Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger?
Abstract
1. Introduction
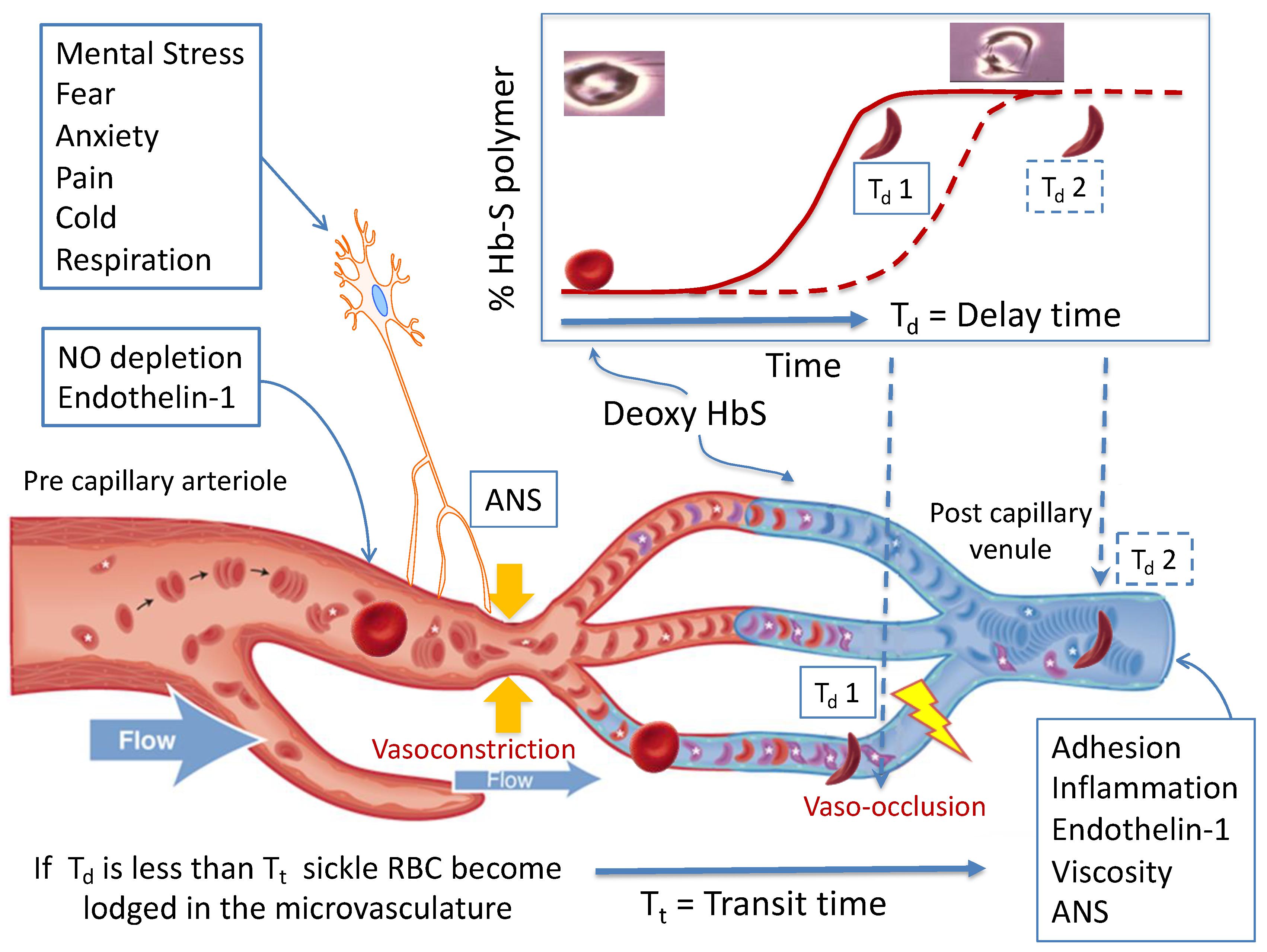
2. Pathophysiology of Sickle Vaso-Occlusion
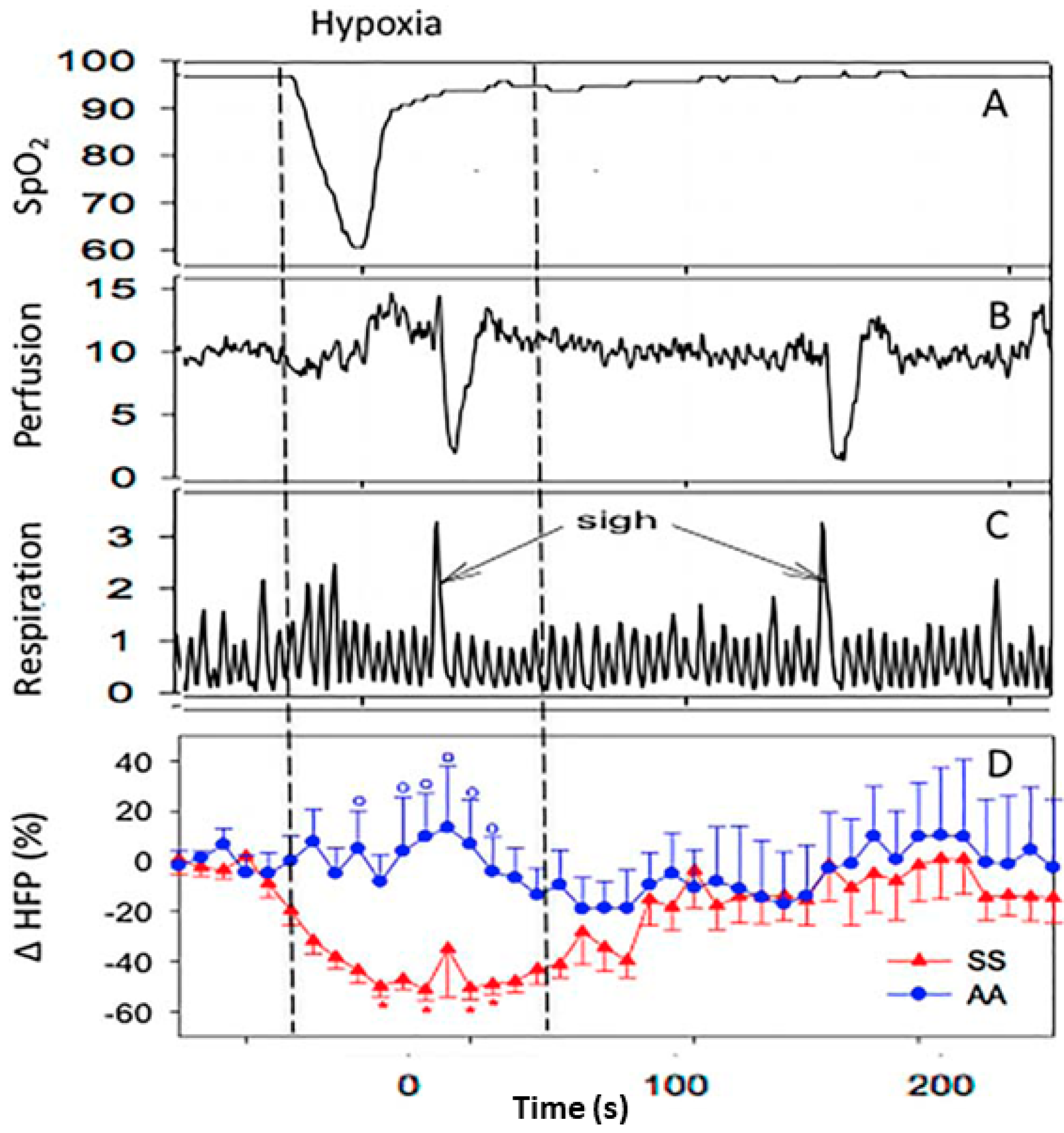
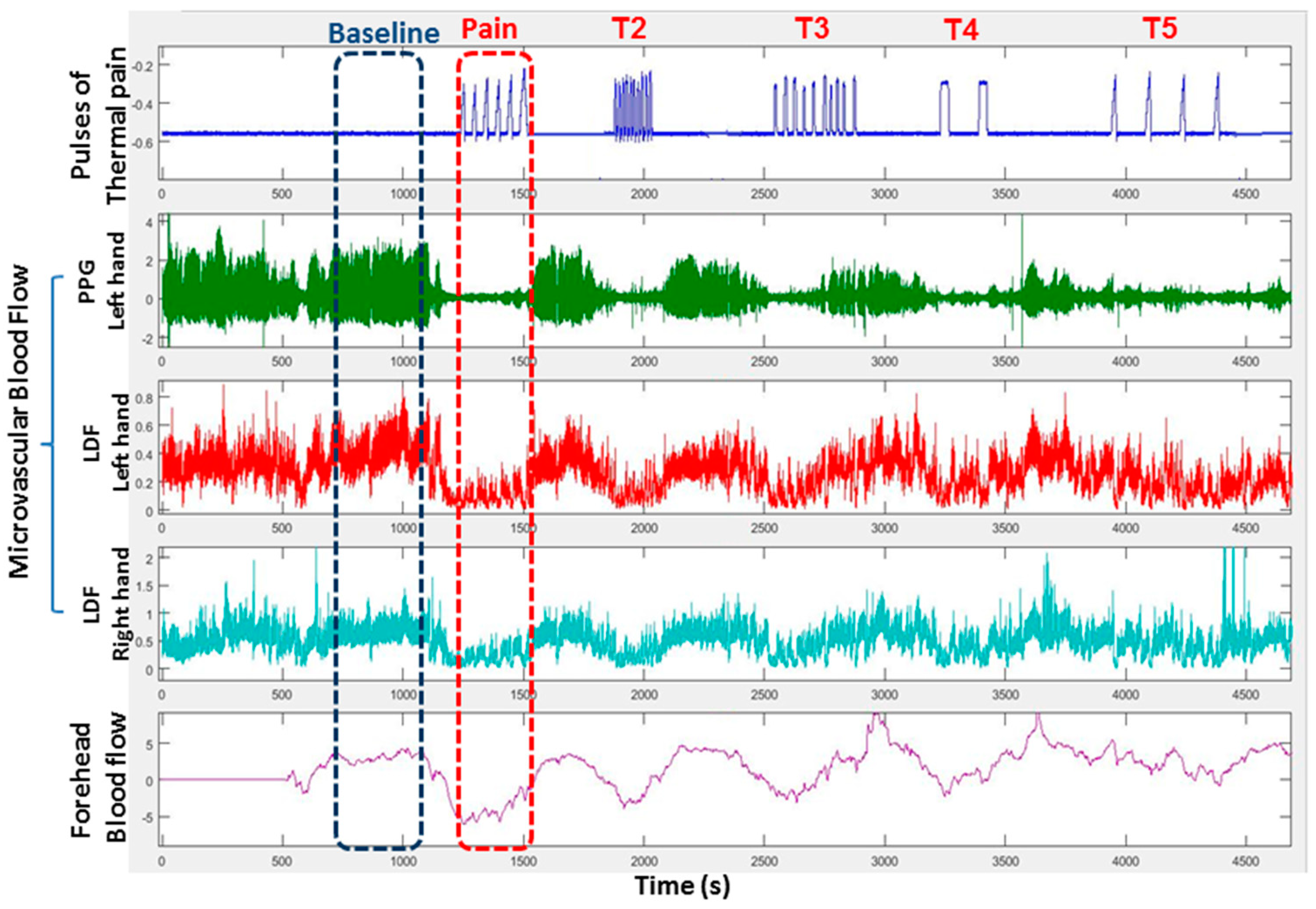
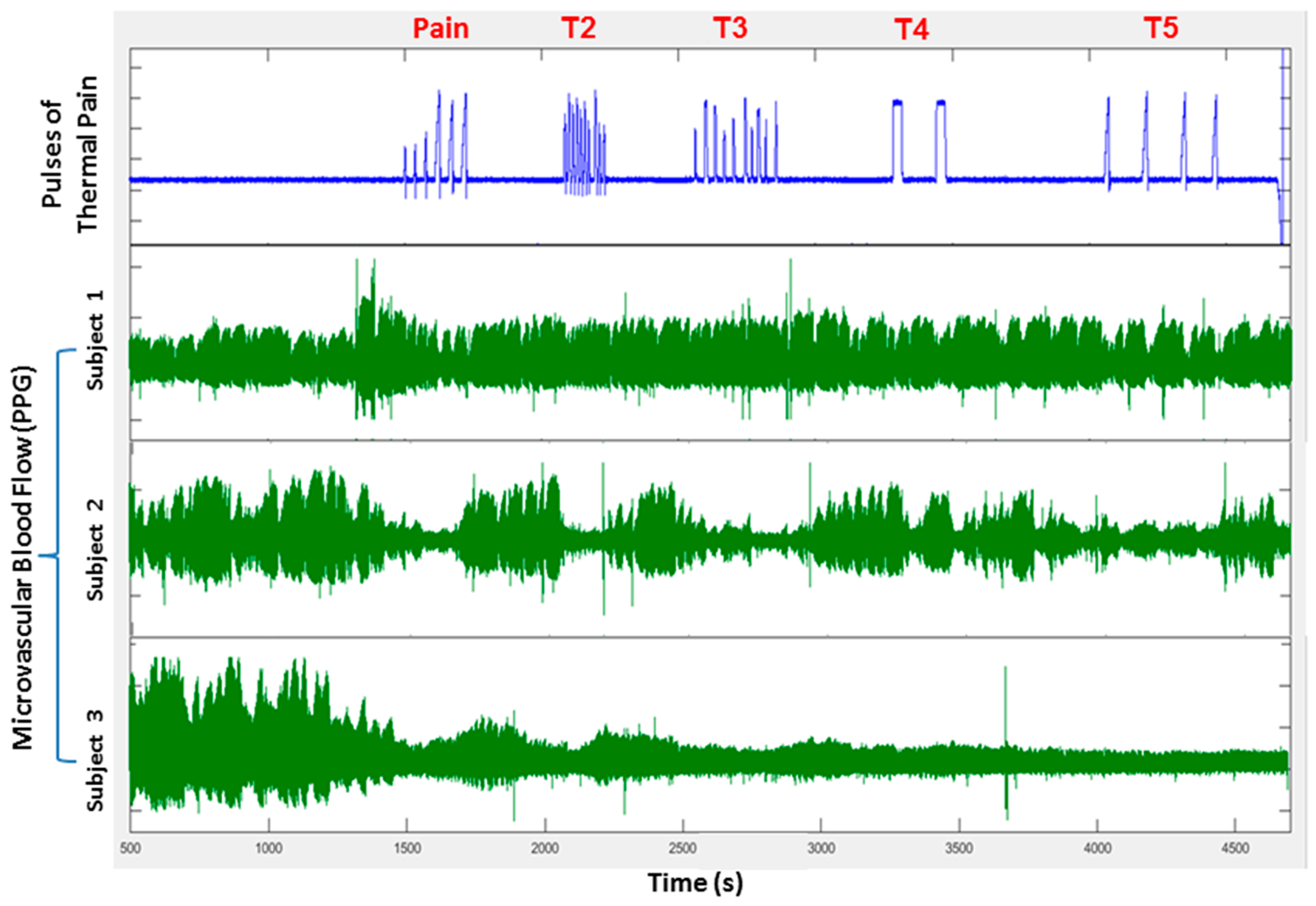
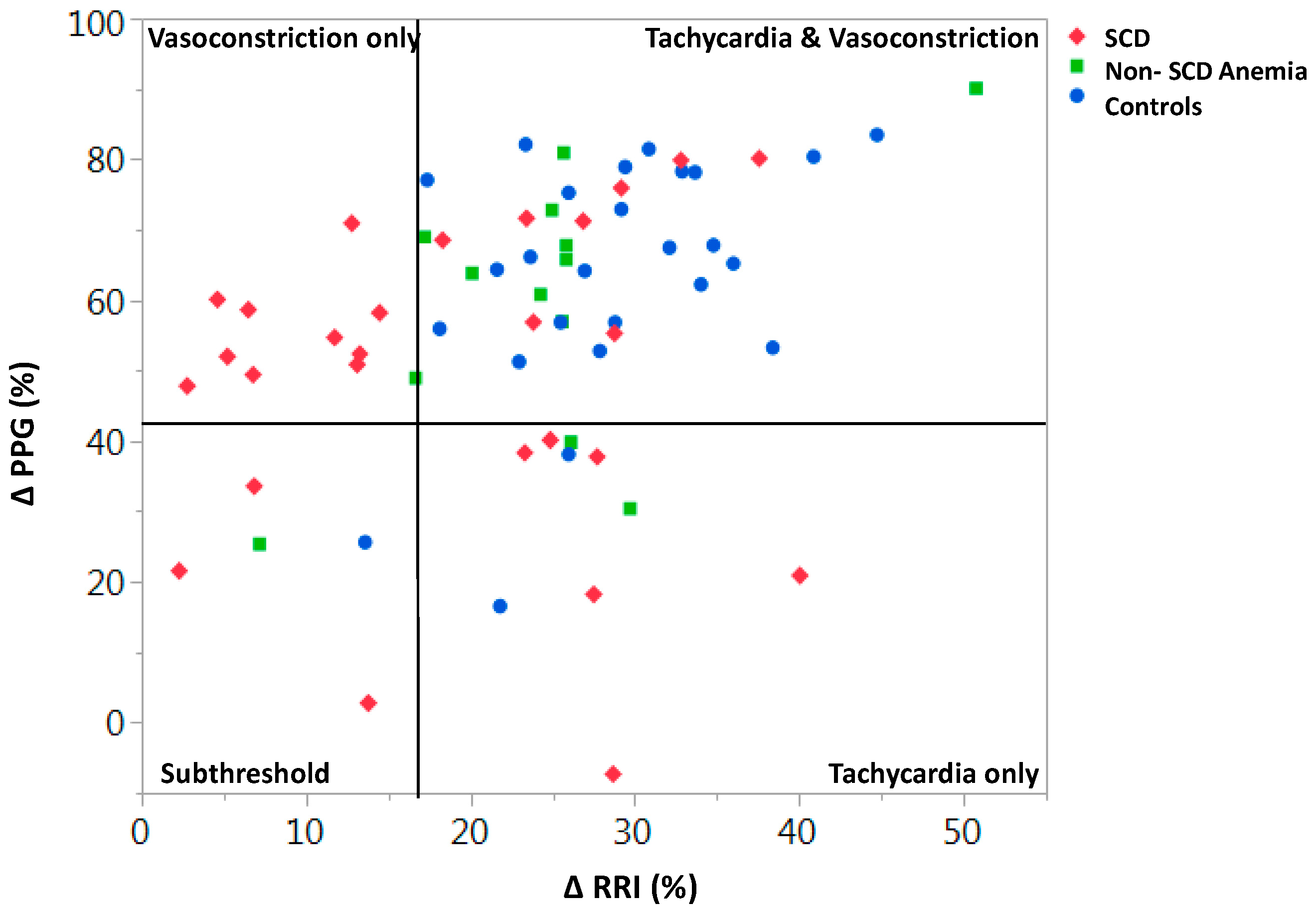
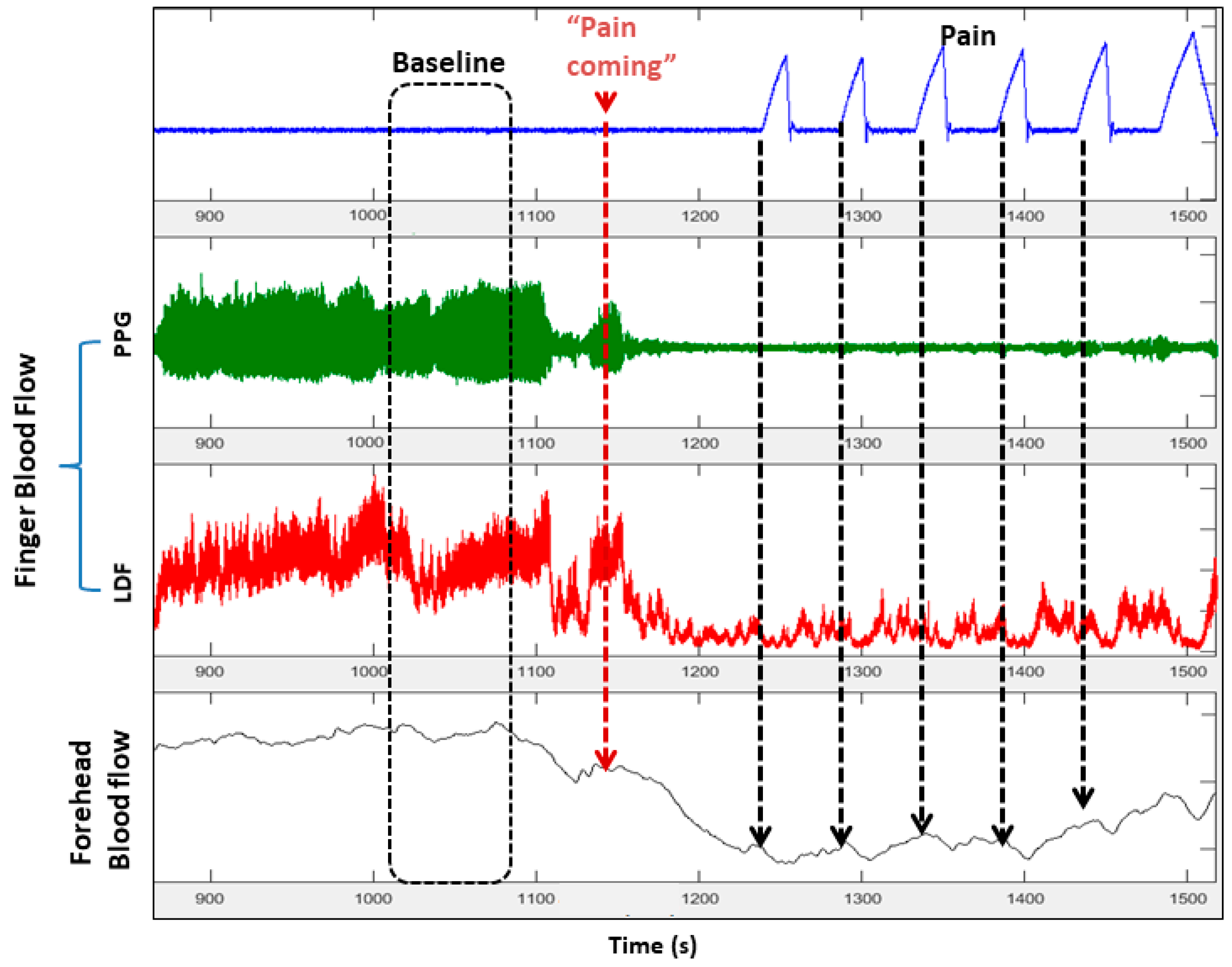
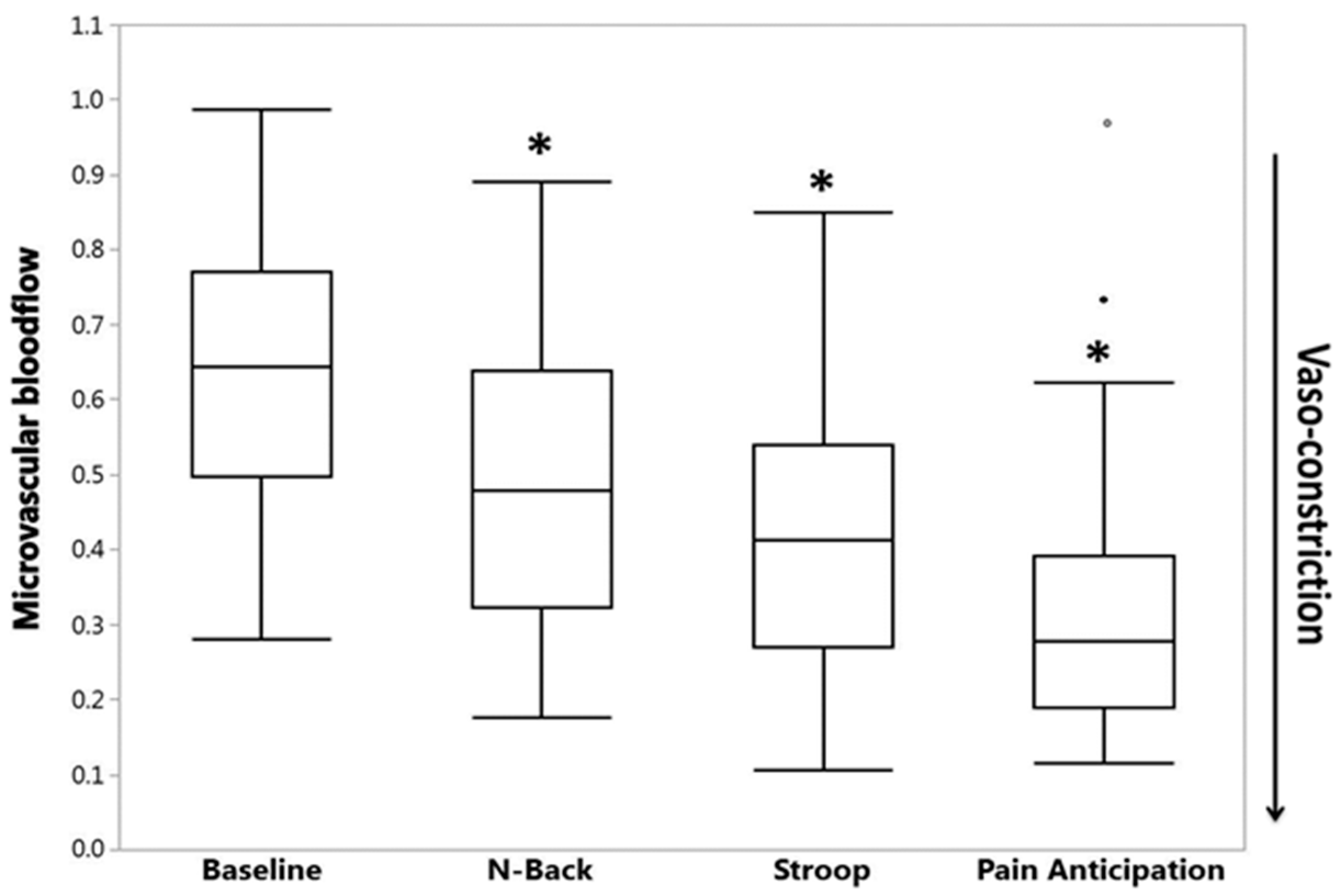
3. Autonomic Dysfunction and Peripheral Vasoreactivity in SCD
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pauling, L.; Itano, H.A.; Singer, S.J.; Wells, I.C. Sickle cell anemia a molecular disease. Science 1949, 110, 543–548. [Google Scholar] [CrossRef]
- Ingram, V.M. Abnormal human haemoglobins. III the chemical difference between normal and sickle cell haemoglobins. Biochim. Biophys. Acta 1959, 36, 402–411. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Vichinsky, E.P.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.; Hofrichter, J. Hemoglobin S gelation and sickle cell disease. Blood 1987, 70, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K.; Mohandas, N. Sickle red cell microrheology and sickle blood rheology. Microcirculation 2004, 11, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.E.; John, K.M.; Karnovsky, M.J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J. Clin. Investig. 1976, 58, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Alexy, T.; Sangkatumvong, S.; Connes, P.; Pais, E.; Tripette, J.; Barthelemy, J.C.; Coates, T.D.; Fisher, T.C.; Meiselman, H.J.; Khoo, M.C. Sickle cell disease: Selected aspects of pathophysiology. Clin. Hemorheol. Microcirc. 2010, 44, 155–166. [Google Scholar]
- Jimenez, M.A.; Kato, G.J.; Sundd, P. Neutrophil-Platelet Aggregation Enables Vaso-Occlusion in Sickle Cell Disease. Blood 2016, 128, 1295. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef]
- Platt, O.S.; Thorington, B.D.; Brambilla, D.J.; Milner, P.F.; Rosse, W.F.; Vichinsky, E.; Kinney, T.R. Pain in sickle cell disease. N. Engl. J. Med. 1991, 325, 11–16. [Google Scholar] [CrossRef]
- Steinberg, M.H. Predicting clinical severity in sickle cell anaemia. Br. J. Haematol. 2005, 129, 465–481. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Rodeghier, M.; Cohen, R.; Kirkham, F.J.; Rosen, C.L.; Roberts, I.; Warner, J.O.; Cooper, B.; Stocks, J.; Wilkey, O.; et al. Factors predicting future ACS episodes in children with sickle cell anemia. Am. J. Hematol. 2014, 89, E212–E217. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.A.; Hofrichter, J.; Ross, P.D. Editorial: Delay time of gelation: A possible determinant of clinical severity in sickle cell disease. Blood 1976, 47, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, F.A.; Hofrichter, J.; Eaton, W.A. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J. Mol. Biol. 1985, 183, 611–631. [Google Scholar] [CrossRef]
- Ferrone, F.A.; Hofrichter, J.; Eaton, W.A. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J. Mol. Biol. 1985, 183, 591–610. [Google Scholar] [CrossRef]
- Hofrichter, J. Kinetics of sickle hemoglobin polymerization. III. Nucleation rates determined from stochastic fluctuations in polymerization progress curves. J. Mol. Biol. 1986, 189, 553–571. [Google Scholar] [CrossRef]
- Christoph, G.W.; Hofrichter, J.; Eaton, W.A. Understanding the shape of sickled red cells. Biophys. J. 2005, 88, 1371–1376. [Google Scholar] [CrossRef]
- Coates, T.D. So what if blood is thicker than water? Blood 2011, 117, 745–746. [Google Scholar] [CrossRef]
- Coates, T.D.; Chalacheva, P.; Zeltzer, L.; Khoo, M.C. Autonomic nervous system involvement in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 251–262. [Google Scholar] [CrossRef]
- Eaton, W.A.; Bunn, H.F. Treating sickle cell disease by targeting HbS polymerization. Blood 2017, 129, 2719–2726. [Google Scholar] [CrossRef]
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Diuguid, D.L.; Hassab, H.; Achebe, M.M.; Alkindi, S.; et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Alexy, T.; Detterich, J.; Romana, M.; Hardy-Dessources, M.D.; Ballas, S.K. The role of blood rheology in sickle cell disease. Blood Rev. 2016, 30, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Gladwin, M.T.; Steinberg, M.H. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, P.; Lobysheva, I.I.; Feron, O.; McMahon, T.J. Transport and peripheral bioactivities of nitrogen oxides carried by red blood cell hemoglobin: Role in oxygen delivery. Physiology 2007, 22, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Day, T.G.; Drasar, E.R.; Fulford, T.; Sharpe, C.C.; Thein, S.L. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica 2012, 97, 201–205. [Google Scholar] [CrossRef]
- Detterich, J.A.; Kato, R.M.; Rabai, M.; Meiselman, H.J.; Coates, T.D.; Wood, J.C. Chronic transfusion therapy improves but does not normalize systemic and pulmonary vasculopathy in sickle cell disease. Blood 2015, 126, 703–710. [Google Scholar] [CrossRef]
- Hebbel, R.P.; Yamada, O.; Moldow, C.F.; Jacob, H.S.; White, J.G.; Eaton, J.W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: Possible mechanism for microvascular occlusion in sickle cell disease. J. Clin. Investig. 1980, 65, 154–160. [Google Scholar] [CrossRef]
- Turhan, A.; Weiss, L.A.; Mohandas, N.; Coller, B.S.; Frenette, P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: A new paradigm. Proc. Natl. Acad. Sci. USA 2002, 99, 3047–3051. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, C.; Manwani, D.; Frenette, P.S. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 2016, 127, 801–809. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Gualandro, S.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Bigal, M.E.; Kurth, T.; Hu, H.; Santanello, N.; Lipton, R.B. Migraine and cardiovascular disease: Possible mechanisms of interaction. Neurology 2009, 72, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.N.; Russo, A.F. Vascular contributions to migraine: Time to revisit? Front. Cell. Neurosci. 2018, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, F.J.; Hewes, D.K.M.; Prengler, M.; Wade, A.; Lane, R.; Evans, J.P.M. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet 2001, 357, 1656–1659. [Google Scholar] [CrossRef]
- Hargrave, D.R.; Wade, A.; Evans, J.P.; Hewes, D.K.; Kirkham, F.J. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood 2003, 101, 846–848. [Google Scholar] [CrossRef]
- Sangkatumvong, S.; Khoo, M.C.; Kato, R.; Detterich, J.A.; Bush, A.; Keens, T.G.; Coates, T.D.; Meiselman, H.J.; Wood, J.C. Peripheral vasoconstriction and abnormal parasympathetic response to sighs and transient hypoxia in sickle cell disease. Am. J. Respir. Crit. Care Med. 2011, 184, 474–481. [Google Scholar] [CrossRef]
- Coates, T.D. You don’t always get what you want: Does hypoxia cause sickle cell crisis? Am. J. Hematol. 2018, 93, 475–477. [Google Scholar] [CrossRef]
- Bolton, B.; Carmichael, E.A.; Sturup, G. Vaso-constriction following deep inspiration. J. Physiol. 1936, 86, 83–94. [Google Scholar] [CrossRef]
- Willen, S.M.; Rodeghier, M.; Rosen, C.L.; DeBaun, M.R. Sleep disordered breathing does not predict acute severe pain episodes in children with sickle cell anemia. Am. J. Hematol. 2018, 93, 478–485. [Google Scholar] [CrossRef]
- Eckberg, D.L. Sympathovagal balance: A critical appraisal. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef]
- Ondicova, K.; Mravec, B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: A minireview. Endocr. Regul. 2010, 44, 69–75. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Stauss, H.M. Heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R927–R931. [Google Scholar] [CrossRef] [PubMed]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Mestre, J.R.; Hernandez, A.; Agramonte, O.; Hernandez, P. Cardiovascular autonomic dysfunction in sickle cell anemia: A possible risk factor for sudden death? Clin. Auton. Res. 1997, 7, 121–125. [Google Scholar] [CrossRef]
- Pearson, S.R.; Alkon, A.; Treadwell, M.; Wolff, B.; Quirolo, K.; Boyce, W.T. Autonomic reactivity and clinical severity in children with sickle cell disease. Clin. Auton. Res. 2005, 15, 400–407. [Google Scholar] [CrossRef]
- Nebor, D.; Bowers, A.; Hardy-Dessources, M.D.; Knight-Madden, J.; Romana, M.; Reid, H.; Reid, M.; Barthélémy, J.-C.; Cumming, V.; Hue, O.; et al. Frequency of pain crises in sickle cell anemia and its relationship with the sympatho-vagal balance, blood viscosity and inflammation. Haematologica 2011, 96, 1589–1594. [Google Scholar] [CrossRef]
- Oguanobi, N.I.; Onwubere, B.J.; Anisiuba, B.C.; Ike, S.O.; Ejim, E.C.; Ibegbulam, O.G. Clinical findings associated with cardiovascular autonomic dysfunction in adult sickle cell anaemia patients. Acta Cardiol. 2012, 67, 169–175. [Google Scholar] [CrossRef]
- Knight-Madden, J.M.; Connes, P.; Bowers, A.; Nebor, D.; Hardy-Dessources, M.D.; Romana, M.; Elion, J.; Reid, H.; Pichon, A.P.; Cumming, V.B.; et al. Relationship between acute chest syndrome and the sympatho-vagal balance in adults with hemoglobin SS disease; a case control study. Clin. Hemorheol. Microcirc. 2013, 53, 231–238. [Google Scholar]
- Charlot, K.; Hierso, R.; Lemonne, N.; Romana, M.; Tressières, B.; Lalanne-Mistrih, M.L.; Connes, P.; Etienne-Julan, M.; Tarer, V.; Ferracci, S. Changes in autonomic nervous activity during vaso-occlusive crisis in patients with sickle cell anaemia. Br. J. Haematol. 2017, 177, 484–486. [Google Scholar] [CrossRef][Green Version]
- Bini, G.; Hagbarth, K.E.; Wallin, B.G. Cardiac rhythmicity of skin sympathetic activity recorded from peripheral nerves in man. J. Auton. Nerv. Syst. 1981, 4, 17–24. [Google Scholar] [CrossRef]
- Cataldo, G.; Rajput, S.; Gupta, K.; Simone, D.A. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015, 156, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Martin, C.; Barthelemy, J.C.; Monchanin, G.; Atchou, G.; Forsuh, A.; Pichot, V.; Massarelli, R.; Wouassi, D.; Thiriet, P. Nocturnal autonomic nervous system activity impairment in sickle cell trait carriers. Clin. Physiol. Funct. Imaging 2006, 26, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Chalacheva, P.; Khaleel, M.; Sunwoo, J.; Shah, P.; Detterich, J.A.; Kato, R.M.; Wood, J.C.; Thuptimdang, W.; Meiselman, H.J.; Sposto, R.; et al. Biophysical markers of the peripheral vasoconstriction response to pain in sickle cell disease. PLoS ONE 2017, 12, e0178353. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, M.; Puliyel, M.; Shah, P.; Sunwoo, J.; Kato, R.M.; Chalacheva, P.; Zeltzer, L.; Thuptimdang, W.; Detterich, J.; Wood, J.C.; et al. Individuals with sickle cell disease have a significantly greater vasoconstriction response to thermal pain than controls and have significant vasoconstriction in response to anticipation of pain. Am. J. Hematol. 2017, 92, 1137–1145. [Google Scholar] [CrossRef]
- Chalacheva, P.; Kato, R.M.; Shah, P.; Veluswamy, S.; Denton, C.C.; Sunwoo, J.; Khoo, M.C.; Thuptimdang, W.; Wood, J.C.; Coates, T.D.; et al. Sickle Cell Disease Subjects Have a Distinct Abnormal Autonomic Phenotype Characterized by Peripheral Vasoconstriction With Blunted Cardiac Response to Head-Up Tilt. Front. Physiol. 2019, 10, 381. [Google Scholar] [CrossRef]
- Mahdi, N.; Al-Ola, K.; Khalek, N.A.; Almawi, W.Y. Depression, anxiety, and stress comorbidities in sickle cell anemia patients with vaso-occlusive crisis. J. Pediatric Hematol. Oncol. 2010, 32, 345–349. [Google Scholar] [CrossRef]
- Gil, K.M.; Carson, J.W.; Porter, L.S.; Ready, J.; Valrie, C.; Redding-Lallinger, R.; Daeschner, C. Daily stress and mood and their association with pain, health-care use, and school activity in adolescents with sickle cell disease. J. Pediatric Psychol. 2003, 28, 363–373. [Google Scholar] [CrossRef]
- Von Hehn, C.A.; Baron, R.; Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012, 73, 638–652. [Google Scholar] [CrossRef]
- Shah, P.; Khaleel, M.; Thuptimdang, W.; Sunwoo, J.; Veluswamy, S.; Chalacheva, P.; Sposto, R.; Kato, R.M.; Wood, J.C.; Zeltzer, L.; et al. Mental stress causes vasoconstriction in sickle cell disease and normal controls. Haematologica 2019. [Google Scholar] [CrossRef]
- Connes, P.; Hue, O.; Hardy-Dessources, M.D.; Boucher, J.H.; Pichot, V.; Barthelemy, J.C. Hemorheology and heart rate variability: Is there a relationship? Clin. Hemorheol. Microcirc. 2008, 38, 257–265. [Google Scholar]
- Niss, O.; Fleck, R.; Makue, F.; Alsaied, T.; Desai, P.; Towbin, J.A.; Quinn, C.T.; Malik, P.; Taylor, M.D. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood 2017, 130, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Veline, S.L.; Cox, S.E.; Simpson, D.; Gill, C.; Makani, J.; Soka, D.; Clough, G.F.; Mgaya, J.; Kirkham, F.J. Peripheral vascular response to inspiratory breath hold in paediatric homozygous sickle cell disease. Exp. Physiol. 2013, 98, 49–56. [Google Scholar]
- Affes, Z.; Lionnet, F.; Livrozet, M.; Santin, A.; Frochot, V.; Letavernier, E.; Haymann, J.P.; Lefaucheur, J.P. High prevalence of altered sudomotor function in homozygous sickle cell patients: Influence of age and anaemia. Br. J. Haematol. 2019, 186, e50–e52. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.C.K.; Chalacheva, P. Model-derived markers of autonomic cardiovascular dysfunction in sleep-disordered breathing. Sleep Med. Clin. 2016, 11, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Kumar, G.K. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir. Physiol. Neurobiol. 2010, 174, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Andrade, D.C.; Del Rio, R. Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front. Physiol. 2014, 5, 468. [Google Scholar] [CrossRef]
- Connes, P.; Coates, T.D. Autonomic nervous system dysfunction: Implication in sickle cell disease. Comptes Rendus Biol. 2013, 336, 142–147. [Google Scholar] [CrossRef]
- Bhatt, R.R.; Martin, S.R.; Evans, S.; Lung, K.; Coates, T.D.; Zeltzer, L.K.; Tsao, J.C. The effect of hypnosis on pain and peripheral blood flow in sickle-cell disease: A pilot study. J. Pain Res. 2017, 10, 1635–1644. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veluswamy, S.; Shah, P.; Denton, C.C.; Chalacheva, P.; Khoo, M.C.K.; Coates, T.D. Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger? J. Clin. Med. 2019, 8, 1690. https://doi.org/10.3390/jcm8101690
Veluswamy S, Shah P, Denton CC, Chalacheva P, Khoo MCK, Coates TD. Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger? Journal of Clinical Medicine. 2019; 8(10):1690. https://doi.org/10.3390/jcm8101690
Chicago/Turabian StyleVeluswamy, Saranya, Payal Shah, Christopher C. Denton, Patjanaporn Chalacheva, Michael C. K. Khoo, and Thomas D. Coates. 2019. "Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger?" Journal of Clinical Medicine 8, no. 10: 1690. https://doi.org/10.3390/jcm8101690
APA StyleVeluswamy, S., Shah, P., Denton, C. C., Chalacheva, P., Khoo, M. C. K., & Coates, T. D. (2019). Vaso-Occlusion in Sickle Cell Disease: Is Autonomic Dysregulation of the Microvasculature the Trigger? Journal of Clinical Medicine, 8(10), 1690. https://doi.org/10.3390/jcm8101690




