Evaluation of the Mistakes in Self-Diagnosis of Sexual Dysfunctions in 11,000 Male Outpatients: A Real-Life Study in An Andrology Clinic
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Jannini, E.A.; McCabe, M.P.; Salonia, A.; Montorsi, F.; Sachs, B.D. Organic vs. psychogenic? The Manichean diagnosis in sexual medicine. J. Sex. Med. 2010, 7, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Goldstein, I.; Hatzichristou, D.G.; Krane, R.J.; McKinlay, J.B. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J. Urol. 1994, 51, 54–61. [Google Scholar] [CrossRef]
- Corona, G.; Lee, D.M.; Forti, G.; O’Connor, D.B.; Maggi, M.; O’Neill, T.W.; Finn, J.D.; Pendleton, N.; Barrfi, G.; Boonen, S.; et al. Age-related changes in general and sexual health in middle-aged and older men: Results from the European Male Ageing Study (EMAS). J. Sex. Med. 2010, 7, 1362–1380. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Sternbach, N.; Limoncin, E.; Ciocca, G.; Gravina, G.L.; Tripodi, F.; Simonelli, C.; Petruccelli, I.; Keijzer, S.; Isherwood, G.; et al. Health-related characteristics and unmet needs of men with erectile dysfunction: A survey in five European countries. J. Sex. Med. 2014, 11, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, W.; Phillips, M. Organic causes of erectile dysfunction in men under 40. Urol. Int. 2014, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, P.; Colicchia, M.; Ventimiglia, E.; Castagna, G.; Clementi, M.C.; Suardi, N.; Montorsi, F.; Castiglione, F.; Briganti, A.; Cantiello, F.; et al. One patient out of four with newly diagnosed erectile dysfunction is a young man—Worrisome picture from the everyday clinical practice. J. Sex. Med. 2013, 10, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Heruti, R.; Shochat, T.; Tekes-Manova, D.; Ashkenazi, I.; Justo, D. Prevalence of erectile dysfunction among young adults: Results of a large-scale survey. J. Sex. Med. 2004, 1, 284–291. [Google Scholar] [CrossRef]
- McMahon, C.G.; Althof, S.E.; Waldinger, M.D.; Porst, H.; Dean, J.; Sharlip, I.D.; Dabees, K.; Adaikan, P.G.; Becher, E.; Broderick, G.A.; et al. An evidence-based definition of lifelong premature ejaculation: Report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J. Sex. Med. 2008, 5, 1590–1606. [Google Scholar] [CrossRef]
- Althof, S.E.; McMahon, C.G.; Waldinger, M.D.; Serefoglu, E.C.; Shindel, A.W.; Adaikan, P.G.; Giraldi, A.; Becher, E.; Dean, J.; Giuliano, F.; et al. An Update of the International Society of Sexual Medicine’s Guidelines for the Diagnosis and Treatment of Premature Ejaculation (PE). J. Sex. Med. 2014, 11, 1392–1422. [Google Scholar] [CrossRef]
- McMahon, C.G.; Jannini, E.A.; Serefoglu, E.C.; Hellstrom, W.J. The pathophysiology of acquired premature ejaculation. Transl. Androl. Urol. 2016, 5, 434–449. [Google Scholar] [CrossRef]
- Paduch, D.A.; Polzer, P.; Morgentaler, A.; Althof, S.; Donatucci, C.; Ni, X.; Patel, A.B.; Basaria, S. Clinical and demographic correlates of ejaculatory dysfunctions other than premature ejaculation: A prospective, observational study. J. Sex. Med. 2015, 12, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, S.; Mollaioli, D.; Gravina, G.L.; Ciocca, G.; Limoncin, E.; Carosa, E.; Lenzi, A.; Jannini, E.A. Epidemiology of delayed ejaculation. Transl. Androl. Urol. 2016, 5, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Jockenhovel, F. Late-onset hypogonadism in the aging male (LOH): Definition, diagnostic and clinical aspects. J. Endocrinol. Investig. 2005, 28, 23–27. [Google Scholar]
- Travison, T.G.; Morley, J.E.; Araujo, A.B.; O’Donnell, A.B.; McKinlay, J.B. The relationship between libido and testosterone levels in aging men. J. Clin. Endocrinol. Metab. 2006, 91, 2509–2513. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; Giwercman, A.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Nimbi, F.M.; Tripodi, F.; Rossi, R.; Simonelli, C. Testing a Conceptual Model for Men’s Sexual Desire Referring to Automatic Thoughts, Emotions, Sexual Function, and Sexism. J. Sex. Med. 2018, 15, 1518–1526. [Google Scholar] [CrossRef]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Hayes, R.P.; Henne, J.; Kinchen, K.S. Establishing the content validity of the Sexual Arousal, Interest, and Drive Scale and the Hypogonadism Energy Diary. Int. J. Clin. Pract. 2015, 69, 454–465. [Google Scholar] [CrossRef]
- Goldstein, I.; Burnett, A.I.; Rosec, R.C.; Park, P.W.; Stecher, V.J. The serendipitous story of sildenafil: An unexpected oral therapy for erectile dysfunction. Sex. Med. Rev. 2018, 7, 115–128. [Google Scholar] [CrossRef]
- Mulhall, J.P.; Giraldi, A.; Hackett, G.; Hellstrom, W.J.; Jannini, E.A.; Rubio-Aurioles, E.; Hassan, T.A.; Trost, L. The 2018 Revision to the Process of Care Model for Management of Erectile Dysfunction. J. Sex. Med. 2018, 15, 1434–1445. [Google Scholar] [CrossRef]
- Jannini, E.A.; Lenzi, A.; Isidori, A.; Fabbri, A. Subclinical erectile dysfunction: Proposal for a novel taxonomic category in sexual medicine. J. Sex. Med. 2006, 3, 787–794. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.P.; Sharlip, I.D.; Atalla, E.; Balon, R.; Fisher, A.D.; Laumann, E.; Segraves, R.T.; Lee, S.W.; Lewis, R. Definitions of Sexual Dysfunctions in Women and Men: A Consensus Statement From the Fourth International Consultation on Sexual Medicine 2015. J. Sex. Med. 2016, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Symonds, T.; Perelman, M.A.; Althof, S.; Giuliano, F.; Martin, M.; May, K.; Morris, M.; Abraham, L.; Crossland, A. Development and validation of a premature ejaculation diagnostic tool. Eur. Urol. 2007, 52, 565–573. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Consensus Development Conference Statement; National Institutes of Health: Bethesda, MD, USA, 1993; pp. 181–284.
- Fanni, E.; Castellini, G.; Corona, G.; Boddi, V.; Ricca, V.; Rastrelli, G.; Maggi, M.; Fisher, A.D.; Cipriani, S. The role of somatic symptoms in sexual medicine: somatization as important contextual factor in male sexual dysfunction. J. Sex. Med. 2016, 13, 1395–1407. [Google Scholar] [CrossRef]
- Takayanagi, A.; Kobayashi, K.; Fukuta, F.; Matsuki, M.; Matsuda, Y.; Mori, M.; Masumori, N. Changes of sexual function and perception in Japanese men: A 15-year cross-sectional community-based study. Int. J. Urol. 2016, 23, 941–945. [Google Scholar] [CrossRef]
- Zheng, X.; Ji, P.; Mao, H.; Wu, J. Evaluation of penile erection rigidity in healthy men using virtual touch tissue quantification. Radiol. Oncol. 2012, 46, 114–118. [Google Scholar] [CrossRef][Green Version]
- Boybeyi, Ö.; Gunal, Y.D.; Atasoy, P.; Kısa, U.; Aslan, M.K. Investigation of the effect of dorsal penile block to penile tissue. J. Pediatr. Urol. 2015, 11, 268. [Google Scholar] [CrossRef]
- Limanjaya, A.; Song, K.M.; Choi, M.J.; Ghatak, K.; Minh, N.N.; Kang, D.H.; Suh, J.K.; Ock, J.; Yin, G.N.; Chung, H.Y.; et al. Calorie restriction reverses age-related alteration of cavernous neurovascular structure in the rat. Andrology 2017, 5, 1023–1031. [Google Scholar] [CrossRef]
- Amarenco, G.; Sheikh, S.I.; Verollet, D.; Le, F.B.; Lacroix, P.; Fahed, M. Clinical and electrophysiological study of sensory penile alterations. A prospective study of 44 cases. Prog. Urol. 2013, 23, 946–950. [Google Scholar] [CrossRef]
- Wiggins, A.; Farrell, M.R.; Tsambarlis, P.; Levine, L.A. The Penile Sensitivity Ratio: A Novel Application of Biothesiometry to Assess Changes in Penile Sensitivity. J. Sex. Med. 2019, 16, 447–451. [Google Scholar] [CrossRef]
- Clement, P.; Giuliano, F. Physiology and pharmacology of ejaculation. Basic Clin. Pharmacol. Toxicol. 2016, 119, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; Coolen, L.M. Neural mechanisms of sexual behavior in the male rat: Emphasis on ejaculation-related circuits. Pharmacol. Biochem. Behav. 2014, 121, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Jannini, E.A.; Gnessi, L.; Ulisse, S.; Moretti, C.; Isidori, A. Neuroendocrine control of male reproductive function. The opioid system as a model of control at multiple sites. J. Steroid. Biochem. 1989, 32, 145–150. [Google Scholar] [CrossRef]
- McMahon, C.G. Current and Emerging Treatments for Premature Ejaculation. Sex. Med. Rev. 2015, 3, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Waldinger, M.D. The neurobiological approach to premature ejaculation. J. Urol. 2002, 168, 2359–2367. [Google Scholar] [CrossRef]
- Giuliano, F.; Clément, P. Serotonin and premature ejaculation: From physiology to patient management. Eur. Urol. 2006, 50, 454–466. [Google Scholar] [CrossRef]
- Olivier, J.D.; Franco, D.C.E.; Oosting, R.; Waldinger, M.; Sarnyai, Z.; Olivier, B. Tramadol: Effects on sexual behavior in male rats are mainly caused by its 5-HT reuptake blocking effects. Neuropharmacology 2017, 116, 50–58. [Google Scholar] [CrossRef]
- Jannini, E.A.; McMahon, C.; Chen, J.; Aversa, A.; Perelman, M. The controversial role of phosphodiesterase type 5 inhibitors in the treatment of premature ejaculation. J. Sex. Med. 2011, 8, 2135–2143. [Google Scholar] [CrossRef]
- Limoncin, E.; Lotti, F.; Rossi, M.; Maseroli, E.; Gravina, G.L.; Ciocca, G.; Jannini, E.A.; Mollaioli, D.; Sante, S.D.; Maggi, M.; et al. The impact of premature ejaculation on the subjective perception of orgasmic intensity: Validation and standardisation of the ‘Orgasmometer’. Andrology 2016, 4, 921–926. [Google Scholar] [CrossRef]
- Petrone, L.; Mannucci, E.; Corona, G.; Bartolini, M.; Forti, G.; Giommi, R.; Maggi, M. Structured interview on erectile dysfunction (SIEDY): A new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int. J. Impot. Res. 2003, 15, 210–220. [Google Scholar] [CrossRef]
- Boddi, V.; Fanni, E.; Castellini, G.; Fisher, A.D.; Corona, G.; Maggi, M. Conflicts within the family and within the couple as contextual factors in the determinism of male sexual dysfunction. J. Sex. Med. 2015, 12, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Petrone, L.; Mannucci, E.; Magini, A.; Lotti, F.; Ricca, V.; Maggi, M.; Chiarini, V.; Forti, G. Assessment of the relational factor in male patients consulting for sexual dysfunction: The concept of couple sexual dysfunction. J. Androl. 2006, 27, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz-Meydan, A.; Ayalon, L. Physicians’ response to sexual dysfunction presented by a younger vs.an older adult. Int. J. Geriatr. Psychiatry 2017, 32, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
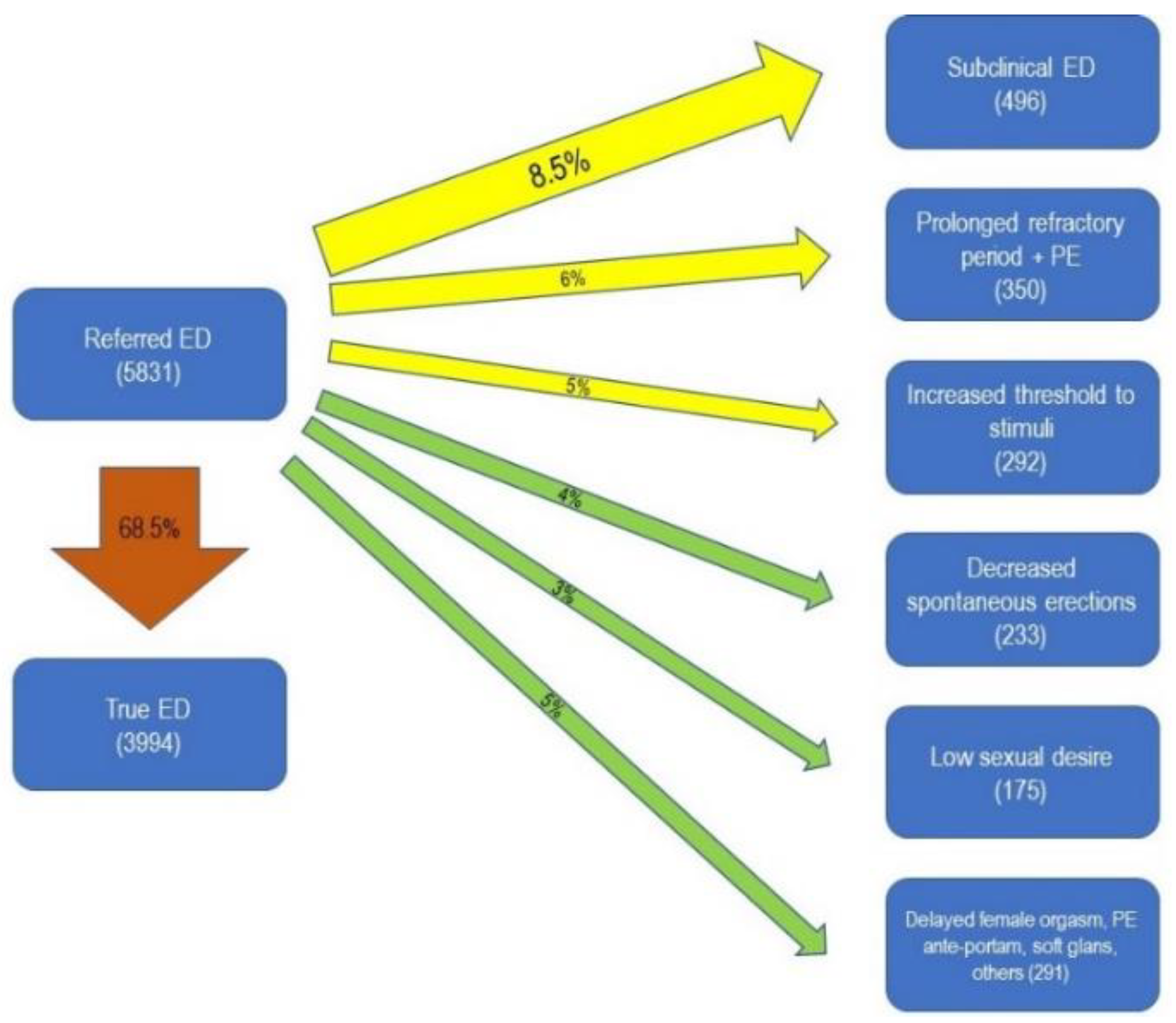
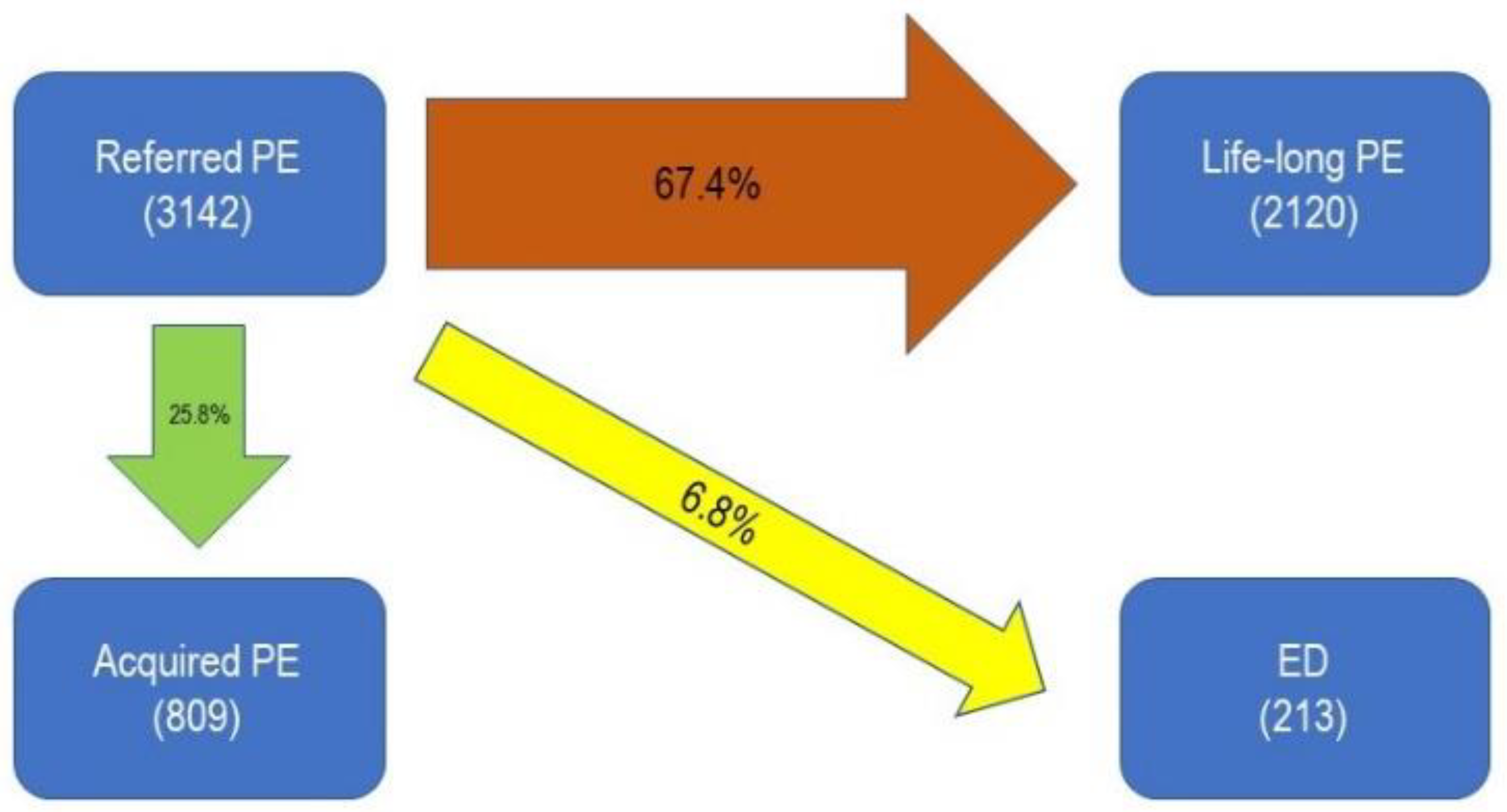
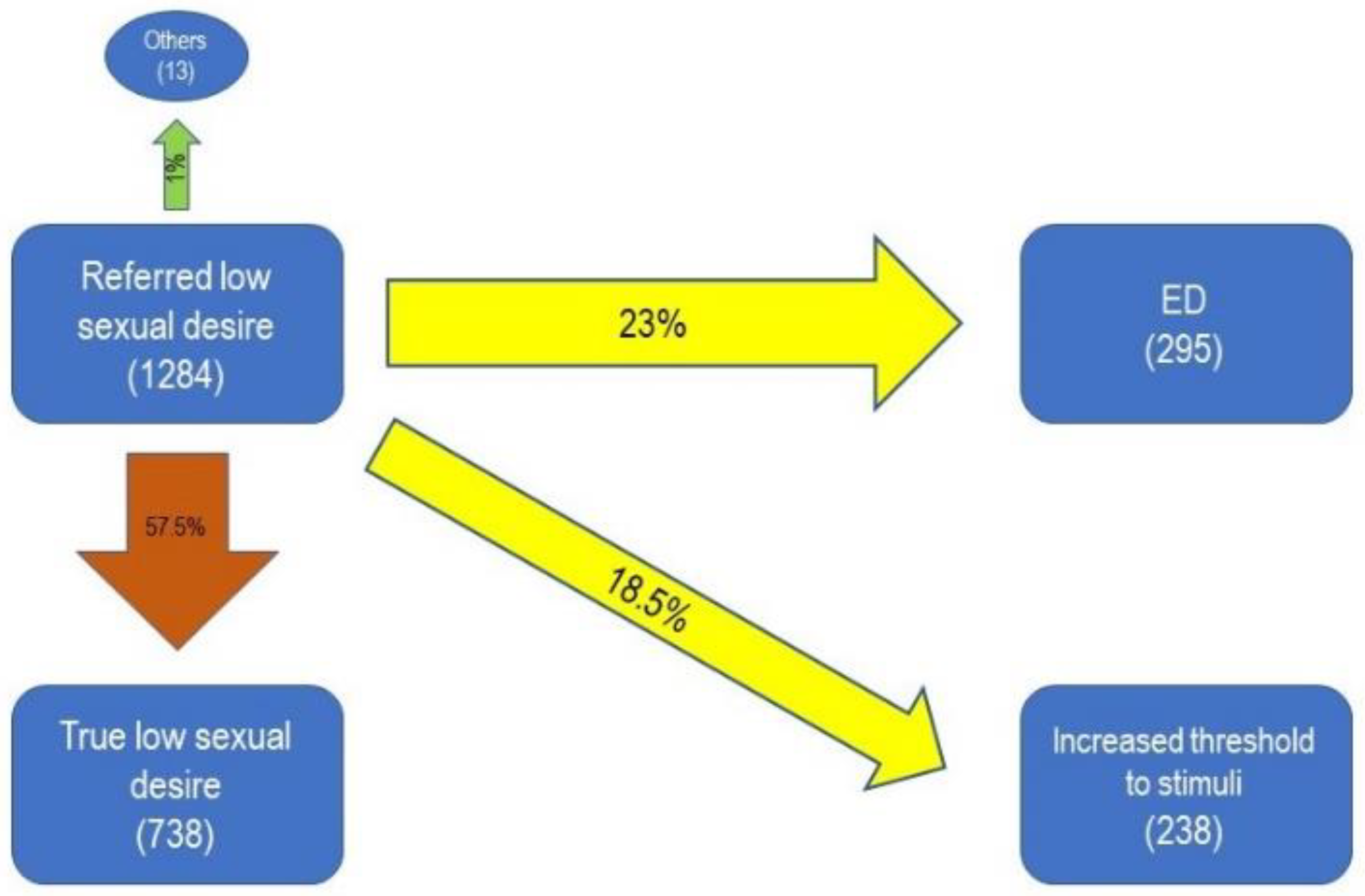
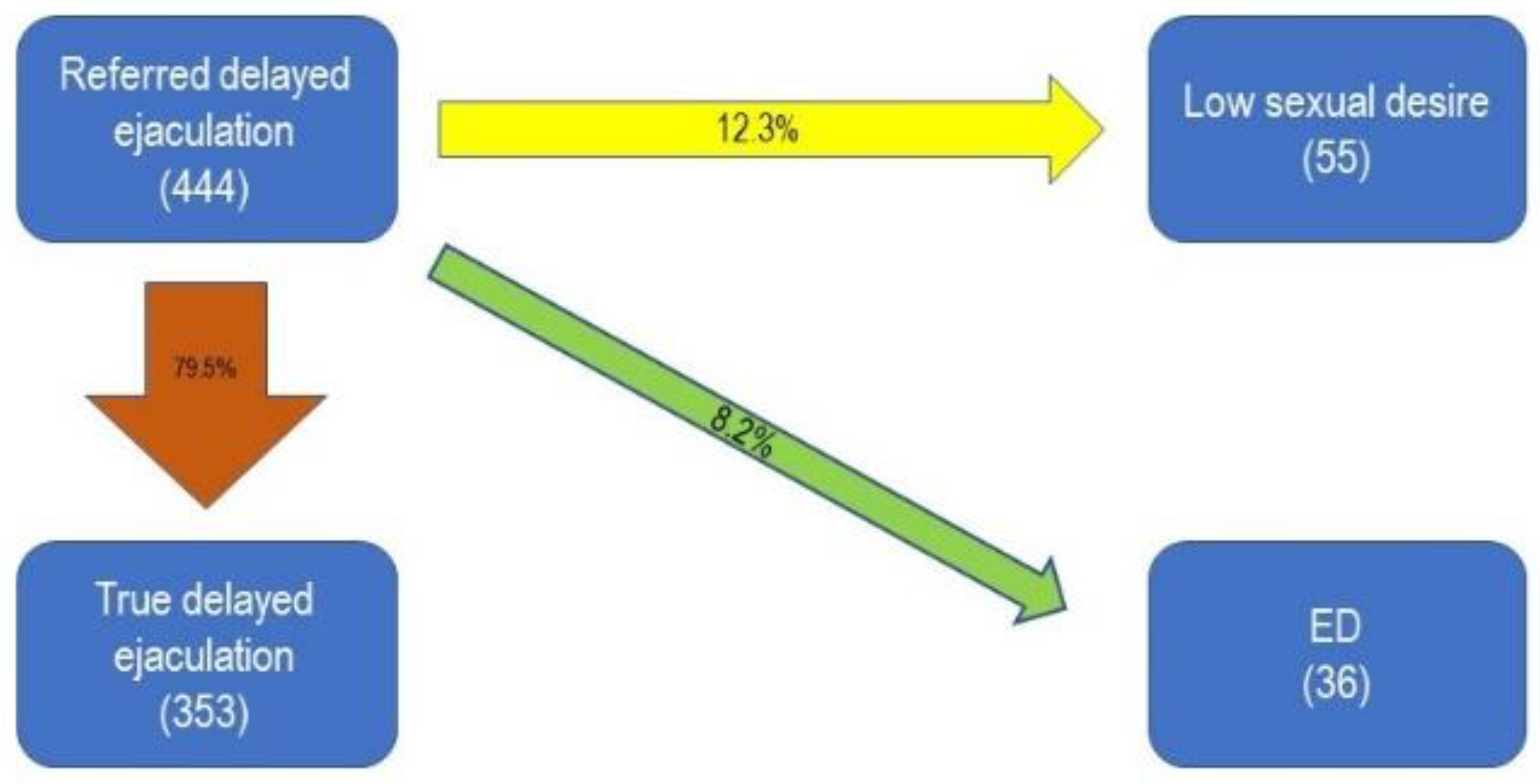
| Type of Sexual Dysfunction | Number of Patients | Percentage |
|---|---|---|
| Erectile Dysfunction | 5831 | 52% |
| Premature Ejaculation (PE) | 3142 | 28.1% |
| • Life-Long PE | 1017 | 9.1% |
| • Acquired PE | 2125 | 19% |
| Low Sexual Desire | 1284 | 11.5% |
| Delayed Ejaculation | 444 | 4% |
| Others (Low Ejaculate Volume, Dysmorphophobia, Decreased Ejaculatory Power, “Unmet Needs” *) | 499 | 4.4% |
| Type of Sexual Dysfunction | Number of Patients | Percentage |
|---|---|---|
| Erectile Dysfunction | 4557 | 40.7% |
| Life-long Premature Ejaculation | 2161 | 19.3% |
| Low Sexual Desire | 968 | 8.6% |
| Acquired Premature Ejaculation | 809 | 7.3% |
| Increased Threshold to Stimuli | 530 | 4.7% |
| Subclinical Erectile Dysfunction | 496 | 4.4% |
| Delayed Female Orgasm | 440 | 3.9% |
| Delayed Ejaculation | 353 | 3.2% |
| Prolonged Refractory Period + Premature Ejaculation | 350 | 3.1% |
| Reduced Spontaneous Erections | 233 | 2.1% |
| Soft Glans | 17 | 0.2% |
| Other Diagnoses | 286 | 2.5% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgio, G.; Giammusso, B.; Calogero, A.E.; Mollaioli, D.; Condorelli, R.A.; Jannini, E.A.; La Vignera, S. Evaluation of the Mistakes in Self-Diagnosis of Sexual Dysfunctions in 11,000 Male Outpatients: A Real-Life Study in An Andrology Clinic. J. Clin. Med. 2019, 8, 1679. https://doi.org/10.3390/jcm8101679
Burgio G, Giammusso B, Calogero AE, Mollaioli D, Condorelli RA, Jannini EA, La Vignera S. Evaluation of the Mistakes in Self-Diagnosis of Sexual Dysfunctions in 11,000 Male Outpatients: A Real-Life Study in An Andrology Clinic. Journal of Clinical Medicine. 2019; 8(10):1679. https://doi.org/10.3390/jcm8101679
Chicago/Turabian StyleBurgio, Giovanni, Bruno Giammusso, Aldo E. Calogero, Daniele Mollaioli, Rosita A. Condorelli, Emmanuele A. Jannini, and Sandro La Vignera. 2019. "Evaluation of the Mistakes in Self-Diagnosis of Sexual Dysfunctions in 11,000 Male Outpatients: A Real-Life Study in An Andrology Clinic" Journal of Clinical Medicine 8, no. 10: 1679. https://doi.org/10.3390/jcm8101679
APA StyleBurgio, G., Giammusso, B., Calogero, A. E., Mollaioli, D., Condorelli, R. A., Jannini, E. A., & La Vignera, S. (2019). Evaluation of the Mistakes in Self-Diagnosis of Sexual Dysfunctions in 11,000 Male Outpatients: A Real-Life Study in An Andrology Clinic. Journal of Clinical Medicine, 8(10), 1679. https://doi.org/10.3390/jcm8101679









