GSTM1 and Liver Iron Content in Children with Sickle Cell Anemia and Iron Overload
Abstract
1. Background
2. Methods
2.1. Patient Selection
2.2. Data Collection
2.3. MRI Studies
2.4. GSTM1 Assay
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
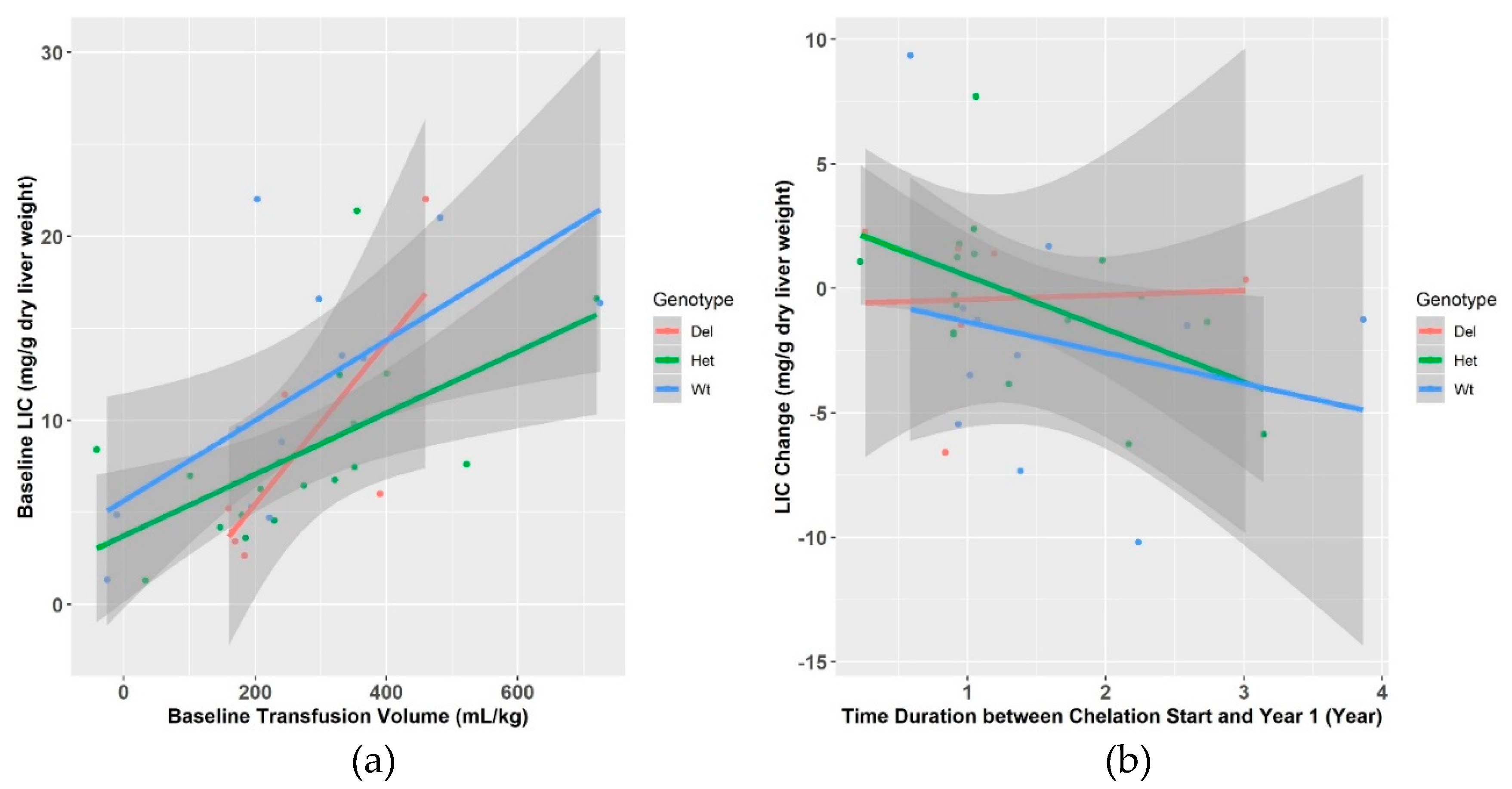
3.2. Liver Iron Content (LIC) and GSTM1 Genotype
3.3. Serum Ferritin and GSTM1 Genotype
3.4. Cardiac Tissue Iron and GSTM1 Genotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adams, R.J.; Brambilla, D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N. Engl. J. Med. 2005, 353, 2769–2778. [Google Scholar] [PubMed]
- DeBaun, M.R. Secondary prevention of overt strokes in sickle cell disease: Therapeutic strategies and efficacy. Hematol. Am. Soc. Hematol. Educ. Program 2011, 2011, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Coates, T.D.; Wood, J.C. How we manage iron overload in sickle cell patients. Br. J. Haematol. 2017, 177, 703–716. [Google Scholar] [CrossRef]
- Xie, Y.; Pivnick, E.K.; Cohen, H.L.; Adams-Graves, P.E.; Pourcyrous, M.; Aygun, B.; Hankins, J.S. Phenocopy of warfarin syndrome in an infant born to a mother with sickle cell anemia and severe transfusional iron overload. J. Pediatr. Hematol. Oncol. 2013, 35, e265–e268. [Google Scholar] [CrossRef] [PubMed]
- Darbari, D.S.; Kwagyan, J.; Rana, S.; Gordeuk, V.R.; Castro, O.; Kple-Faget, P.; Kple-Faget, P. Circumstances of death in adult sickle cell disease patients. Am. J. Hematol. 2006, 81, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Perronne, V.; Roberts-Harewood, M.; Bachir, D.; Roudot-Thoraval, F.; Delord, J.-M.; Thuret, I.; Schaeffer, A.; Davies, S.C.; Galactéros, F.; Godeau, B. Patterns of mortality in sickle cell disease in adults in France and England. Hematol. J. 2002, 3, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Files, B.; Brambilla, D.; Kutlar, A.; Miller, S.; Vichinsky, E.; Wang, W.; Granger, S.; Adams, R.J. Longitudinal changes in ferritin during chronic transfusion: A report from the Stroke Prevention Trial in Sickle Cell Anemia (STOP). J. Pediatr. Hematol. Oncol. 2002, 24, 284–290. [Google Scholar] [CrossRef]
- Brown, K.; Subramony, C.; May, W.; Megason, G.; Liu, H.; Bishop, P.; Walker, T.; Nowicki, M.J. Hepatic iron overload in children with sickle cell anemia on chronic transfusion therapy. J. Pediatr. Hematol. Oncol. 2009, 31, 309–312. [Google Scholar] [CrossRef]
- Harmatz, P.; Butensky, E.; Quirolo, K.; Williams, R.; Ferrell, L.; Moyer, T.; Golden, D.; Neumayr, L.; Vichinsky, E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 2000, 96, 76–79. [Google Scholar] [CrossRef]
- Olivieri, N.F. Progression of iron overload in sickle cell disease. Semin. Hematol. 2001, 38 (Suppl. 1), 57–62. [Google Scholar] [CrossRef]
- Flanagan, J.M.; Steward, S.; Hankins, J.S.; Howard, T.M.; Neale, G.; Ware, R.E. Microarray analysis of liver gene expression in iron overloaded patients with sickle cell anemia and beta-thalassemia. Am. J. Hematol. 2009, 84, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Chang, J.G.; Ho, Y.J.; Wu, S.F.; Peng, C.T. Glutathione S-transferase M1 gene polymorphisms are associated with cardiac iron deposition in patients with beta-thalassemia major. Hemoglobin 2006, 30, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ellithy, H.N.; Yousri, S.; Shahin, G.H. Relation between glutathione S-transferase genes (GSTM1, GSTT1, and GSTP1) polymorphisms and clinical manifestations of sickle cell disease in Egyptian patients. Hematology 2015, 20, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.E.; Kahler, K.H.; Frech, F.; Chan, K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006, 15, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.M.; Leonard, S.; Jonassaint, J.; Lunyera, J.; Bonner, M.; Shah, N. Mobile health intervention for youth with sickle cell disease: Impact on adherence, disease knowledge, and quality of life. Pediatr. Blood Cancer 2018, 65, e27081. [Google Scholar] [CrossRef]
- Estepp, J.H.; Winter, B.; Johnson, M.; Smeltzer, M.P.; Howard, S.C.; Hankins, J.S. Improved hydroxyurea effect with the use of text messaging in children with sickle cell anemia. Pediatr. Blood Cancer 2014, 61, 2031–2036. [Google Scholar] [CrossRef]
- Brousseau, D.C.; Richardson, T.; Hall, M.; Ellison, A.M.; Shah, S.S.; Raphael, J.L.; Bundy, D.G.; Arnold, S. Hydroxyurea Use for Sickle Cell Disease Among Medicaid-Enrolled Children. Pediatrics 2019, 144. [Google Scholar] [CrossRef]
- Krafft, A.J.; Loeffler, R.B.; Song, R.; Bian, X.; McCarville, M.B.; Hankins, J.S.; Hillenbrand, C.M. Does fat suppression via chemically selective saturation affect R2*-MRI for transfusional iron overload assessment? A clinical evaluation at 1.5T and 3T. Magn. Reson. Med. 2016, 76, 591–601. [Google Scholar] [CrossRef]
- Loeffler, R.B.; McCarville, M.B.; Wagstaff, A.W.; Smeltzer, M.P.; Krafft, A.J.; Song, R.; Hillenbrand, C.M. Can multi-slice or navigator-gated R2* MRI replace single-slice breath-hold acquisition for hepatic iron quantification? Pediatr. Radiol. 2017, 47, 46–54. [Google Scholar] [CrossRef]
- McCarville, M.B.; Hillenbrand, C.M.; Loeffler, R.B.; Smeltzer, M.P.; Song, R.; Li, C.-S.; Hankins, J.S. Comparison of whole liver and small region-of-interest measurements of MRI liver R2* in children with iron overload. Pediatr. Radiol. 2010, 40, 1360–1367. [Google Scholar] [CrossRef]
- Westwood, M.A.; Anderson, L.J.; Firmin, D.N.; Gatehouse, P.D.; Lorenz, C.H.; Wonke, B.; Pennell, D.J. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J. Magn. Reson. Imaging 2003, 18, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Faranesh, A.Z.; Boxerman, J.L.; McVeigh, E.R. In vivo measurement of T*2 and field inhomogeneity maps in the human heart at 1.5 T. Magn. Reson. Med. 1998, 39, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Liem, R.I.; Rigsby, C.K.; Labotka, R.J.; DeFreitas, R.A.; Thompson, A.A. Assessing cardiac and liver iron overload in chronically transfused patients with sickle cell disease. Br. J. Haematol. 2016, 175, 705–713. [Google Scholar] [CrossRef]
- Origa, R.; Satta, S.; Matta, G.; Galanello, R. Glutathione S-transferase gene polymorphism and cardiac iron overload in thalassaemia major. Br. J. Haematol. 2008, 142, 143–145. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, B.; Saxena, R. Glutathione S-transferase gene deletions and their effect on iron status in HbE/beta thalassemia patients. Ann. Hematol. 2010, 89, 411–414. [Google Scholar] [CrossRef]
- Kaushik, N.; Eckrich, M.J.; Parra, D.; Yang, E. Chronically transfused pediatric sickle cell patients are protected from cardiac iron overload. Pediatr. Hematol. Oncol. 2012, 29, 254–260. [Google Scholar] [CrossRef]
| Demographic Variables | Total (n = 38) | GSTM1 Homozygotes (n = 7) | GSTM1 Heterozygotes (n = 19) | GSTM1 Wild Type (n = 12) | p-Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Median age at baseline (Range) (Years) | 7.5 (3.5–18.1) | 5.84 (4.7–18.1) | 7.6 (3.47–15.8) | 8.5 (4.0–17.3) | 0.6 |
| Sex (Male/Female) | 15/23 | 1/6 | 7/12 | 7/5 | 0.2 |
| Sickle genotype (HbSS/HbSβ0-thalassemia) | 37/1 | 7/0 | 18/1 | 12/0 | 1 |
| Transfusion Regimen | |||||
| Simple transfusions (%) | 23 (60.5) | 4 (57.1) | 14 (73.7) | 5 (41.7) | 0.2 |
| Simple and Exchange transfusions (%) | 15 (39.5) | 3 (42.9) | 5 (26.3) | 7 (58.3) | 0.2 |
| Mean Cumulative Blood Transfusion Volume | |||||
| Up to baseline (mL/kg) | 238.7 (−41.3–725.2) | 183.5 (159.7–459.1) | 244.2 (−41.3–720.1) | 231.1 (−25.4–725.2) | 0.9 |
| Up to Year 1 (mL/kg) | 370.9 (−106.0–2269.8) | 335.05 (228.33–627.8) | 467.6 (−106.0–2269.8) | 323.7 (182.22–946.7) | 0.5 |
| Iron Overload Treatment Regimen | 0.6 | ||||
| Desferoxamine(%) | 1 (2.8) | 0 (0) | 0 (0) | 1 (9.1) | |
| Deferasirox(%) | 32 (91.4) | 6 (100) | 17 (94.4) | 9 (81.8) | |
| Deferasirox and Desferoxamine(%) | 1 (2.9) | 0 (0) | 0 (0) | 1 (9.1) | |
| Therapeutic Phlebotomy (%) | 1 (2.9) | 0 (0) | 1 (5.6) | 0(0) | |
| Splenectomy * (%) | 0.11 | ||||
| Yes | 12 (31.6) | 3 (42.9) | 3 (15.8) | 6 (50) | |
| No | 26 (68.4) | 4 (57.1) | 16 (84.2) | 6 (50) | |
| Iron Overload Variables and Chelation | Total (n = 38) | GSTM1 Homozygotes (n = 7) | GSTM1 Heterozygotes (n = 19) | GSTM1 Wild Type (n = 12) | p-Value |
|---|---|---|---|---|---|
| R2*MRI LIC (mg/g of Liver Dry Weight) | |||||
| Baseline | 9.2 ± 5.9 | 7.8 ± 6.9 | 8.2 ± 4.85 | 11.4 ± 6.85 | 0.09 |
| Year 1 | 8.1 ± 5.4 | 7.9± 4.7 | 7.2 ± 4.5 | 9.5± 6.8 | 0.82 |
| Mean change | -0.7 ± 3.8 | 0.1 ± 3.3 | -0.3 ± 3.2 | −1.9 ± 4.9 | 0.047 |
| Serum Ferritin (ng/dL) | |||||
| Baseline | 2879.7 ± 1528 | 2413.6 ± 1133.7 | 2763.6 ± 1284.4 | 3335.3 ± 2016. | 0.29 |
| Year 1 | 3130.2 ± 2012.7 | 2576.1 ± 1025.5 | 3184.6 ± 1962.9 | 3367.2 ± 2546.6 | 0.71 |
| Mean change | 250.6 ± 1129.9 | 162.6 ± 845.4 | 421.1 ± 1197.5 | 31.9 ± 1204.5 | 0.11 |
| MRI Heart T2* (msec) | |||||
| Baseline # | 37.5 ± 6.7 | 36.9 ± 3.54 | 37.7 ± 7.6 | 37.8 ± 7.8 | 0.86 |
| Year 1 | 38.3± 6.03 | 40.7± 2.1 | 38.7 ± 5.8 | 36.2 ± 7.6 | 0.31 |
| Mean change | 0.9 ± 6.0 | 3.92 ± 2.6 | 0.95 ± 7.9 | −1.2 ± 3.2 | 0.2 |
| Duration of chelation (Months) | 17.76 ± 10.4 | 14.38 ± 11.3 | 18.01 ± 9.9 | 19.2 ± 11.4 | 0.45 |
| Iron chelation MPR (%) ## | 81 ± 16 | 83 ± 19 | 82 ± 15 | 78 ± 17 | 0.84 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, L.; Flanagan, J.M.; Kang, G.; Ding, J.; Bi, W.; McCarville, B.M.; Loeffler, R.B.; Tipirneni-Sajja, A.; Villavicencio, M.; Crews, K.R.; et al. GSTM1 and Liver Iron Content in Children with Sickle Cell Anemia and Iron Overload. J. Clin. Med. 2019, 8, 1878. https://doi.org/10.3390/jcm8111878
Puri L, Flanagan JM, Kang G, Ding J, Bi W, McCarville BM, Loeffler RB, Tipirneni-Sajja A, Villavicencio M, Crews KR, et al. GSTM1 and Liver Iron Content in Children with Sickle Cell Anemia and Iron Overload. Journal of Clinical Medicine. 2019; 8(11):1878. https://doi.org/10.3390/jcm8111878
Chicago/Turabian StylePuri, Latika, Jonathan M. Flanagan, Guolian Kang, Juan Ding, Wenjian Bi, Beth M. McCarville, Ralf B. Loeffler, Aaryani Tipirneni-Sajja, Martha Villavicencio, Kristine R. Crews, and et al. 2019. "GSTM1 and Liver Iron Content in Children with Sickle Cell Anemia and Iron Overload" Journal of Clinical Medicine 8, no. 11: 1878. https://doi.org/10.3390/jcm8111878
APA StylePuri, L., Flanagan, J. M., Kang, G., Ding, J., Bi, W., McCarville, B. M., Loeffler, R. B., Tipirneni-Sajja, A., Villavicencio, M., Crews, K. R., Hillenbrand, C. M., & Hankins, J. S. (2019). GSTM1 and Liver Iron Content in Children with Sickle Cell Anemia and Iron Overload. Journal of Clinical Medicine, 8(11), 1878. https://doi.org/10.3390/jcm8111878






