Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Data Source, and Case Identification
2.2. Study Variables
2.3. Definitions
2.4. Statistical Analysis
3. Results
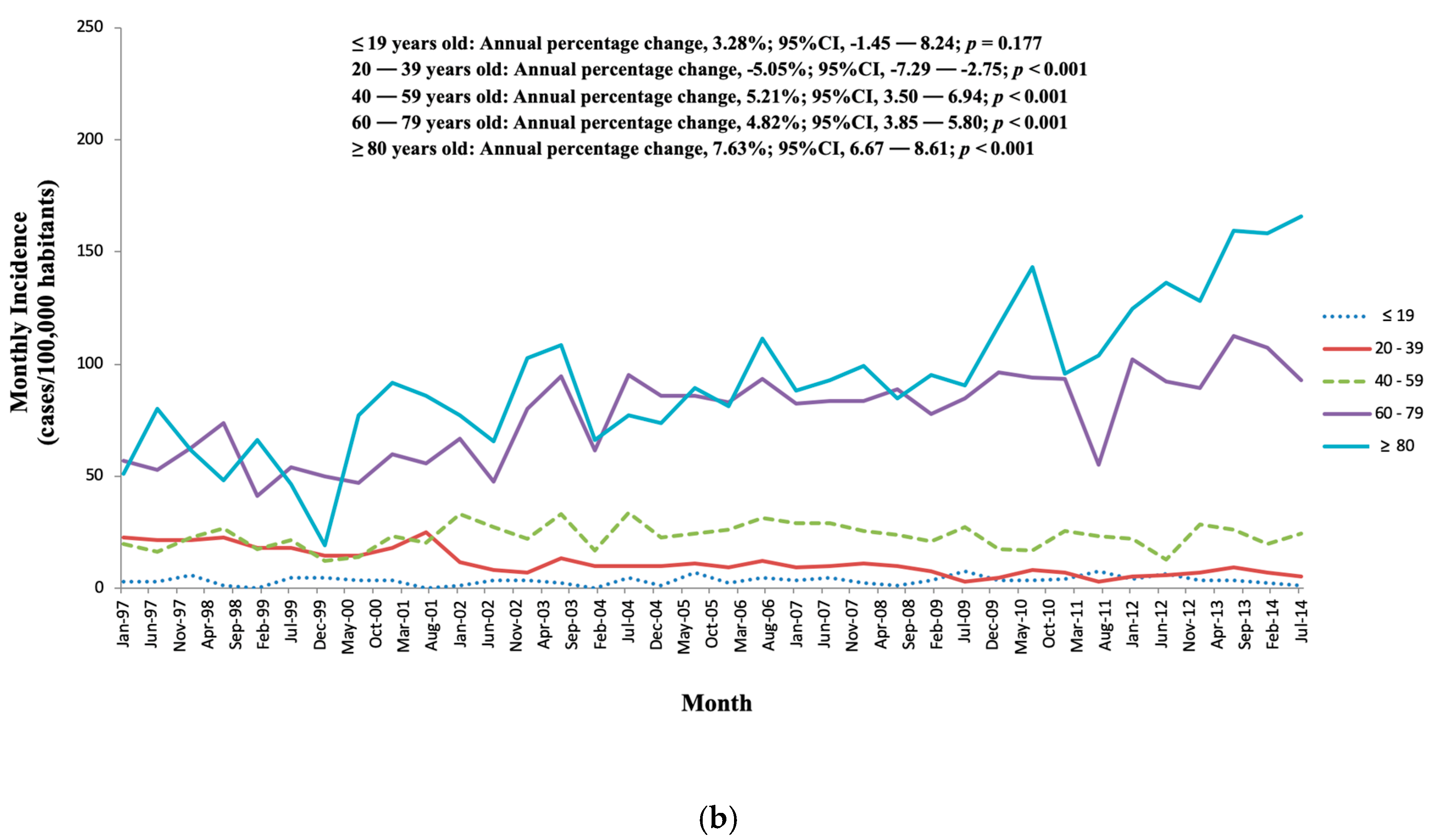
3.1. Incidence
3.2. Population Description
3.3. Evolution and Tendencies of IE
3.4. Comparison between NIE and CIE
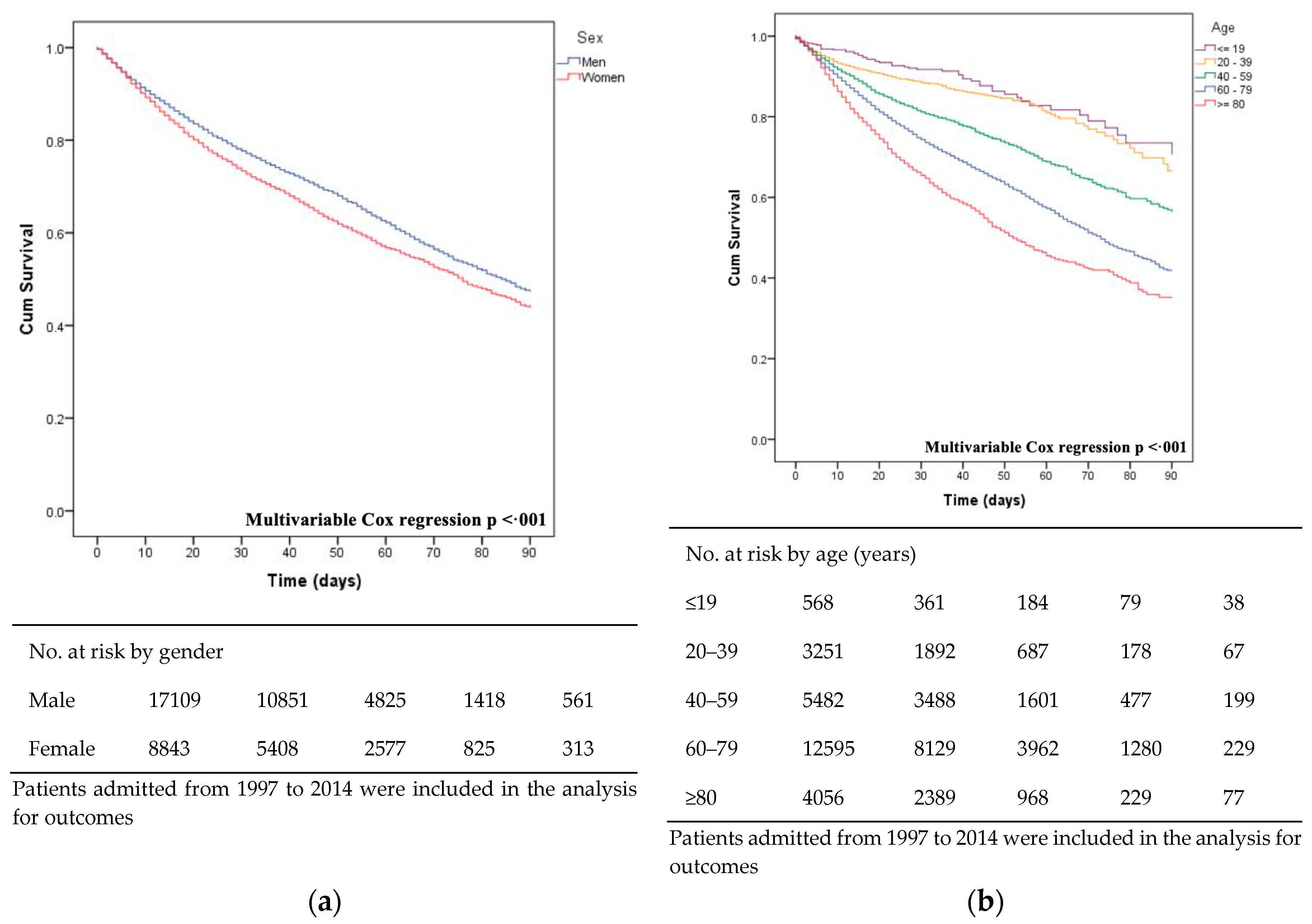
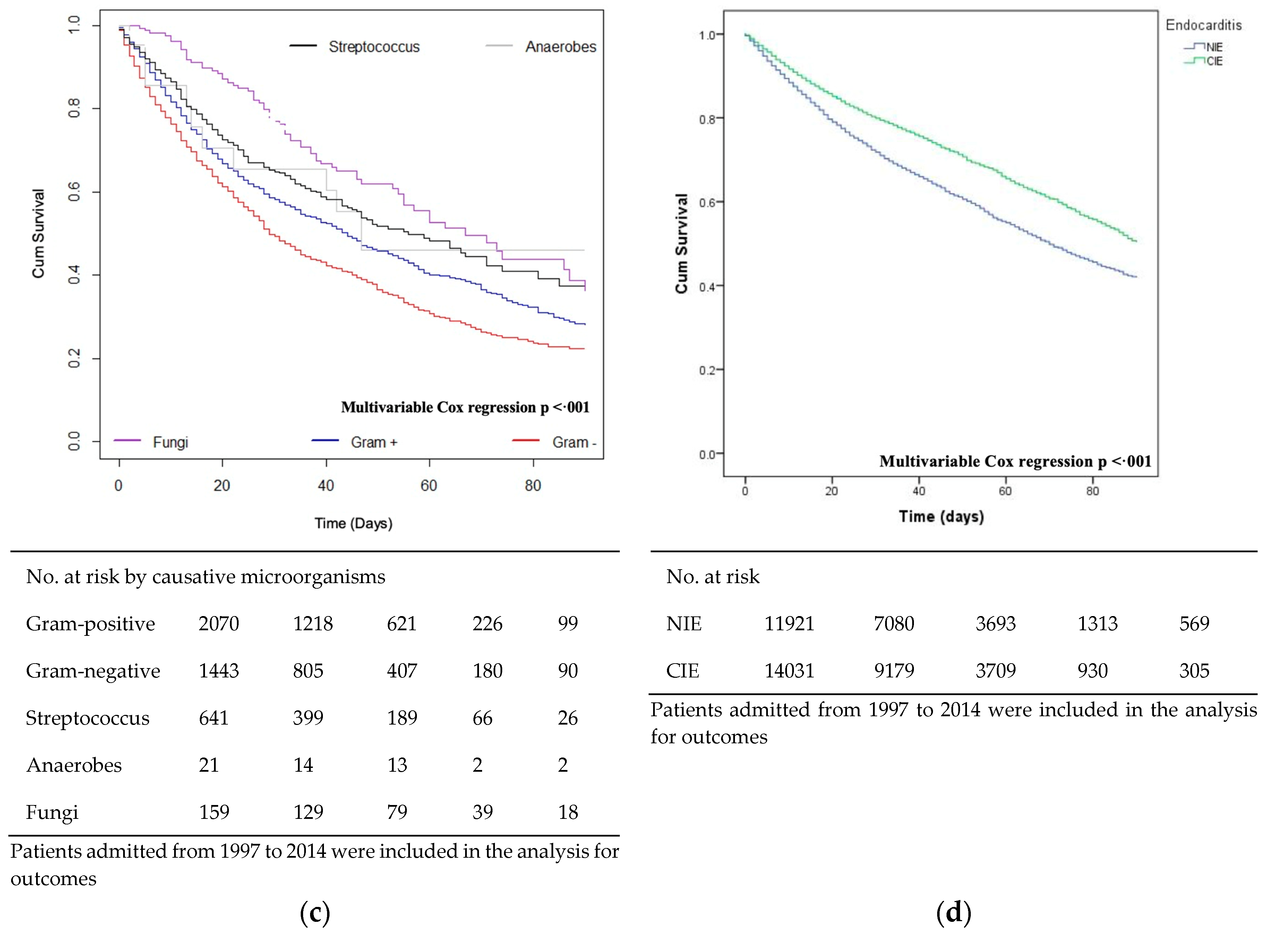
3.5. Mortality and Survival
3.6. Risk Factors of Mortality for NIE and CIE
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Osler, W. The gulstonian lectures, on malignant endocarditis. Br. Med. J. 1885, 1, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The international collaboration on endocarditis-prospective cohort study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Park, S.W.; Cho, E.J.; Lee, G.Y.; Kim, E.K.; Chang, S.A.; Park, S.J.; Lee, S.C.; Kang, C.I.; Chung, D.R.; et al. Risk factors for poor prognosis in nosocomial infective endocarditis. Korean J. Intern. Med. 2018, 33, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Hidalgo, N.; Tornos Mas, P. Epidemiology of infective endocarditis in spain in the last 20 years. Rev. Esp. Cardiol. 2013, 66, 728–733. [Google Scholar] [CrossRef]
- Olmos, C.; Vilacosta, I.; Fernandez-Perez, C.; Bernal, J.L.; Ferrera, C.; Garcia-Arribas, D.; Perez-Garcia, C.N.; San Roman, J.A.; Maroto, L.; Macaya, C.; et al. The evolving nature of infective endocarditis in spain: A population-based study (2003 to 2014). J. Am. Coll. Cardiol. 2017, 70, 2795–2804. [Google Scholar] [CrossRef]
- Bikdeli, B.; Wang, Y.; Kim, N.; Desai, M.M.; Quagliarello, V.; Krumholz, H.M. Trends in hospitalization rates and outcomes of endocarditis among medicare beneficiaries. J. Am. Coll. Cardiol. 2013, 62, 2217–2226. [Google Scholar] [CrossRef]
- Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; de Jesus Antunes, M.; Thilen, U.; Lekakis, J.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (New Version 2009): The task force on the prevention, diagnosis, and treatment of infective endocarditis of the european society of cardiology (ESC). Endorsed by the european society of clinical microbiology and infectious diseases (ESCMID) and the international society of chemotherapy (ISC) for infection and cancer. Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the american heart association: A guideline from the american heart association rheumatic fever, endocarditis, and kawasaki disease committee, council on cardiovascular disease in the young, and the council on clinical cardiology, council on cardiovascular surgery and anesthesia, and the quality of care and outcomes research interdisciplinary working group. Circulation 2007, 116, 1736–1754. [Google Scholar]
- Blanco, N.; Perencevich, E.; Li, S.S.; Morgan, D.J.; Pineles, L.; Johnson, J.K.; Robinson, G.; Anderson, D.J.; Jacob, J.T.; Maragakis, L.L.; et al. Effect of meteorological factors and geographic location on methicillin-resistant staphylococcus aureus and vancomycin-resistant enterococci colonization in the us. PLoS ONE 2017, 12, e0178254. [Google Scholar] [CrossRef]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in infective endocarditis in california and new york state, 1998–2013. JAMA 2017, 317, 1652–1660. [Google Scholar] [CrossRef]
- Shih, C.J.; Chu, H.; Chao, P.W.; Lee, Y.J.; Kuo, S.C.; Li, S.Y.; Tarng, D.C.; Yang, C.Y.; Yang, W.C.; Ou, S.M.; et al. Long-term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: A nationwide population-based study. Circulation 2014, 130, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Giannitsioti, E.; Skiadas, I.; Antoniadou, A.; Tsiodras, S.; Kanavos, K.; Triantafyllidi, H.; Giamarellou, H.; Hellenic Endocarditis Study Group. Nosocomial vs. Community-acquired infective endocarditis in greece: Changing epidemiological profile and mortality risk. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007, 13, 763–769. [Google Scholar]
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in infective endocarditis incidence, microbiology, and valve replacement in the united states from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Cresti, A.; Chiavarelli, M.; Scalese, M.; Nencioni, C.; Valentini, S.; Guerrini, F.; D’Aiello, I.; Picchi, A.; De Sensi, F.; Habib, G. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc. Diagn. Ther. 2017, 7, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Bor, D.H.; Woolhandler, S.; Nardin, R.; Brusch, J.; Himmelstein, D.U. Infective endocarditis in the U.S., 1998–2009: A nationwide study. PLoS ONE 2013, 8, e60033. [Google Scholar] [CrossRef]
- Dayer, M.J.; Jones, S.; Prendergast, B.; Baddour, L.M.; Lockhart, P.B.; Thornhill, M.H. Incidence of infective endocarditis in england, 2000–2013: A secular trend, interrupted time-series analysis. Lancet 2015, 385, 1219–1228. [Google Scholar] [CrossRef]
- De Sa, D.D.C.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; Bachuwar, A.; Pazdernik, M.; Steckelberg, J.M.; Wilson, W.R.; et al. Epidemiological trends of infective endocarditis: A population-based study in olmsted county, minnesota. Mayo Clin. Proc. 2010, 85, 422–426. [Google Scholar] [CrossRef]
- Fedeli, U.; Schievano, E.; Buonfrate, D.; Pellizzer, G.; Spolaore, P. Increasing incidence and mortality of infective endocarditis: A population-based study through a record-linkage system. BMC Infect. Dis. 2011, 11, 48. [Google Scholar] [CrossRef]
- Marsteller, J.A.; Hsu, Y.J.; Weeks, K. Evaluating the impact of mandatory public reporting on participation and performance in a program to reduce central line-associated bloodstream infections: Evidence from a national patient safety collaborative. Am. J. Infect. Control 2014, 42, S209–S215. [Google Scholar] [CrossRef]
- Benito, N.; Miro, J.M.; de Lazzari, E.; Cabell, C.H.; del Rio, A.; Altclas, J.; Commerford, P.; Delahaye, F.; Dragulescu, S.; Giamarellou, H.; et al. Health care-associated native valve endocarditis: Importance of non-nosocomial acquisition. Ann. Intern. Med. 2009, 150, 586–594. [Google Scholar] [CrossRef]
- Wu, K.S.; Lee, S.S.; Tsai, H.C.; Wann, S.R.; Chen, J.K.; Sy, C.L.; Wang, Y.H.; Tseng, Y.T.; Chen, Y.S. Non-nosocomial healthcare-associated infective endocarditis in taiwan: An underrecognized disease with poor outcome. BMC Infect. Dis. 2011, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, E.; Gualis, J.; Florez, S.; Castrodeza, J.; Eiros Bouza, J.M.; Alvarez, F.J. Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. J. Thorac. Cardiovasc. Surg. 2008, 136, 1522–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selton-Suty, C.; Celard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.C.; Sahoo, S.; Marrone, G.; Pathak, A.; Lundborg, C.S.; Tamhankar, A.J. Climatic factors and community—Associated methicillin-resistant staphylococcus aureus skin and soft-tissue infections—A time-series analysis study. Int. J. Environ. Res. Public Health 2014, 11, 8996–9007. [Google Scholar] [CrossRef] [PubMed]
- Day, M.D.; Gauvreau, K.; Shulman, S.; Newburger, J.W. Characteristics of children hospitalized with infective endocarditis. Circulation 2009, 119, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Sasaki, Y.; Hirai, H.; Fukui, T.; Hosono, M.; Suehiro, S. Early surgery for hospital-acquired and community-acquired active infective endocarditis. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, J.; Tamayo, E.; Florez, S.; Telleria, J.J.; Bustamante, E.; Lopez, J.; San Roman, J.A.; Alvarez, F.J. Toll-like receptor 2 r753q polymorphisms are associated with an increased risk of infective endocarditis. Rev. Esp. Cardiol. 2011, 64, 1056–1059. [Google Scholar] [CrossRef]
- DeSimone, D.C.; Tleyjeh, I.M.; De Sa, D.D.C.; Anavekar, N.S.; Lahr, B.D.; Sohail, M.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. Temporal trends in infective endocarditis epidemiology from 2007 to 2013 in Olmsted County, MN. Am. Heart J. 2015, 170, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Francischetto, O.; Silva, L.A.; Senna, K.M.; Vasques, M.R.; Barbosa, G.F.; Weksler, C.; Ramos, R.G.; Golebiovski, W.F.; Lamas Cda, C. Healthcare-associated infective endocarditis: A case series in a referral hospital from 2006 to 2011. Arq. Bras. Cardiol. 2014, 103, 292–298. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Yu, J.; Shao, L.; Zhou, P.; Zhu, L.; Chen, S.; Zhang, W.; Weng, X.; Zhang, J.; et al. Epidemiology and the prognosis of healthcare-associated infective endocarditis in china: The significance of non-nosocomial acquisition. Emerg. Microbes Infect. 2015, 4, e38. [Google Scholar] [CrossRef]
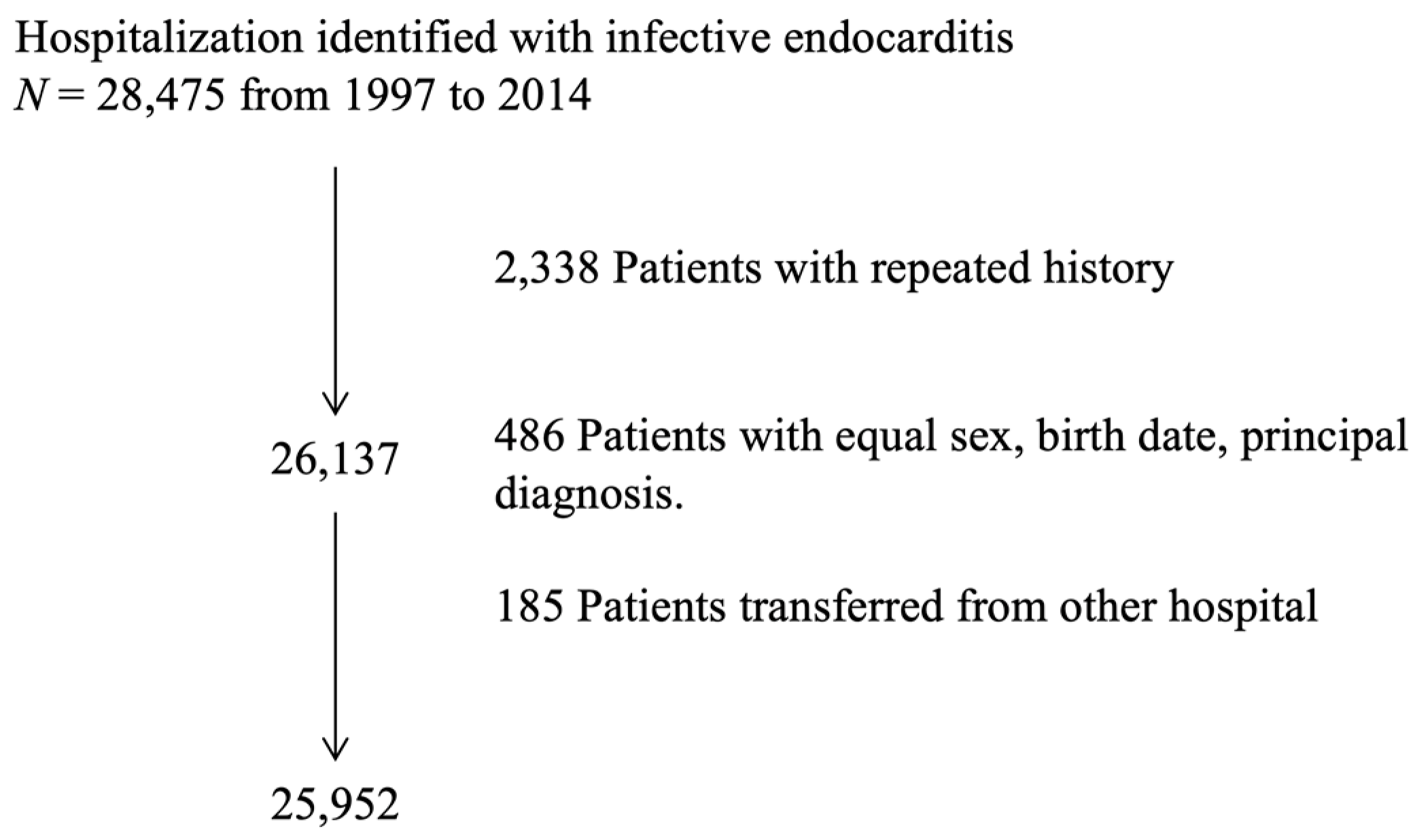


| Variable | Global (N = 25,952) | 1997–1999 (n = 3385) | 2000–2004 (n = 6151) | 2005–2009 (n = 7651) | 2010–2014 (n = 8785) | p-Value | |
|---|---|---|---|---|---|---|---|
| Incidence | 3.28 | 2.83 | 2.94 | 3.36 | 3.73 | 0.724 | |
| Sex (% male) | 17,109 (65.9) | 2319 (68.5) | 4102 (66.7) | 4942 (64.8) | 5746 (65.4) | 0.002 | |
| Mean age in years (±SD) | 62.2 (18.6) | 53.6 (20.1) | 59.8 (18.5) | 62.8 (18) | 66.6 (16.9) | <0.001 | |
| Age Group | ≤19 | 568 (2.2) | 90 (2.7) | 127 (2.1) | 170 (2.2) | 181 (2.1) | <0.001 |
| 20–39 | 3251 (12.5) | 966 (28.5) | 1000 (16.3) | 799 (10.5) | 486 (5.5) | ||
| 40–59 | 5482 (21.1) | 707 (20.9) | 1372 (22.3) | 1699 (22.3) | 1704 (19.4) | ||
| 60–79 | 12595 (48.5) | 1376 (40.6) | 3000 (48.8) | 3790 (49.7) | 4429 (50.4) | ||
| ≥80 | 4056 (15.6) | 246 (7.3) | 652 (10.6) | 1173 (15.4) | 1985 (22.6) | ||
| Seasons | Spring | 6673 (25.7) | 845 (25.0) | 1626 (26.4) | 1987 (26.0) | 2215 (25.2) | 0.244 |
| Summer | 6539 (25.2) | 907 (26.8) | 1564 (25.4) | 1889 (24.8) | 2179 (24.8) | ||
| Autumn | 6142 (23.7) | 787 (23.2) | 1438 (23.4) | 1819 (23.8) | 2098 (23.9) | ||
| Winter | 6598 (25.4) | 846 (25.0) | 1523 (24.8) | 1936 (25.4) | 2293 (26.1) | ||
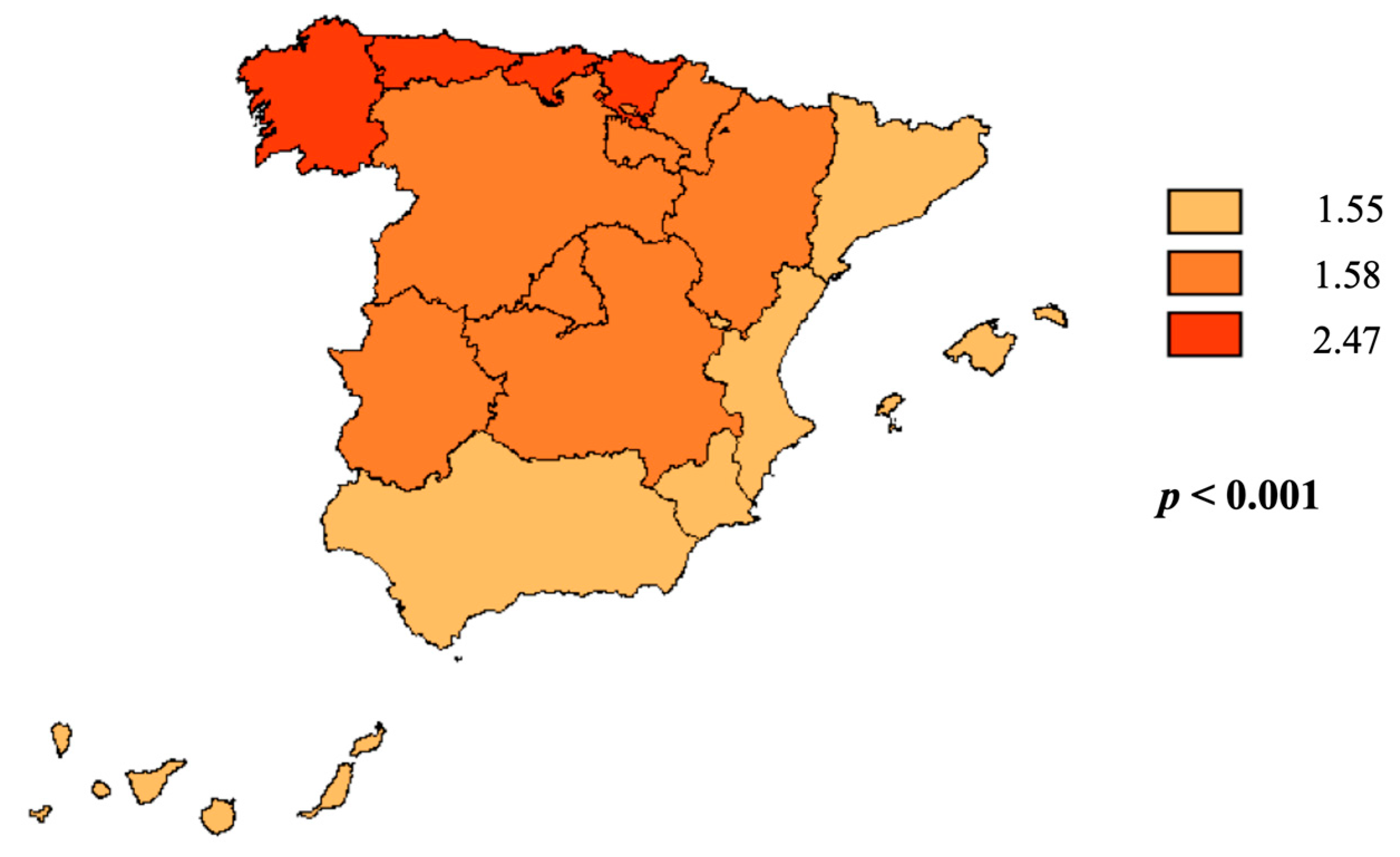
| Locations | North | 5489 (22.0) | 646 (24.0) | 1351 (22.5) | 1613 (21.4) | 1879 (21.6) | <0.001 |
| Center | 7498 (30.1) | 941 (35.0) | 1692 (28.2) | 2186 (29.0) | 2679 (30.8) | ||
| South | 11935 (47.9) | 1104 (41.0) | 2951 (49.2) | 3737 (49.6) | 4143 (47.6) | ||
| Comorbidities | |||||||
| Charlson Index Score (±SD) | 1.1 (1.4) | 0.7 (1.1) | 1 (1.4) | 1.2 (1.4) | 1.3 (1.4) | <0.001 | |
| 0 | 10,825 (41.7) | 2000 (59.1) | 2823 (45.9) | 3027 (39.7) | 2975 (33.9) | <0.001 | |
| Charlson | 1 | 7761 (29.9) | 795 (23.5) | 1711 (27.8) | 2310 (30.3) | 2945 (33.5) | <0.001 |
| 2 | 4151 (16) | 344 (10.2) | 871 (14.2) | 1247 (16.3) | 1689 (19.2) | <0.001 | |
| ≥3 | 3215 (12.4) | 246 (7.3) | 746 (12.1) | 1047 (13.7) | 1176 (13.4) | <0.001 | |
| Hypertension | 6145 (23.7) | 346 (10.2) | 1158 (18.8) | 1974 (25.9) | 2667 (30.4) | <0.001 | |
| Diabetes | 4396 (16.9) | 282 (8.3) | 883 (14.4) | 1390 (18.2) | 1841 (21) | <0.001 | |
| Mild to Moderate Diabetes | 3478 (13.4) | 233 (6.9) | 709 (11.5) | 1107 (14.5) | 1429 (16.3) | <0.001 | |
| Diabetes with Chronic Complications | 940 (3.6) | 49 (1.4) | 177 (2.9) | 292 (3.8) | 422 (4.8) | <0.001 | |
| Coronary Artery Disease | 1904 (7.3) | 132 (3.9) | 374 (6.1) | 620 (8.1) | 778 (8.9) | <0.001 | |
| Peripheral Vascular Disease | 1307 (5) | 75 (2.2) | 280 (4.6) | 406 (5.3) | 546 (6.2) | <0.001 | |
| Rheumatic Disease | 415 (1.6) | 27 (0.8) | 83 (1.3) | 139 (1.8) | 166 (1.9) | <0.001 | |
| COPD | 3537 (13.6) | 253 (7.5) | 708 (11.5) | 1101 (14.4) | 1475 (16.8) | <0.001 | |
| Renal Disease | 1446 (5.6) | 230 (6.8) | 492 (8) | 536 (7) | 188 (2.1) | <0.001 | |
| Hemodialysis | 1808 (7.0) | 82 (2.4) | 244 (4.0) | 533 (7.0) | 949 (10.8) | <0.001 | |
| Liver Disease | 1175 (4.5) | 109 (3.2) | 230 (3.7) | 385 (5) | 451 (5.1) | < 0.001 | |
| Cancer | 475 (1.8) | 32 (0.9) | 88 (1.4) | 158 (2.1) | 197 (2.2) | <0.001 | |
| Human Immunodeficiency Virus | 1361 (5.2) | 459 (13.6) | 428 (7.0) | 313 (4.1) | 161 (1.8) | <0.001 | |
| Receiving Intravenous Therapy | 1152 (4.5) | 79 (2.3) | 204 (3.3) | 394 (5.2) | 485 (5.5) | <0.001 | |
| Sepsis | 3458 (13.3) | 274 (8.1) | 714 (11.6) | 1115 (14.6) | 1355 (15.4) | <0.001 | |
| Shock | 2583 (10.0) | 208 (6.1) | 498 (8.1) | 807 (10.6) | 1070 (12.2) | <0.001 | |
| AMI | 1231 (4.7) | 89 (2.6) | 313 (5.1) | 427 (5.6) | 402 (4.6) | 0.002 | |
| HF | 6139 (23.7) | 497 (14.7) | 1263 (20.5) | 1821 (23.9) | 2558 (29.1) | <0.001 | |
| Stroke | 1099 (4.2) | 111 (3.3) | 267 (4.3) | 298 (3.9) | 423 (4.8) | 0.001 | |
| Hemiplegia | 441 (1.7) | 46 (1.4) | 87 (1.4) | 96 (1.3) | 212 (2.4) | <0.001 | |
| Dementia | 369 (1.4) | 28 (0.8) | 93 (1.5) | 103 (1.3) | 145 (1.7) | 0.005 | |
| Predisposing factor | |||||||
| History of Congenital Heart Disease | 822 (3.2) | 68 (2.2) | 167 (2.7) | 256 (3.4) | 331 (3.8) | <0.001 | |
| History of Valve Surgery | 2502 (9.6) | 300 (8.9) | 488 (7.9) | 743 (9.7) | 971 (11.1) | <0.001 | |
| History of Implanted Pacemaker or Defibrillator | 1681 (6.5) | 134 (4.0) | 321 (5.2) | 501 (6.6) | 725 (8.3) | <0.001 | |
| Disease Type | |||||||
| Cardiac device-related endocarditis | 5506 (21.2) | 378 (11.2) | 1050 (17.1) | 1670 (21.9) | 2408 (27.4) | <0.001 | |
| Drug abuse-related endocarditis | 1757 (6.8) | 560 (16.5) | 550 (8.9) | 457 (6.0) | 190 (2.2) | <0.001 | |
| Number of Organ Failure | 0 | 19,453 (75) | 2888 (85.3) | 4926 (80.1) | 5627 (73.7) | 6012 (68.4) | <0.001 |
| 1 | 5022 (19.4) | 417 (12.3) | 972 (15.8) | 1540 (20.2) | 2093 (23.8) | <0.001 | |
| 2 | 1171 (4.5) | 66 (1.9) | 206 (3.3) | 377 (4.9) | 522 (5.9) | <0.001 | |
| ≥ 3 | 306 (1.2) | 14 (0.4) | 47 (0.8) | 87 (1.1) | 158 (1.8) | < 0.001 | |
| Mode of Acquisition | |||||||
| NIE | 11,921 (45.9) | 1357 (40.1) | 2503 (40.7) | 3490 (45.7) | 4571 (52.0) | <0.001 | |
| CIE | 14,031 (54.1) | 2028 (59.9) | 3648 (59.3) | 4141 (54.3) | 4214 (48.0) | <0.001 | |
| Causative organism | |||||||
| Staphylococcus | 8487 (32.7) | 1098 (32.4) | 1987 (32.3) | 2510 (32.9) | 2892 (32.9) | 0.834 | |
| Staphylococcus aureus | 5099 (19.6) | 621 (18.3) | 1293 (21.0) | 1541 (20.2) | 1644 (18.7) | <0.001 | |
| Methicillin-resistant | 997 (3.8) | 61 (1.8) | 297 (4.8) | 226 (3.0) | 413 (4.7) | <0.001 | |
| Methicillin-sensitive | 4102 (15.8) | 560 (16.5) | 996 (16.2) | 1315 (17.2) | 1231 (14.0) | <0.001 | |
| Streptococcus | 641 (2.5) | 113 (3.3) | 123 (2.0) | 165 (2.2) | 240 (2.7) | <0.001 | |
| Gram-negative bacilli | 4150 (16.0) | 244 (7.2) | 763 (12.4) | 1275 (15.7) | 1868 (21.3) | <0.001 | |
| Anaerobes | 21 (0.1) | 2 (0.1) | 4 (0.1) | 4 (0.1) | 11 (0.1) | 0.181 | |
| Fungi | 234 (0.9) | 27 (0.8) | 55 (0.9) | 64 (0.8) | 88 (1.0) | 0.629 | |
| Unspecified | 12,419 (47.8) | 1091 (56.2) | 3219 (52.3) | 3633 (48.3) | 3686 (42.0) | < 0.001 | |
| Admission | Urgent | 21227 (81.8) | 2813 (83.1) | 5029 (81.8) | 6214 (81.4) | 7171 (81.6) | 0.116 |
| Elective | 4671 (18) | 547 (16.2) | 1111 (18.1) | 1412 (18.5) | 1601 (18.2) | 0.028 | |
| Others-unknown | 54 (0.2) | 25 (0.7) | 11 (0.2) | 5 (0.1) | 13 (0.1) | <0.001 | |
| Intervention | 6364 (24.5) | 634 (18.7) | 1581 (25.7) | 1986 (26) | 2163 (24.6) | <0.001 | |
| Re-admission | 4605 (17.7) | 491 (14.5) | 975 (15.9) | 1348 (17.7) | 1791 (20.4) | <0.001 | |
| LOS | 30.5 (24.8) | 28.8 (21.0) | 30.2 (26.1) | 31.8 (25.9) | 30.2 (24.2) | 0.014 | |
| Mortality | |||||||
| Global Mortality | 6952 (27.2) | 741 (22.3) | 1560 (25.6) | 2095 (27.7) | 2556 (29.8) | <0.001 | |
| <90 days | 6801 (26.2) | 732 (21.6) | 1530 (24.9) | 2043 (26.8) | 2496 (28.4) | <0.001 | |
| Variable | Total (N = 25,952) | NIE (n = 11,921) | CIE (n = 14,031) | p-Value | |
|---|---|---|---|---|---|
| Sex (% male) | 17,109 (65.9) | 7623 (64.0) | 9486 (67.6) | <0.001 | |
| Mean age in years (±SD) | 62.2 ± 18.6 | 63.8 ±18.2 | 60.8 ± 18.7 | <0.001 | |
| Age Group | ≤19 | 566 (2.2) | 298 (2.5) | 270 (1.9) | <0.001 |
| 20–39 | 3251 (12.5) | 1166 (9.8) | 2085 (14.9) | ||
| 40–59 | 5482 (21.2) | 2298 (19.3) | 3184 (22.7) | ||
| 60–79 | 12595 (48.5) | 6144 (51.5) | 6451 (46.0) | ||
| ≥80 | 4056 (15.6) | 2015 (16.9) | 2041 (14.6) | ||
| Seasons | Spring | 6673 (25.7) | 3041 (25.5) | 2632 (25.9) | 0.014 |
| Summer | 6539 (25.2) | 2908 (24.4) | 3631 (25.9) | ||
| Autumn | 6142 (23.7) | 2871 (24.1) | 3271 (23.3) | ||
| Winter | 6598 (25.4) | 3101 (26.0) | 3497 (24.9) | ||
| Location | North | 5489 (22.0) | 2581 (22.4) | 2908 (21.7) | <0.001 |
| Center | 7498 (30.1) | 3647 (31.7) | 3851 (28.7) | ||
| South | 11935 (47.9) | 5279 (45.9) | 6656 (49.6) | ||
| Comorbidities | |||||
| Charlson Index Score (±SD) | 1.1 ± 1.4 | 1.22 ± 1.5 | 1.03 ± 1.3 | <0.001 | |
| 0 | 10825 (41.7) | 4496 (37.7) | 6329 (45.1) | <0.001 | |
| Charlson | 1 | 7761 (29.9) | 3725 (31.2) | 4036 (28.8) | |
| 2 | 4151 (16) | 2051 (17.2) | 2100 (15.0) | ||
| ≥3 | 3215 (12.4) | 1649 (13.8) | 1566 (11.2) | ||
| Hypertension | 6145 (23.7%) | 2862 (24.0) | 3283 (23.4) | 0.249 | |
| Diabetes | 4396 (16.9%) | 2137 (17.9) | 2259 (16.1) | <0.001 | |
| Mild to Moderate Diabetes | 3478 (13.4%) | 1660 (13.9) | 1818 (13.0) | 0.023 | |
| Diabetes with Chronic Complications | 940 (3.6%) | 488 (4.1) | 452 (3.2) | <0.001 | |
| Coronary Artery Disease | 1904 (7.3) | 1039 (8.7) | 865 (6.2) | <0.001 | |
| Peripheral Vascular Disease | 1307 (5) | 676 (5.7) | 631 (4.5) | <0.001 | |
| Rheumatic Disease | 415 (1.6) | 211 (1.8) | 204 (1.4) | 0.043 | |
| COPD | 3537 (13.6) | 1660 (13.9) | 1877 (13.4) | 0.200 | |
| Renal Disease | 1446 (5.6) | 654 (5.5) | 792 (5.6) | 0.579 | |
| Hemodialysis | 1808 (7.0) | 990 (8.3) | 818 (5.8) | <0.001 | |
| Liver Disease | 1175 (4.5) | 518 (4.4) | 657 (4.7) | 0.193 | |
| Cancer | 475 (1.8) | 244 (2.0) | 231 (1.6) | 0.016 | |
| Human Immunodeficiency Virus | 1361 (5.2) | 629 (5.3) | 732 (5.2) | 0.831 | |
| Receiving Intravenous Therapy | 1162 (4.5) | 819 (6.9) | 343 (2.4) | <0.001 | |
| Sepsis | 3458 (13.3) | 2183 (18.3) | 1275 (9.1) | <0.001 | |
| Shock | 2583 (10.0) | 1524 (12.8) | 1059 (7.5) | <0.001 | |
| AMI | 1231 (4.7) | 753 (6.3) | 478 (3.4) | <0.001 | |
| HF | 6139 (23.7) | 2960 (24.8) | 3179 (22.7) | <0.001 | |
| Stroke | 1099 (4.2) | 598 (5.0) | 501 (3.6) | <0.001 | |
| Hemiplegia | 441 (1.7) | 251 (2.1) | 190 (1.4) | <0.001 | |
| Dementia | 369 (1.4) | 186 (1.6) | 183 (1.3) | 0.008 | |
| Predisposing Factor | |||||
| History of Congenital Heart Disease | 822 (3.2) | 298 (2.5) | 524 (3.7) | <0.001 | |
| History of Valve Surgery | 2502 (9.6) | 1350 (11.3) | 1152 (8.2) | <0.001 | |
| History of Implanted Pacemaker or Defibrillator | 1681 (6.5) | 1039 (8.7) | 642 (4.6) | <0.001 | |
| Disease Type | |||||
| Cardiac device-related endocarditis | 5506 (21.2) | 4702 (39.4) | 804 (5.7) | <0.001 | |
| Drug abuse-related endocarditis | 1757 (6.8) | 579 (4.9) | 1178 (8.4) | <0.001 | |
| Number of Organ Failure | 0 | 19453 (75) | 8504 (71.3) | 10949 (78.0) | <0.001 |
| 1 | 5022 (19.4) | 2555 (21.4) | 2467 (17.6) | <0.001 | |
| 2 | 1171 (4.5) | 678 (5.7) | 493 (3.5) | <0.001 | |
| ≥3 | 306 (1.2) | 184 (1.5) | 122 (0.9) | <0.001 | |
| Causative Organism | |||||
| Staphylococcus | 8487 (32.7) | 4277 (35.9) | 4210 (30.0) | <0.001 | |
| Staphylococcus aureus | 5099 (19.6) | 2446 (20.5) | 2653 (18.9) | <0.001 | |
| Methicillin-resistant | 997 (3.8) | 598 (5.0) | 399 (2.8) | <0.001 | |
| Methicillin-sensitive | 4102 (15.8) | 2446 (20.5) | 2653 (18.9) | <0.001 | |
| Streptococcus | 641 (2.5) | 373 (3.1) | 268 (1.9) | <0.001 | |
| Gram-negative bacilli | 4150 (16.0) | 1807 (15.2) | 2343 (16.7) | <0.001 | |
| Anaerobes | 21 (0.1) | 12 (0.1) | 9 (0.1) | 0.303 | |
| Fungi | 234 (0.9) | 168 (1.4) | 66 (0.5) | <0.001 | |
| Unspecified | 12,419 (47.8) | 5284 (44.3) | 7135 (50.8) | <0.001 | |
| Admission | Urgent | 21227 (81.8) | 9607 (80.6) | 11620 (82.8) | <0.001 |
| Elective | 4671 (18) | 2289 (19.2) | 2382 (17.0) | ||
| Others-unknown | 54 (0.2) | 25 (0.02) | 29 (0.02) | ||
| Intervention | 6364 (24.5) | 3357 (28.2) | 3007 (21.4) | <0.001 | |
| Re-admission | 4605 (17.7) | 2393 (20.1) | 2212 (15.8) | <0.001 | |
| LOS | 30.5 (24.8) | 31.3 (29.0) | 30.0 (20.6) | <0.001 | |
| Mortality | |||||
| Global | 6952 (27.2) | 3868 (32.9) | 3084 (22.4) | <0.001 | |
| <90 d | 6801 (26.2) | 3761 (31.5) | 3040 (21.7) | <0.001 | |
| Variable | Global (N = 6952) | 1997–1999 (n = 741) | 2000–2004 (n = 1560) | 2005–2009 (n = 2095) | 2010–2014 (n = 2556) | p-Value | |
|---|---|---|---|---|---|---|---|
| Sex | Men (%) | 4302 (25.6) | 457 (20.1) | 974 (24.0) | 1285 (26.3) | 1586 (28.3) | <0.001 |
| Women (%) | 2650 (30.4) | 284 (27.0) | 586 (28.9) | 810 (30.4) | 970 (32.6) | ||
| Age Group | ≤19 | 63 (11.1) | 12 (13.5) | 16 (12.6) | 14 (8.2) | 21 (11.6) | <0.001 |
| 20–39 | 365 (11.5) | 102 (10.8) | 117 (11.8) | 94 (12.0) | 52 (11.1) | ||
| 40–59 | 1139 (21.1) | 141 (20.3) | 271 (20.0) | 358 (21.3) | 369 (22.2) | ||
| 60–79 | 3886 (31.3) | 393 (28.9) | 920 (31.0) | 1196 (31.7) | 1377 (31.8) | ||
| ≥80 | 1499 (31.3) | 93 (38.1) | 236 (36.4) | 433 (37.3) | 737 (38.1) | ||
| Mode of Acquisition | NIE | 3868 (32.9) | 367 (27.4) | 745 (30.1) | 1162 (33.5) | 1594 (35.5) | <0.001 |
| CIE | 3084 (22.4) | 374 (18.8) | 815 (22.5) | 933 (22.8) | 962 (23.5) | ||
| Variable | NIE | CIE | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Female sex | 1.08 (1.01–1.15) | 0.022 | 1.32 (1.22–1.42) | <0.001 |
| Age | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.02–1.03) | <0.001 |
| Pacemaker or defibrillator placement | 0.98 (0.87–1.09) | 0.675 | 1.28 (1.09–1.49) | 0.002 |
| Diabetes | 1.17 (1.08–1.27) | <0.001 | 1.28 (1.17–1.40) | <0.001 |
| COPD | 1.23 (1.23–1.34) | <0.001 | 1.25 (1.13–1.37) | <0.001 |
| Coronary artery disease | 1.00 (0.90–1.13) | 0.893 | 1.25 (1.10–1.42) | 0.001 |
| Renal disease | 1.17 (1.02–1.33) | <0.001 | 1.47 (1.29–1.66) | <0.001 |
| Hemodialysis | 1.13 (1.17–1.45) | <0.001 | 1.68 (1.48–1.90) | <0.001 |
| Shock | 3.17 (2.96–3.40) | <0.001 | 5.29 (4.88–5.74) | <0.001 |
| Charlson | 1.14 (1.12–1.16) | <0.001 | 1.20 (1.18–1.23) | <0.001 |
| Heart Failure | 1.65 (1.55–1.77) | <0.001 | 1.94 (1.80–2.09) | <0.001 |
| Stroke | 1.74 (1.55–1.95) | <0.001 | 2.27 (1.99–2.59) | <0.001 |
| Type of admission | 0.71 (0.64–0.77) | <0.001 | 0.139 (0.20–0.99) | <0.001 |
| Gram-positive | 1.83 (1.68–1.98) | <0.001 | 2.05 (1.83–2.30) | <0.001 |
| Gram-negative | 2.16 (1.98–2.34) | <0.001 | 3.10 (2.76–3.45) | <0.001 |
| Variable | NIE | CIE | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.02 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Charlson Index | 1.09 (1.07–1.12) | <0.001 | 1.10 (1.07–1.13) | <0.001 |
| Hemodialysis | 1.20 (1.08–1.34) | 0.001 | 1.33 (1.17–1.51) | 0.001 |
| Shock | 3.19 (2.98–3.42) | <0.001 | 5.14 (4.73–5.58) | <0.001 |
| Heart Failure | 1.32 (1.23–1.41) | <0.001 | 1.40 (1.29–1.52) | <0.001 |
| Stroke | 1.76 (1.57–1.98) | <0.001 | 1.94 (1.69–2.22) | <0.001 |
| Female sex | 1.11 (1.03–1.20) | 0.005 | ||
| Renal Disease | 1.15 (1.01–1.33) | 0.049 | ||
| Pacemaker or defibrillator implanted | 1.18 (1.01–1.38) | 0.040 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Loubon, C.; Muñoz-Moreno, M.F.; García, I.A.; Álvarez, F.J.; Gómez-Sánchez, E.; Bustamante-Munguira, J.; Lorenzo-López, M.; Tamayo-Velasco, Á.; Jorge-Monjas, P.; Resino, S.; et al. Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century. J. Clin. Med. 2019, 8, 1755. https://doi.org/10.3390/jcm8101755
Ortega-Loubon C, Muñoz-Moreno MF, García IA, Álvarez FJ, Gómez-Sánchez E, Bustamante-Munguira J, Lorenzo-López M, Tamayo-Velasco Á, Jorge-Monjas P, Resino S, et al. Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century. Journal of Clinical Medicine. 2019; 8(10):1755. https://doi.org/10.3390/jcm8101755
Chicago/Turabian StyleOrtega-Loubon, Christian, María Fe Muñoz-Moreno, Irene Andrés García, Francisco Javier Álvarez, Esther Gómez-Sánchez, Juan Bustamante-Munguira, Mario Lorenzo-López, Álvaro Tamayo-Velasco, Pablo Jorge-Monjas, Salvador Resino, and et al. 2019. "Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century" Journal of Clinical Medicine 8, no. 10: 1755. https://doi.org/10.3390/jcm8101755
APA StyleOrtega-Loubon, C., Muñoz-Moreno, M. F., García, I. A., Álvarez, F. J., Gómez-Sánchez, E., Bustamante-Munguira, J., Lorenzo-López, M., Tamayo-Velasco, Á., Jorge-Monjas, P., Resino, S., Tamayo, E., & Heredia-Rodríguez, M. (2019). Nosocomial Vs. Community-Acquired Infective Endocarditis in Spain: Location, Trends, Clinical Presentation, Etiology, and Survival in the 21st Century. Journal of Clinical Medicine, 8(10), 1755. https://doi.org/10.3390/jcm8101755







