Identification of Clinical and Laboratory Parameters Associated with the Development of Acute Chest Syndrome during Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Preliminary Step before Assessing Specific and Early Treatment Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Management at the Hospital
2.3. Definitions
2.4. Collected Data
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Patient History and Laboratory Parameters at Steady State According to the Development of ASC after Hospitalization for VOEs in the 176 Children
3.2. Risk Factors of Acute Chest Syndrome Complication and Predictive Score
3.3. Short-Term Outcome
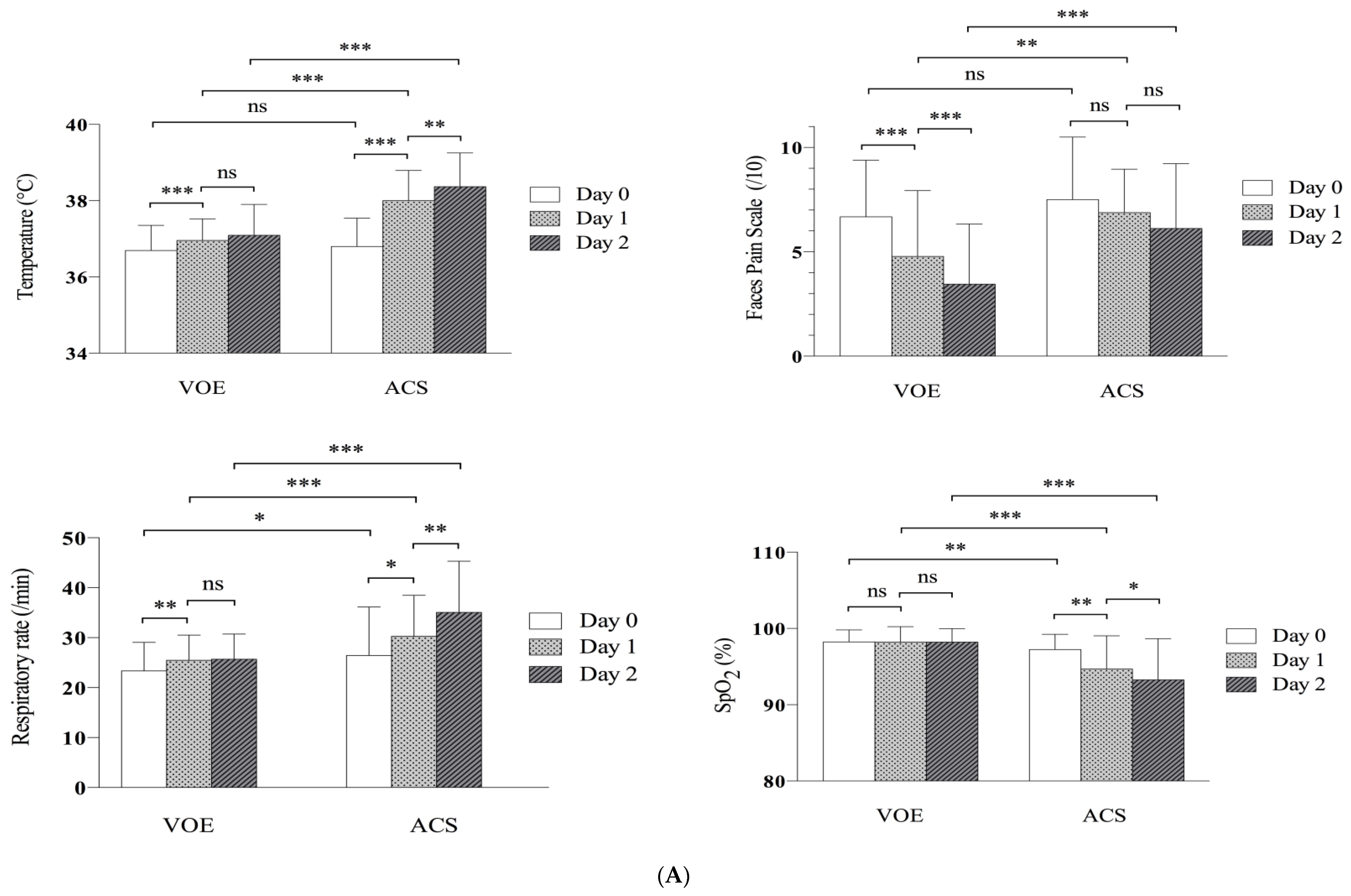
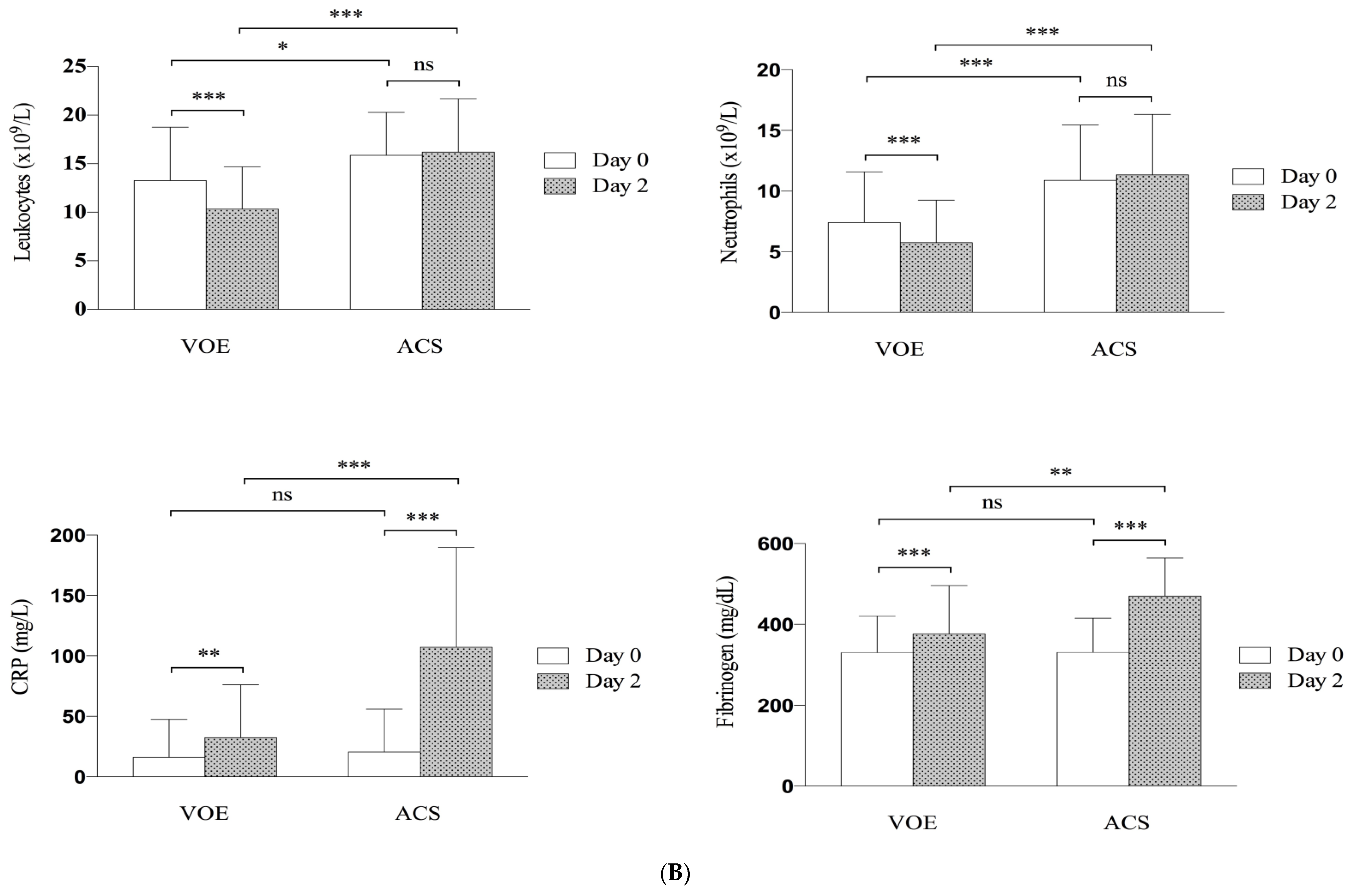
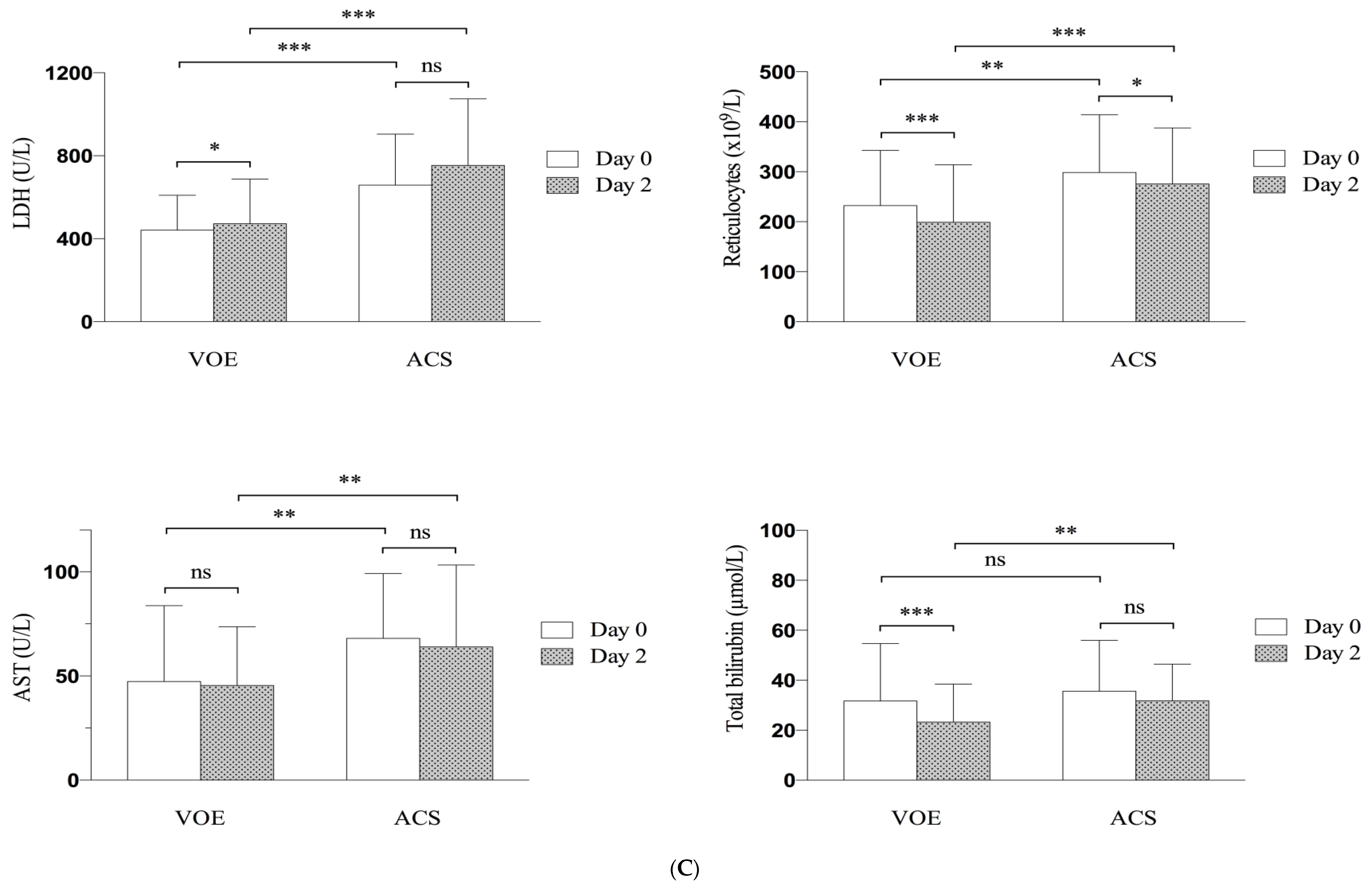
3.4. Evolution of Clinical and Biological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brousse, V.; Arnaud, C.; Lesprit, E.; Quinet, B.; Odièvre, M.H.; Etienne-Julan, M.; Guillaumat, C.; Elana, G.; Belloy, M.; Garnier, N.; et al. Evaluation of Outcomes and Quality of Care in Children with Sickle Cell Disease Diagnosed by Newborn Screening: A Real-World Nation-Wide Study in France. J. Clin. Med. 2019, 8, 1594. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K.; Lieff, S.; Benjamin, L.J.; Dampier, C.D.; Heeney, M.M.; Hoppe, C.; Johnson, C.S.; Rogers, Z.R.; Smith-Whitley, K.; Wang, W.C.; et al. Definitions of the phenotypic manifestations of sickle cell disease. Am. J. Hematol. 2010, 85, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Styles, L.A.; Colangelo, L.H.; Wright, E.C.; Castro, O.; Nickerson, B. Acute chest syndrome in sickle cell disease: Clinical presentation and course: Cooperative Study of Sickle Cell Disease. Blood 1997, 89, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.P.; Neumayr, L.D.; Earles, A.N.; Williams, R.; Lennette, E.T.; Dean, D.; Nickerson, B.; Orringer, E.; McKie, V.; Bellevue, R.; et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000, 342, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.N.; Castro, O.L.; Aggarwal, A.; Oneal, P.A. Acute chest syndrome: Sickle cell disease. Eur. J. Haematol. 2011, 87, 191–207. [Google Scholar] [CrossRef]
- Jain, S.; Bakshi, N.; Krishnamurti, L. Acute Chest Syndrome in Children with Sickle Cell Disease. Pediatr. Allergy. Immunol. Pulmonol. 2017, 30, 191–201. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bauchner, H. Socioeconomic status and length of hospital stay in children with vaso-occlusive crises of sickle cell disease. J. Natl. Med. Assoc. 2007, 99, 192–196. [Google Scholar]
- Raphael, J.L.; Mei, M.; Mueller, B.U.; Giordano, T. High resource hospitalizations among children with vaso-occlusive crises in sickle cell disease. Pediatr. Blood Cancer. 2012, 58, 584–590. [Google Scholar] [CrossRef]
- Howard, J.; Hart, N.; Roberts-Harewood, M.; Awogbade, M.; Davis, B.; BCSH Committee. Guidelines on the management of acute chest syndrome in sickle cell disease. Br. J. Haematol. 2015, 169, 492–505. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Zhi, H.; Yuen, P.S.; Star, R.A.; Banks, S.M.; Schechter, A.N.; Natanson, C.; Gladwin, M.T.; Solomon, S.B. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Investig. 2005, 115, 3409–3417. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Ofori-Acquah, S.F. Erythroid DAMPs drive inflammation in SCD. Blood 2014, 123, 3689–3690. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Vichinsky, E. Pulmonary complications of sickle cell disease. N. Engl. J. Med. 2008, 20, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Bellet, P.S.; Kalinyak, K.A.; Shukla, R.; Gelfand, M.J.; Rucknagel, D.L. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N. Engl. J. Med. 1995, 333, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, C.; Merckx, A.; Brousse, V.; Allali, S.; Hubert, P.; de Montalembert, M.; Lesage, F. Early Noninvasive Ventilation and Nonroutine Transfusion for Acute Chest Syndrome in Sickle Cell Disease in Children: A Descriptive Study. Pediatr. Crit. Care. Med. 2018, 19, e235–e241. [Google Scholar] [CrossRef]
- Yawn, B.P.; Buchanan, G.R.; Afenyi-Annan, A.N.; Ballas, S.K.; Hassell, K.L.; James, A.H.; Jordan, L.; Lanzkron, S.M.; Lottenberg, R.; Savage, W.J.; et al. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 2014, 312, 1033–1048. [Google Scholar] [CrossRef]
- Emre, U.; Miller, S.T.; Gutierez, M.; Steiner, P.; Rao, S.P.; Rao, M. Effect of transfusion in acute chest syndrome of sickle cell disease. J. Pediatr. 1995, 127, 901–904. [Google Scholar] [CrossRef]
- Mallouh, A.A.; Asha, M. Beneficial effect of blood transfusion in children with sickle cell chest syndrome. Am. J. Dis. Child. 1988, 142, 178–182. [Google Scholar] [CrossRef]
- Wayne, A.S.; Kevy, S.V.; Nathan, D.G. Transfusion management of sickle cell disease. Blood 1993, 81, 1109–1123. [Google Scholar] [CrossRef]
- Lanzkowsky, P.; Shende, A.; Karayalcin, G.; Kim, Y.J.; Aballi, A.J. Partial exchange transfusion in sickle cell anemia. Use in children with serious complications. Am. J. Dis. Child. 1978, 132, 1206–1208. [Google Scholar] [CrossRef]
- Dastgiri, S.; Dolatkhah, R. Blood transfusions for treating acute chest syndrome in people with sickle cell disease. Cochrane. Database. Syst. Rev. 2016, 8, CD007843. [Google Scholar] [CrossRef]
- Castro, O.; Brambilla, D.J.; Thorington, B.; Reindorf, C.A.; Scott, R.B.; Gillette, P.; Vera, J.C.; Levy, P.S. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 1994, 84, 643–649. [Google Scholar] [CrossRef]
- Takahashi, T.; Okubo, Y.; Handa, A. Acute chest syndrome among children hospitalized with vaso-occlusive crisis: A nationwide study in the United States. Pediatr. Blood Cancer. 2018, 65, e26885. [Google Scholar] [CrossRef]
- PNDS2010HAS. Available online: https://www.has-sante.fr/portail/upload/docs/application/pdf/2010-04/ald_10_pnds_drepano_enfant_web.pdf (accessed on 14 January 2010).
- Hicks, C.L.; von Baeyer, C.L.; Spafford, P.A.; van Korlaar, I.; Goodenough, B. The Faces Pain Scale-Revised: Toward a common metric in pediatric pain measurement. Pain 2001, 93, 173–183. [Google Scholar] [CrossRef]
- Narbey, D.; Habibi, A.; Chadebech, P.; Mekontso-Dessap, A.; Khellaf, M.; Lelièvre, J.D.; Godeau, B.; Michel, M.; Galactéros, F.; Djoudi, R.; et al. Incidence and predictive score for delayed hemolytic transfusion reaction in adult patients with sickle cell disease. Am. J. Hematol. 2017, 92, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, I.D.; Woodward, M.; Reed, G.W. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatr. Blood Cancer. 2005, 45, 716–724. [Google Scholar] [CrossRef]
- Bartolucci, P.; Habibi, A.; Khellaf, M.; Roudot-Thoraval, F.; Melica, G.; Lascaux, A.S.; Moutereau, S.; Loric, S.; Wagner-Ballon, O.; Berkenou, J.; et al. Score Predicting Acute Chest Syndrome During Vaso-occlusive Crises in Adult Sickle-cell Disease Patients. eBioMedicine 2016, 10, 305–311. [Google Scholar] [CrossRef]
- Stankovic Stojanovic, K.; Steichen, O.; Lefevre, G.; Bachmeyer, C.; Avellino, V.; Grateau, G.; Girot, R.; Lionnet, F. High lactate dehydrogenase levels at admission for painful vaso-occlusive crisis is associated with severe outcome in adult SCD patients. Clin. Biochem. 2012, 45, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Lewing, K.; Britton, K.; DeBaun, M.; Woods, G. The impact of parenteral narcotic choice in the development of acute chest syndrome in sickle cell disease. J. Pediatr. Hematol. Oncol. 2011, 33, 255–260. [Google Scholar] [CrossRef]
- Birken, C.S.; Khambalia, A.; Dupuis, A.; Pastor, A.; Lee, M.; Padavattan, K.; Mekky, M.; Odame, I.; Parkin, P.C. Morphine is associated with acute chest syndrome in children hospitalized with sickle cell disease. Hosp. Pediatr. 2013, 3, 149–155. [Google Scholar] [CrossRef]
- Daswani, D.D.; Shah, V.P.; Avner, J.R.; Manwani, D.G.; Kurian, J.; Rabiner, J.E. Accuracy of Point-of-care Lung Ultrasonography for Diagnosis of Acute Chest Syndrome in Pediatric Patients with Sickle Cell Disease and Fever. Acad. Emerg. Med. 2016, 23, 932–940. [Google Scholar] [CrossRef]
- Ballas, S.K.; Gupta, K.; Adams-Graves, P. Sickle cell pain: A critical reappraisal. Blood 2012, 120, 3647–3656. [Google Scholar] [CrossRef]
- Ballas, S.K.; Smith, E.D. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood 1992, 79, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Hafiani, E.M.; Arbelot, C.; Blayau, C.; Labbe, V.; Stankovic-Stojanovic, K.; Lionnet, F.; Bonnet, F.; Fulgencio, J.P.; Fartoukh, M.; et al. Morpho-functional evaluation of lung aeration as a marker of sickle-cell acute chest syndrome severity in the ICU: A prospective cohort study. Ann. Intensive Care 2019, 30, 109. [Google Scholar] [CrossRef] [PubMed]
- Fartoukh, M.; Lefort, Y.; Habibi, A.; Bachir, D.; Galacteros, F.; Godeau, B.; Maitre, B.; Brochard, L. Early intermittent noninvasive ventilation for acute chest syndrome in adults with sickle cell disease: A pilot study. Intensive Care Med. 2010, 36, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
| Patient History | All Children n = 176 | Children with VOEs Alone n = 141 | Children with ACS n = 35 | p |
|---|---|---|---|---|
| Age, mean ± SD (n = 141/35) | 9.07 (±5.24) | 9.05 (±5.40) | 9.17 (±4.59) | 0.89 |
| Sex (n = 141/35) | ||||
| Female (n, %) | 89 (50.57) | 74 (52.48) | 15 (42.86) | 0.30 |
| G6PD status (n = 132/33) | ||||
| Normal (n, %) | 151 (91.52) | 122 (92.42) | 29 (87.88) | 0.40 |
| Deficiency (n, %) | 14 (8.48) | 10 (7.58) | 4 (12.12) | |
| Genotype (n = 141/35) | ||||
| SC, Sβ+ (n, %) | 33 (18.75) | 31 (21.99) | 2 (5.71) | 0.027 |
| SS, Sβ0, SDPunjab (n, %) | 143 (81.25) | 110 (78.01) | 33 (94.29) | |
| History of asthma (n = 136/35) | ||||
| Presence (n, %) | 19 (11.11) | 13 (9.56) | 6 (17.14) | 0.20 |
| History of ACS (n = 136/35) | ||||
| Presence (n, %) | 81 (47.37) | 59 (43.38) | 22 (62.86) | 0.04 |
| Hydroxyurea treatment (n = 141/35) | ||||
| Yes | 74 (42.05) | 58 (41.3) | 16 (45.71) | 0.62 |
| No | 102 (57.95) | 83 (58.87) | 19 (54.29) | |
| Markers at steady state (Mean, SD) | ||||
| Hemoglobin, g/dL (n = 130/35) | 8.78 (1.50) | 8.91 (1.49) | 8.30 (1.44) | 0.03 |
| Fetal hemoglobin, % (n = 128/33) | 14.22 (8.93) | 14.26 (9.22) | 14.08 (7.86) | 0.91 |
| Leukocyte count, 109/L (n = 129/35) | 12.66 (5.10) | 12.03 (4.68) | 14.96 (5.94) | 0.002 |
| Neutrophil count, 109/L (n = 125/35) | 5.44 (3.21) | 4.94 (2.59) | 7.23 (4.40) | 0.0001 |
| Platelet count, 109/L (n = 128/35) | 346.926 (121.561) | 337.968 (114.771) | 379.685 (140.706) | 0.071 |
| Mean corpuscular volume, fl (n = 128/35) | 74.22 (8.65) | 73.87 (8.52) | 75.52 (9.10) | 0.31 |
| Reticulocyte count, 109/L (n = 128/35) | 264.120 (126.255) | 245.922 (121.912) | 330.670 (120.954) | 0.0004 |
| Lactate dehydrogenase level, U/L (n = 108/32) | 582.77 (325.69) | 565.37 (333.48) | 641.53 (295.23) | 0.24 |
| Total bilirubin level, µmol/L (n = 101/29) | 25.92 (16.60) | 24.78(16.15) | 29.89(17.80) | 0.14 |
| Clinical Variables | Episodes Limited to VOEs n = 156 | Episodes with ACS During VOEs n = 35 | p |
|---|---|---|---|
| Symptoms duration before admission, day | 1.51 (±2.98) | 0.85 (±1.16) | 0.19 |
| Temperature, °C | 36.7 (±0.6) | 36.8 (±0.7) | 0.36 |
| Heart rate, /min | 103.4 (±22.8) | 104.0 (±20.8) | 0.88 |
| Respiratory rate, /min | 23.4 (±5.7) | 26.4 (±9.7) | 0.046 |
| Oxygen saturation on air, % | 98.2 (±1.6) | 97.2 (±2.0) | 0.002 |
| Faces Pain Score, /10 | 6.7 (±2.7) | 7.5 (±2.8) | 0.14 |
| Use of morphine, n (%) | 20 (14.3) | 15 (45.4) | <0.001 |
| Pain localization, n (%) | |||
| Abdominal | 36 (23.1) | 16 (45.7) | 0.007 |
| Thoracic | 29 (18.8) | 9 (25.7) | 0.35 |
| Spinal | 36 (23.1) | 16 (45.7) | 0.007 |
| Pain restricted to arms or legs (≤ 2 localizations) * | 62 (39.7) | 3 (8.6) | <0.001 |
| Pain restricted to arms or legs (> 2 localizations) | 9 (5.8) | 2 (5.7) | 1 |
| Laboratory Parameters | |||
| Leukocyte count, 109/L | 13.27 (±5.50) | 15.86 (±4.43) | 0.01 |
| Neutrophil count, 109/L | 7.41 (±4.18) | 10.89 (±4.55) | <0.001 |
| Platelet count, 109/L | 341.892 (±123.215) | 354.647 (±114.248) | 0.58 |
| Hemoglobin level, g/dL | 9.0 (±1.5) | 8.2 (±1.1) | 0.0031 |
| Reticulocyte count, 109/L | 232.295 (±110.571) | 298.574 (±115.262) | 0.0029 |
| Alanine aminotransferase level, U/L | 24.7 (±33.9) | 28.1 (±24.5) | 0.56 |
| Aspartate aminotransferase level, U/L | 47.4 (±36.4) | 68.0 (±31.2) | 0.0038 |
| Total bilirubin level, µmol/L | 31.7 (±22.9) | 35.6 (±20.3) | 0.36 |
| Lactate dehydrogenase level, U/L | 440.4 (±168.2) | 657.1 (±245.4) | <0.001 |
| C-reactive protein level, mg/L | 15.8 (±31.4) | 20.4 (±35.4) | 0.45 |
| Fibrinogen level, mg/dL | 3.3 (±0.9) | 3.3 (±0.8) | <0.78 |
| Fetal hemoglobin, % | 12.2 (±8.6) | 9.7 (±4.3) | 0.12 |
| Variable | Univariate Analysis | Multivariable Analysis | Score | ||||
|---|---|---|---|---|---|---|---|
| Crude OR a | 95%CI | pd | aOR b | 95%CI | pd | ||
| Sex c | |||||||
| Male | 1 | – | 1 | – | |||
| Female | 0.69 | 0.33–1.46 | 0.33 | 0.41 | 0.15–1.10 | 0.08 | |
| Faces Pain Score at day 0 c | |||||||
| <9 | 1 | – | 1 | – | |||
| ≥9 | 3.13 | 1.40–6.96 | 0.005 | 3.65 | 1.37–9.75 | 0.01 | 4 |
| Neutrophil count (109/L) at day 0 c | |||||||
| ≤10 | 1 | – | 1 | – | |||
| >10 | 4.00 | 1.85–8.66 | 0.0004 | 4.84 | 1.78–13.17 | 0.002 | 5 |
| Reticulocytes count (109/L) at day 0 c | |||||||
| <260 | 1 | – | 1 | – | |||
| ≥260 | 2.35 | 1.07–5.16 | 0.03 | 3.07 | 1.14–8.30 | 0.03 | 3 |
| Pain restricted to arms or legs (≤ 2 localizations) at day 0 c | |||||||
| Yes | 1 | – | 1 | – | |||
| No | 7.04 | 2.06–23.98 | 0.002 | 11.23 | 2.21–57.15 | 0.004 | 11 |
| Predictive score e | VOEs with ACS | VOEs without ACS | Total | ||||
| >20 | 5 | 1 | 6 | PPV = 83.3% | High | ||
| 11–20 | 23 | 77 | 100 | NPV = 77% | Intermediate | ||
| <11 | 1 | 42 | 43 | NPV = 97.7% | Low | ||
| Total | 29 | 120 | 149 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhi, F.; Kamdem, A.; Jung, C.; Carlier-Gonod, A.; Biscardi, S.; Busca, J.; Arnaud, C.; Hau, I.; Narbey, D.; Epaud, R.; et al. Identification of Clinical and Laboratory Parameters Associated with the Development of Acute Chest Syndrome during Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Preliminary Step before Assessing Specific and Early Treatment Strategies. J. Clin. Med. 2019, 8, 1839. https://doi.org/10.3390/jcm8111839
Madhi F, Kamdem A, Jung C, Carlier-Gonod A, Biscardi S, Busca J, Arnaud C, Hau I, Narbey D, Epaud R, et al. Identification of Clinical and Laboratory Parameters Associated with the Development of Acute Chest Syndrome during Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Preliminary Step before Assessing Specific and Early Treatment Strategies. Journal of Clinical Medicine. 2019; 8(11):1839. https://doi.org/10.3390/jcm8111839
Chicago/Turabian StyleMadhi, Fouad, Annie Kamdem, Camille Jung, Adele Carlier-Gonod, Sandra Biscardi, Jeremy Busca, Cecile Arnaud, Isabelle Hau, David Narbey, Ralph Epaud, and et al. 2019. "Identification of Clinical and Laboratory Parameters Associated with the Development of Acute Chest Syndrome during Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Preliminary Step before Assessing Specific and Early Treatment Strategies" Journal of Clinical Medicine 8, no. 11: 1839. https://doi.org/10.3390/jcm8111839
APA StyleMadhi, F., Kamdem, A., Jung, C., Carlier-Gonod, A., Biscardi, S., Busca, J., Arnaud, C., Hau, I., Narbey, D., Epaud, R., & Pondarre, C. (2019). Identification of Clinical and Laboratory Parameters Associated with the Development of Acute Chest Syndrome during Vaso-Occlusive Episodes in Children with Sickle Cell Disease: A Preliminary Step before Assessing Specific and Early Treatment Strategies. Journal of Clinical Medicine, 8(11), 1839. https://doi.org/10.3390/jcm8111839





