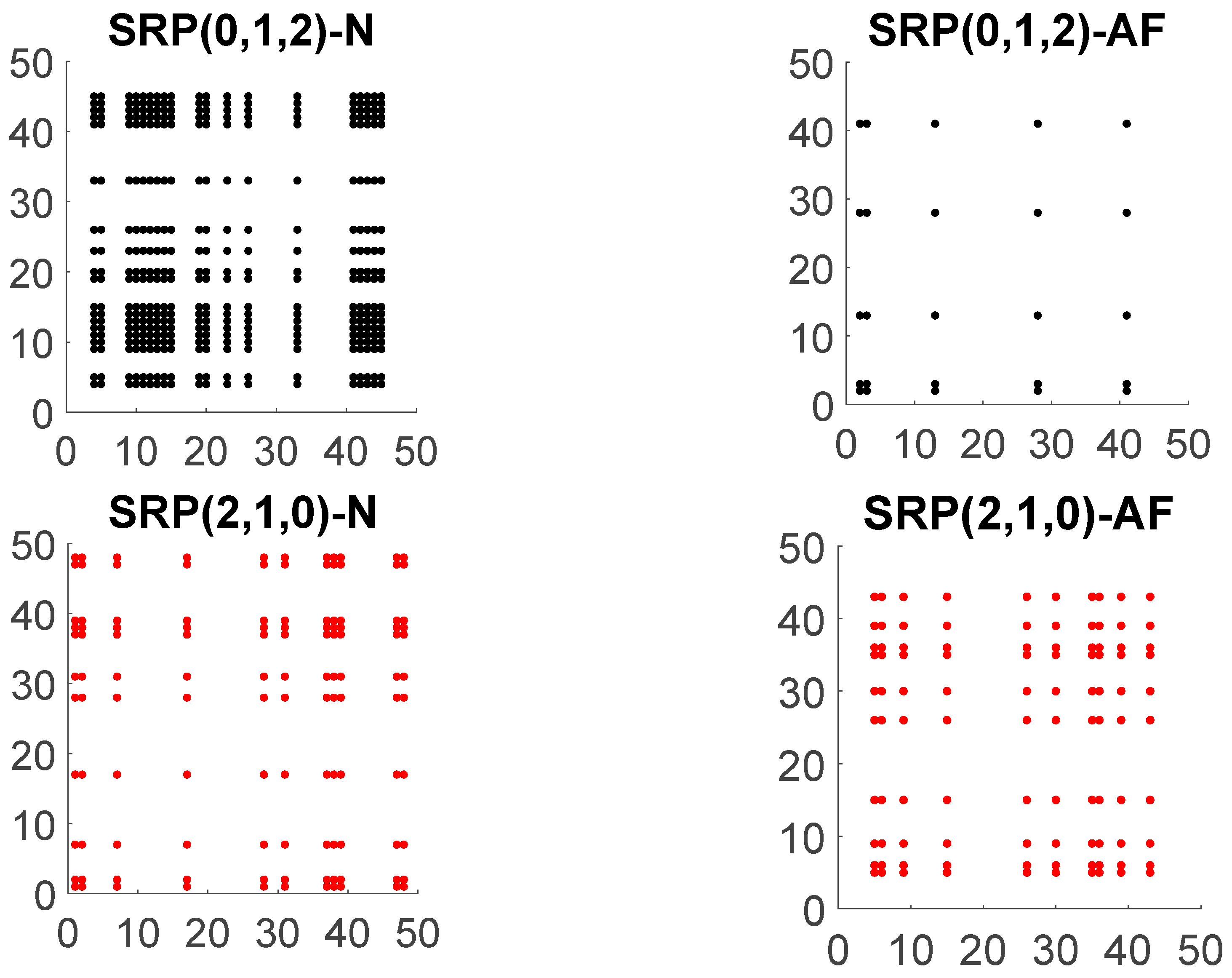
2.3. Symbolic Recurrence Plots of RR Interval Time Series
2.4. Symbolic Recurrence Measures
2.5. A logistic Model to Clasify AF Patients
2.6. Data
3. Results
3.1. Model Estimation
3.2. Classification Power of the Model
- is the number of true positive classified by the model.
- is the number of true negative classified by the model.
- is the number of false negative classified by the model.
- is the number of false positive classified by the model.
- is the true positive rate computed as , also known as sensitivity.
- is the false positive rate computed as . Specificity is known as which measures the proportion of actual negatives that are correctly identified by the model as such.
- determines the model accuracy.
3.3. Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Symbolic Recurrence Measures | N | AF |
|---|---|---|
| 0.1567 | 0.0109 | |
| 0.0213 | 0.0213 | |
| 0.0109 | 0.0352 | |
| 0.0069 | 0.0278 | |
| 0.0017 | 0.0352 | |
| 0.0525 | 0.0434 | |
| 0.6629 | 0.5341 | |
| 0.8326 | 0.5873 | |
| 1.0397 | 0 | |
| 0.6365 | 0 | |
| 4 | 2 | |
| 2.333 | 2 |
| w = 30 | w = 60 | w = 120 | w = 200 | |
|---|---|---|---|---|
| Coeff. | Coeff. | Coeff. | Coeff. | |
| Intercept | 9.25 ** | 16.97 ** | 27.04 ** | 36.44 ** |
| 21.28 ** | 21.47 ** | 26.48 ** | 31.94 ** | |
| −28.02 ** | −28.77 ** | −34.16 ** | −39.54 ** | |
| −32.14 ** | −30.59 ** | −28.02 ** | −27.98 ** | |
| 75.62 ** | 72.96 ** | 69.41 ** | 67.30 ** | |
| −37.69 ** | −63.26 ** | −106.97 ** | −133.84 ** | |
| −27.90 ** | −67.43 ** | −119.86 ** | −142.83 ** | |
| −25.13 ** | −58.88 ** | −102.33 ** | −128.14 ** | |
| −27.53 ** | −62.50 ** | −108.35 ** | −139.10 ** | |
| −30.21 ** | −68.41 ** | −117.66 ** | −145.21 ** | |
| −27.42 ** | −62.58 ** | −114.04 ** | −153.05 ** | |
| 0.33 | 7.99 ** | 21.38 ** | 15.70 * | |
| −2.91 ** | −10.37 ** | −21.45 ** | −20.98 ** | |
| −0.17 | −0.51 ** | −0.74 ** | −0.64 * | |
| 0.13 | −0.31 * | −0.60 ** | −0.68 | |
| −0.03 | 0.02 | −0.19 | −0.56 | |
| 0.02 | −0.04 | −0.16 | −0.20 |
References
- Go, A.S.; Hylek, E.M.; Phillips, K.A.; Chang, Y.; Henault, L.E.; Selby, J.V.; Singer, D.E. Prevalence of Diagnosed Atrial Fibrillation in Adults. JAMA 2001, 285, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Zipes, D.P. Atrial Fibrillation. In Electrocardiography of Arrhythmias: A Comprehensive Review; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 285–308. [Google Scholar]
- Caballero-Pintado, M.V.; Matilla-García, M.; Marín, M.R. Symbolic recurrence plots to analyze dynamical systems. Chaos Interdiscip. J. Nonlinear Sci. 2018, 28, 63112. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Aytemir, K.; Ozer, N.; Atalar, E.; Sade, E.; Aksöyek, S.; Ovünç, K.; Oto, A.; Ozmen, F.; Kes, S. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. 2000, 23, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Censi, F.; Corazza, I.; Reggiani, E.; Calcagnini, G.; Mattei, E.; Triventi, M.; Boriani, G. P-wave Variability and Atrial Fibrillation. Sci. Rep. 2016, 6, 26799. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chang, E.; Yang, W.-H.; Jiang, B.; Yang, A.; Peng, C.-K. Automated Detection of Paroxysmal Atrial Fibrillation Using an Information-Based Similarity Approach. Entropy 2017, 19, 677. [Google Scholar] [CrossRef]
- Dilaveris, P.E.; Gialafos, E.J.; Sideris, S.K.; Theopistou, A.M.; Andrikopoulos, G.K.; Kyriakidis, M.; Gialafos, J.E.; Toutouzas, P.K. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am. Heart J. 1998, 135, 733–738. [Google Scholar] [CrossRef]
- Kennedy, A.; Finlay, D.D.; Guldenring, D.; Bond, R.R.; Moran, K.; Laughlin, J.M. Automated detection of atrial fibrillation using R-R intervals and multivariate-based classification. J. Electrocardiol. 2016, 49, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, G.B.; Mark, R.G. A new method for detecting atrial fibrillation using R-R intervals. Comput. Cardiol. 1983, 10, 227–230. [Google Scholar]
- Teijeiro, T.; Garcia, C.A.; Castro, D.; Félix, P. Arrhythmia Classification from the Abductive Interpretation of Short Single-Lead ECG Records. In Proceedings of the Computing in Cardiology Conference (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
- Andreotti, F.; Carr, O.; Pimentel, M.A.F.; Mahdi, A.; De Vos, M. Comparing Feature Based Classifiers and Convolutional Neural Networks to Detect Arrhythmia from Short Segments of ECG. In Proceedings of the Computing in Cardiology Conference (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
- Limam, M.; Precioso, F. AF Detection and ECG Classification based on Convolutional Recurrent Neural Network. In Proceedings of the Computing in Cardiology Conference (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
- Bin, G.; Shao, M.; Bin, G.; Huang, J.; Zheng, D.; Wu, S. Detection of atrial fibrillation using decision tree ensemble. In Proceedings of the Computing in Cardiology Conference (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
- Lian, J.; Wang, L.; Muessig, D. A Simple Method to Detect Atrial Fibrillation Using RR Intervals. Am. J. Cardiol. 2011, 107, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Tateno, K.; Glass, L. A method for detection of atrial fibrillation using RR intervals. In Proceedings of the Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2000; Volume 27, pp. 391–394. [Google Scholar]
- Dash, S.; Chon, K.H.; Lu, S.; Raeder, E.A. Automatic Real Time Detection of Atrial Fibrillation. Ann. Biomed. Eng. 2009, 37, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
| w = 30 | w = 60 | w = 120 | w = 200 | |
|---|---|---|---|---|
| 0.414 | 0.448 | 0.513 | 0.510 | |
| Se | 0.961 | 0.970 | 0.976 | 0.979 |
| Sp | 0.948 | 0.960 | 0.971 | 0.976 |
| ACC | 0.954 | 0.964 | 0.973 | 0.977 |
| w = 30 | w = 60 | w = 120 | w = 200 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P25 | Me | P75 | P25 | Me | P75 | P25 | Me | P75 | P25 | Me | P75 | |
| 0.399 | 0.417 | 0.420 | 0.419 | 0.457 | 0.465 | 0.460 | 0.479 | 0.510 | 0.498 | 0.505 | 0.526 | |
| Se | 0.927 | 0.965 | 0.992 | 0.942 | 0.969 | 0.991 | 0.962 | 0.973 | 0.988 | 0.975 | 0.979 | 0.986 |
| Sp | 0.940 | 0.956 | 0.973 | 0.936 | 0.962 | 0.976 | 0.957 | 0.965 | 0.982 | 0.962 | 0.967 | 0.980 |
| ACC | 0.929 | 0.945 | 0.965 | 0.950 | 0.956 | 0.965 | 0.967 | 0.969 | 0.975 | 0.967 | 0.974 | 0.984 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Valero, J.; Caballero Pintado, M.V.; Melgarejo, F.; García-Sánchez, A.-J.; Garcia-Haro, J.; García Córdoba, F.; García Córdoba, J.A.; Pinar, E.; García Alberola, A.; Matilla-García, M.; et al. Symbolic Recurrence Analysis of RR Interval to Detect Atrial Fibrillation. J. Clin. Med. 2019, 8, 1840. https://doi.org/10.3390/jcm8111840
Pérez-Valero J, Caballero Pintado MV, Melgarejo F, García-Sánchez A-J, Garcia-Haro J, García Córdoba F, García Córdoba JA, Pinar E, García Alberola A, Matilla-García M, et al. Symbolic Recurrence Analysis of RR Interval to Detect Atrial Fibrillation. Journal of Clinical Medicine. 2019; 8(11):1840. https://doi.org/10.3390/jcm8111840
Chicago/Turabian StylePérez-Valero, Jesús, M. Victoria Caballero Pintado, Francisco Melgarejo, Antonio-Javier García-Sánchez, Joan Garcia-Haro, Francisco García Córdoba, José A. García Córdoba, Eduardo Pinar, Arcadio García Alberola, Mariano Matilla-García, and et al. 2019. "Symbolic Recurrence Analysis of RR Interval to Detect Atrial Fibrillation" Journal of Clinical Medicine 8, no. 11: 1840. https://doi.org/10.3390/jcm8111840
APA StylePérez-Valero, J., Caballero Pintado, M. V., Melgarejo, F., García-Sánchez, A.-J., Garcia-Haro, J., García Córdoba, F., García Córdoba, J. A., Pinar, E., García Alberola, A., Matilla-García, M., Curtin, P., Arora, M., & Ruiz Marín, M. (2019). Symbolic Recurrence Analysis of RR Interval to Detect Atrial Fibrillation. Journal of Clinical Medicine, 8(11), 1840. https://doi.org/10.3390/jcm8111840





