Volumetric Cone Beam Computed Tomography for the Assessment of Oral Manifestations in Systemic Sclerosis: Data from an EUSTAR Cohort
Abstract
1. Introduction
2. Material and Methods
2.1. SSc-Related Parameters
2.2. Oral Manifestations
2.3. Imaging Studies
2.4. Statistical Analysis
3. Results
3.1. SSc-Related Characteristics
3.2. Oral Health
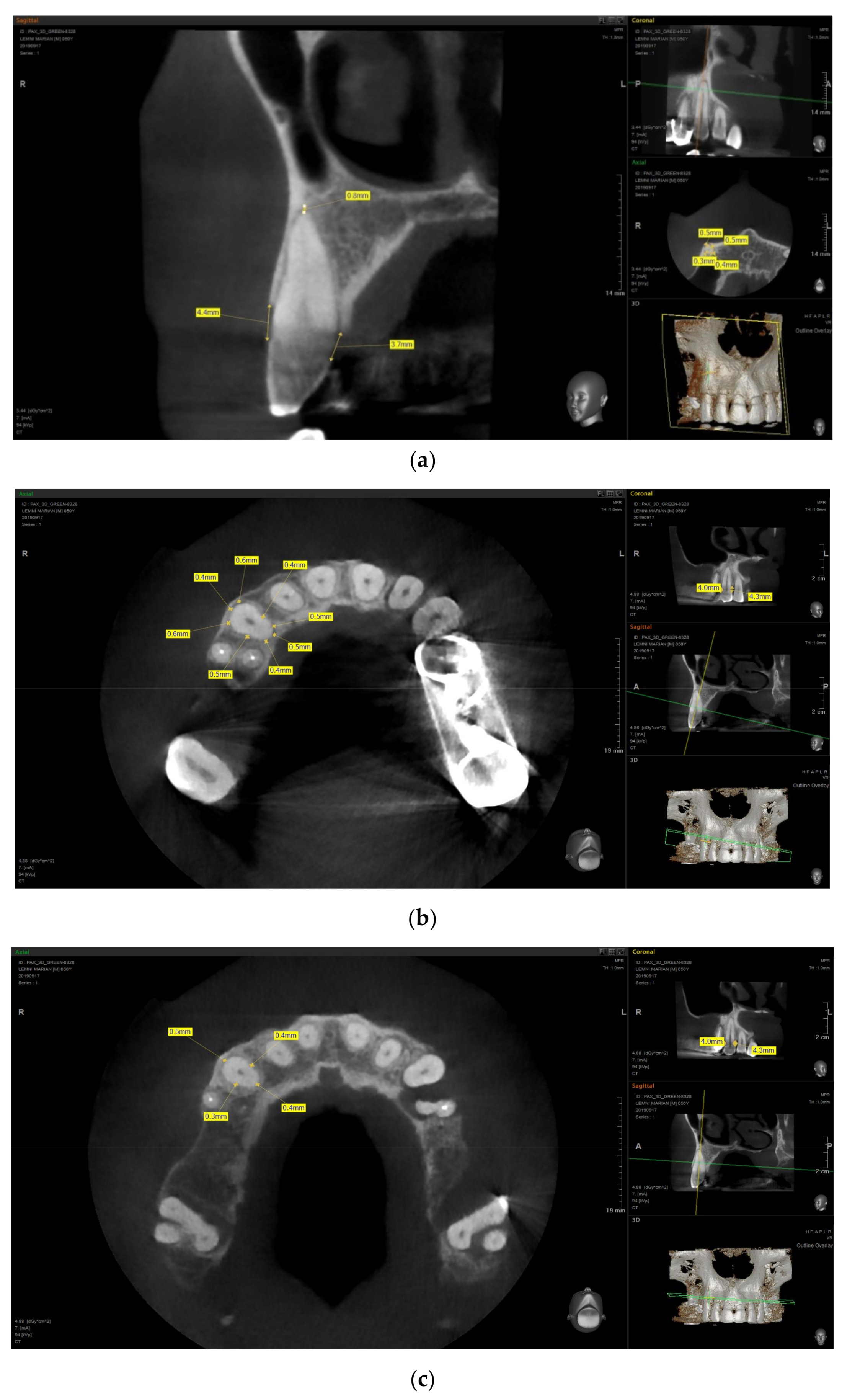
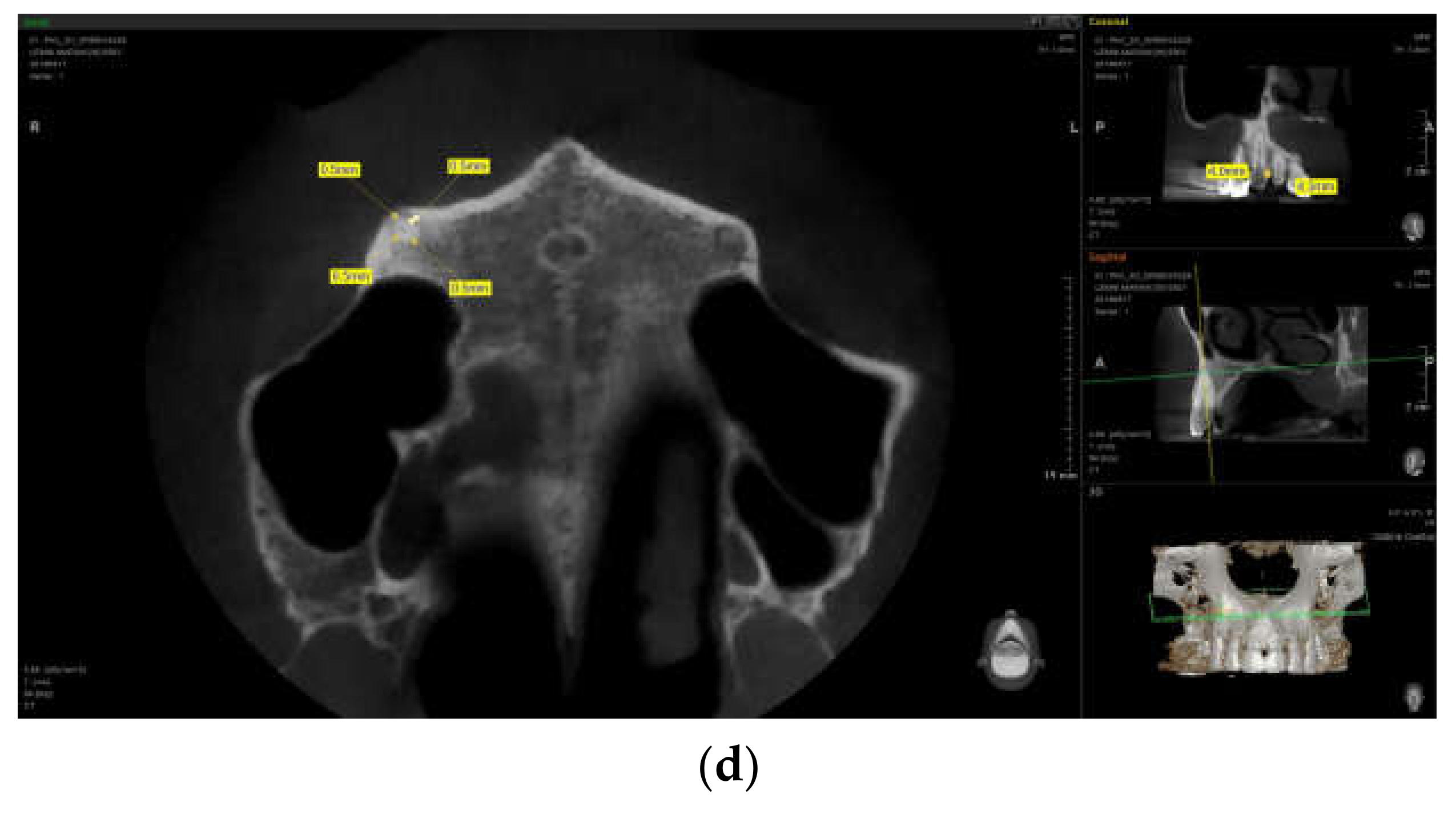
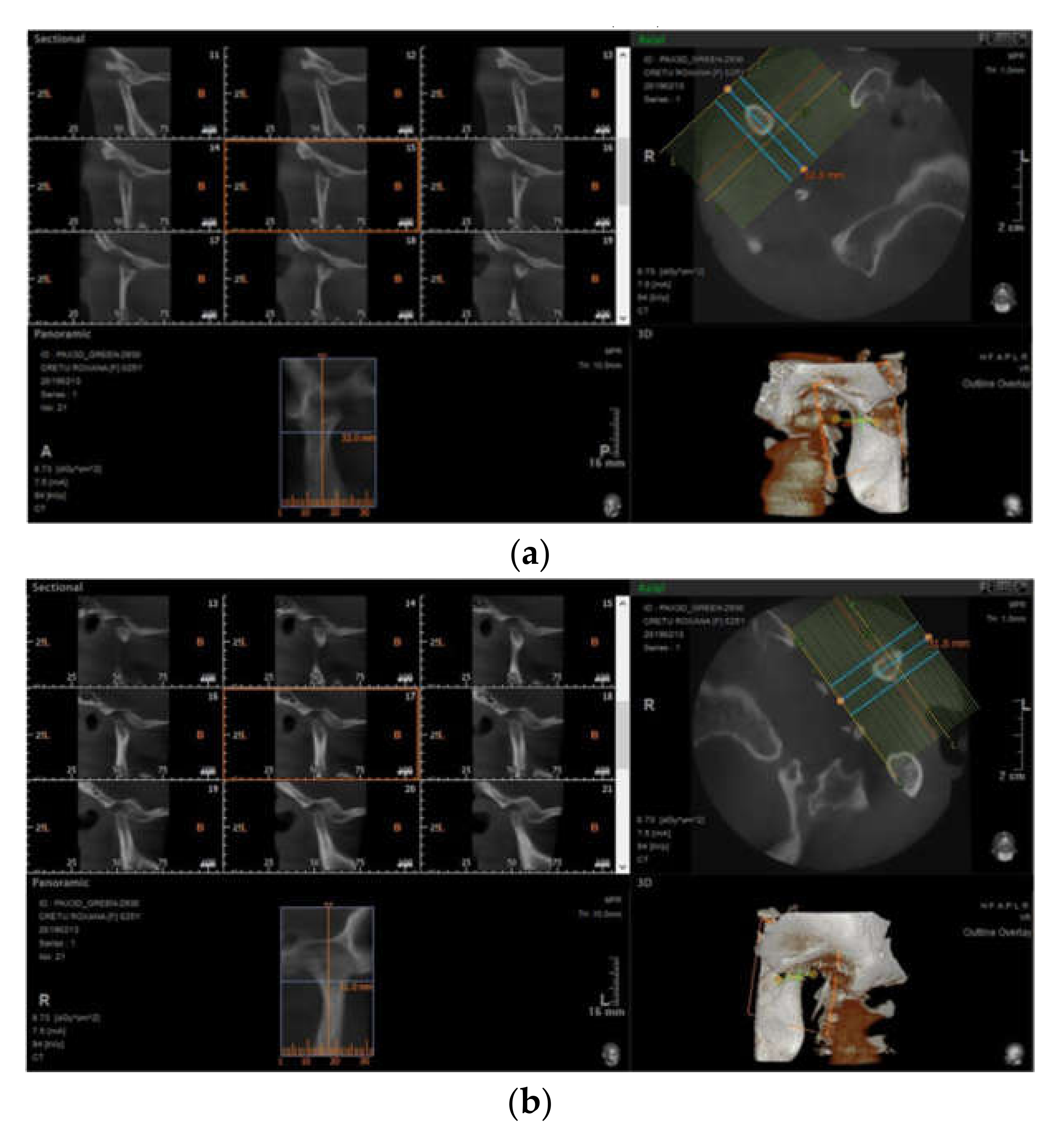
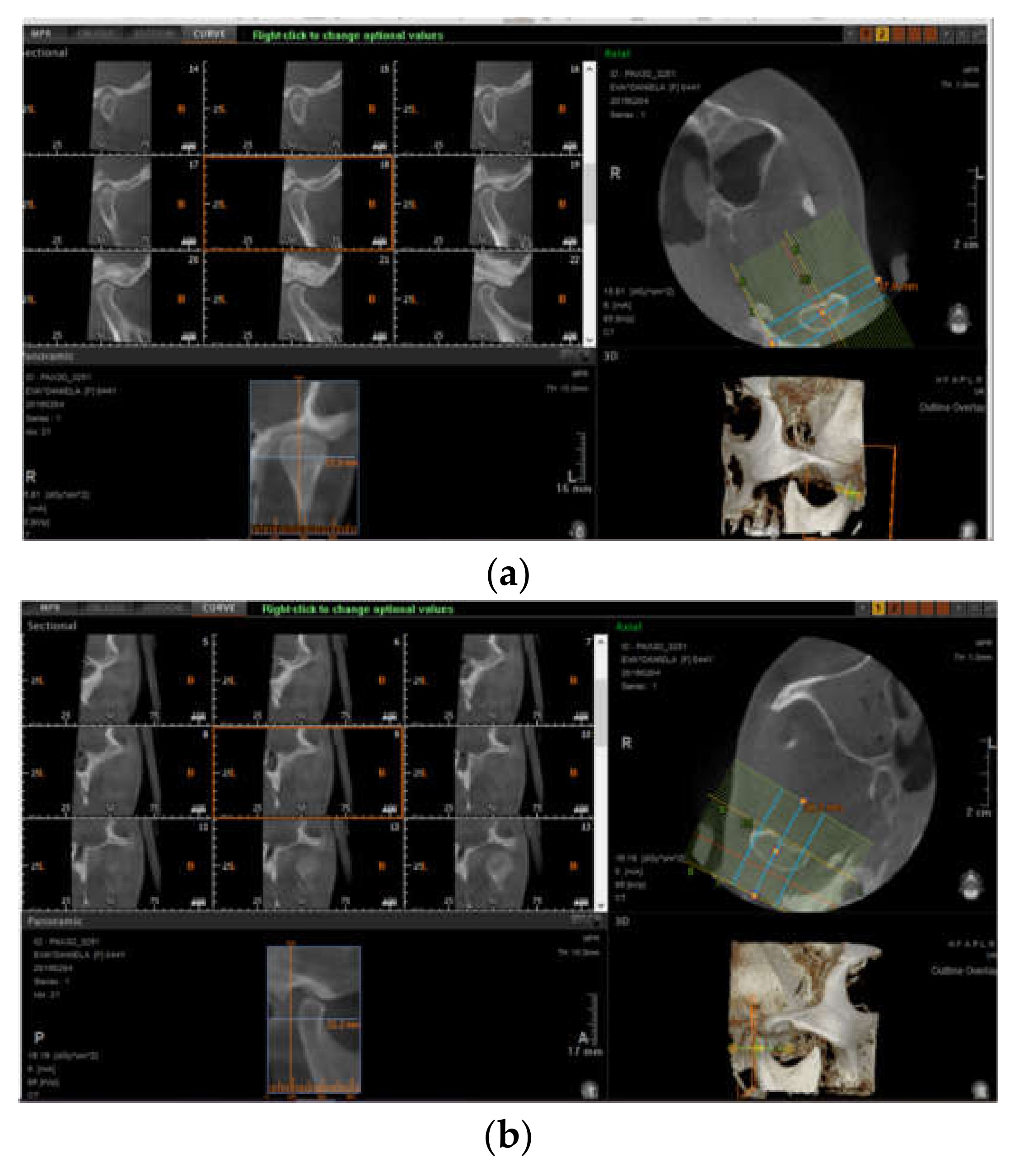
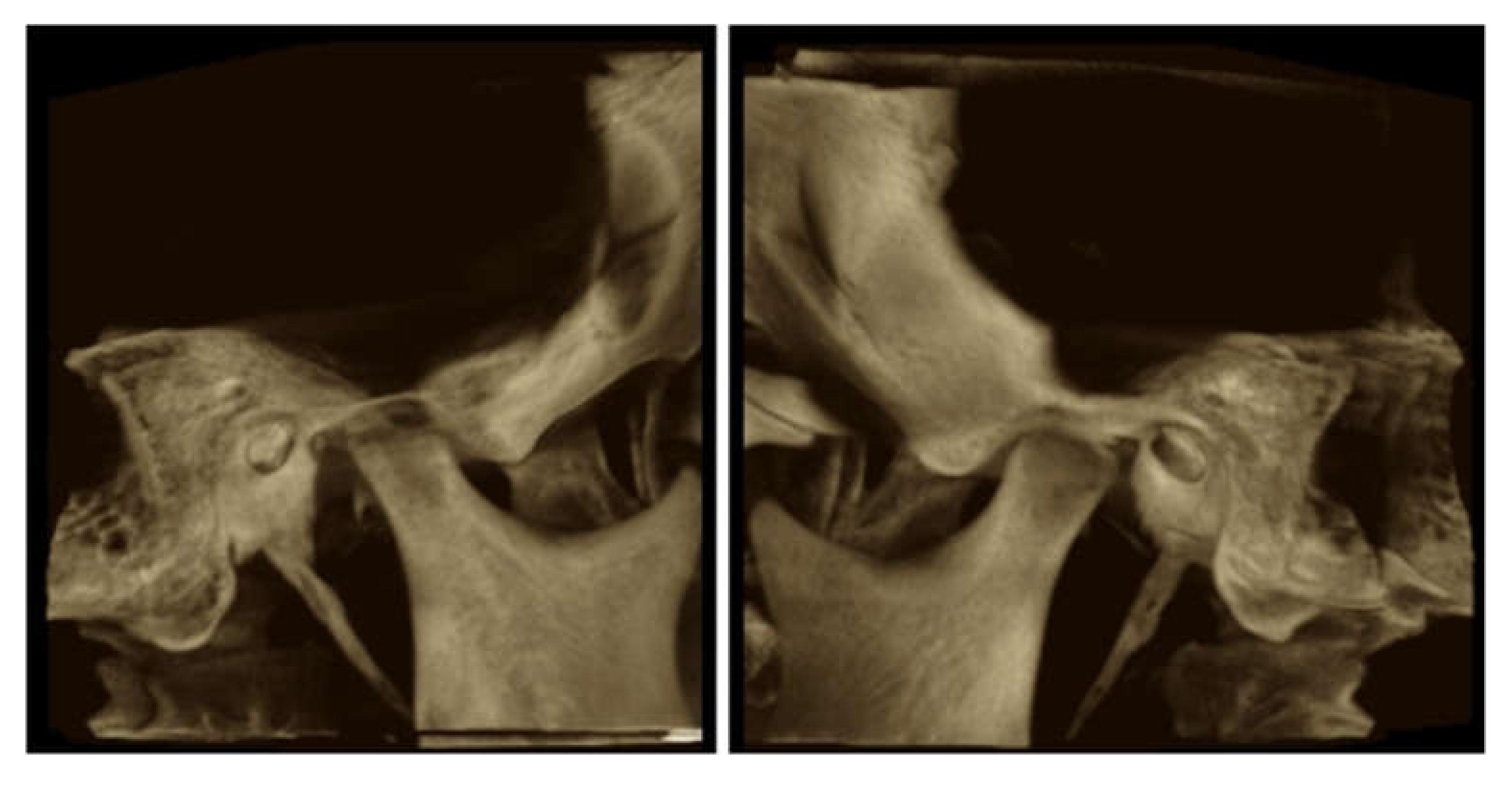
3.3. Imaging Studies
3.3.1. PDL Space Widening
3.3.2. Erosions
3.4. Associations with PDL Space Widening and Erosions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shah, A.A.; Wigley, F.M. My approach to the treatment of scleroderma. Mayo Clin. Proc. 2013, 88, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sepúlveda, A.; Esquinca-González, A.; Benavides-Suárez, S.A.; Sordo-Lima, D.E.; Caballero-Islas, A.E.; Cabral-Castañeda, A.R.; Rodríguez-Reyna, T.S. Systemic Sclerosis Pathogenesis and Emerging Therapies, beyond the Fibroblast. BioMed Res. Int. 2019, 2019, 4569826. [Google Scholar] [CrossRef] [PubMed]
- Smirani, R.; Poursac, N.; Naveau, A.; Schaeverbeke, T.; Devillard, R.; Truchetet, M.-E. Orofacial consequences of systemic sclerosis: A systematic review. J. Scleroderma Relat. Disord. 2018, 3, 81–90. [Google Scholar] [CrossRef]
- Crincoli, V.; Fatone, L.; Fanelli, M.; Rotolo, R.P.; Chialà, A.; Favia, G.; Lapadula, G. Orofacial Manifestations and Temporomandibular Disorders of Systemic Scleroderma: An Observational Study. Int. J. Mol. Sci. 2016, 17, 1189. [Google Scholar] [CrossRef] [PubMed]
- Said, M.H.; Foletti, J.; Graillon, N.; Guyot, L.; Chossegros, C. Orofacial manifestations of scleroderma. A literature review. Revue de Stomatologie, de Chirurgie Maxillo-Faciale et de Chirurgie Orale 2016, 117, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Derbi, H.A. Scleroderma and the Oral Health Implications. Adv. Dent. Oral Health 2018, 7, 555716. [Google Scholar] [CrossRef]
- Yilmaz, S.G.; Bayrakdar, Ş.İ.; Bayrak, S. Maxillofacial Manifestations of Systemic Sclerosis: Case Report. Akd. Med. J. 2018, 4, 1–3. [Google Scholar]
- Smirani, R.; Truchetet, M.É.; Poursac, N.; Naveau, A.; Schaeverbeke, T.; Devillard, R. Impact of systemic sclerosis oral manifestations on patients health-related quality of life: A systematic review. J. Oral Pathol. Med. 2018, 47, 808–815. [Google Scholar] [CrossRef]
- Jung, S.; Martin, T.; Schmittbuhl, M.; Huck, O. The spectrum of orofacial manifestations in systemic sclerosis: A challenging management. Oral Dis. 2017, 23, 424–439. [Google Scholar] [CrossRef]
- Puzio, A.; Przywara-Chowaniec, B.; Postek-Stefańska, L.; Mrowka-Kata, K.; Trzaska, K. Systemic sclerosis and its oral health implications. Adv. Clin. Exp. Med. 2019, 28, 547–554. [Google Scholar] [CrossRef]
- Alexandridis, C.; White, S.C. Periodontal ligament changes in patients with progressive systemic sclerosis. Oral Surg. Oral Med. Oral Pathol. 1984, 58, 113–118. [Google Scholar] [CrossRef]
- Anbiaee, N.; Tafakhori, Z. Localized periodontal ligament space widening as the only presentation of scleroderma—Reliability recheck: Author response. Dentomaxillofac. Radiol. 2012, 41, 441–442. [Google Scholar] [CrossRef]
- Anbiaee, N.; Tafakhori, Z. Early diagnosis of progressive systemic sclerosis (scleroderma) from a panoramic view: Report of three cases. Dentomaxillofac. Radiol. 2011, 40, 457–462. [Google Scholar] [CrossRef]
- Dagenais, M.; Macdonald, D.; Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Gravel, S.; Mohit, S.; Sayegh, T.E.; Pope, J.; et al. The Canadian Systemic Sclerosis Oral Health Study IV: Oral radiographic manifestations in systemic sclerosis compared with the general population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Hudson, M.; Dagenais, M.; Macdonald, D.; Gyger, G.; Sayegh, T.E.; Pope, J.; Fontaine, A.; Masetto, A.; Matthews, D.; et al. Relationship between Disease Characteristics and Oral Radiologic Findings in Systemic Sclerosis: Results from a Canadian Oral Health Study. Arthritis Care Res. 2016, 68, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; Sayegh, T.E.; Pope, J.; Fontaine, A.; et al. Relationship between disease characteristics and orofacial manifestations in systemic sclerosis: Canadian Systemic Sclerosis Oral Health Study III. Arthritis Care Res. 2015, 67, 681–690. [Google Scholar] [CrossRef]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; El Sayegh, T.; Pope, J.; Fontaine, A.; et al. The Canadian systemic sclerosis oral health study: Orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology 2014, 53, 1386–1394. [Google Scholar] [CrossRef]
- Hopper, F.E.; Giles, A.D. Orofacial changes in systemic sclerosis—Report of a case of resorption of mandibular angles and zygomatic arches. Br. J. Oral Surg. 1982, 20, 129–134. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M. Review of common conditions associated with periodontal ligament widening. Imaging Sci. Dent. 2016, 46, 229–237. [Google Scholar] [CrossRef]
- Yalcin, E.D.; Avcu, N.; Uysal, S.; Arslan, U. Evaluation of radiomorphometric indices and bone findings on panoramic images in patients with scleroderma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, e23–e30. [Google Scholar] [CrossRef]
- Stoica, A.M.; Monea, M.; Vlad, R. Cone Beam Computed Tomography Used in the Assessment of the Alveolar Bone in Periodontitis. Eur. Sci. J. 2016, 12, 47–54. [Google Scholar] [CrossRef][Green Version]
- Tyndall, D.A.; Rathore, S. Cone-beam CT diagnostic applications: Caries, periodontal bone assessment, and endodontic applications. Dent. Clin. N. Am. 2008, 52, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Duan, D. Cone-beam computed tomography performance in measuring periodontal bone loss. J. Oral Sci. 2019, 61, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Melsens, K.; De Keyser, F.; Decuman, S.; Piette, Y.; Vandecasteele, E.; Smith, V. Disease activity indices in systemic sclerosis: A systematic literature review. Clin. Exp. Rheumatol. 2016, 34, S186–S192. [Google Scholar]
- Barsotti, S.; Bruni, C.; Orlandi, M.; Rossa, A.D.; Marasco, E.; Codullo, V.; Guiducci, S. One year in review 2017: Systemic sclerosis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 106), S2–S20. [Google Scholar]
- Ross, L.; Baron, M.; Nikpour, M. The challenges and controversies of measuring disease activity in systemic sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 115–121. [Google Scholar] [CrossRef]
- Cox, S.C.; Walker, D.M. Establishing a normal range for mouth opening: Its use in screening for oral submucous fibrosis. Br. J. Oral Maxillofac. Surg. 1997, 35, 40–42. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy II. Correlation between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar]
- Pischon, N.; Hoedke, D.; Kurth, S.; Lee, P.; Dommisch, H.; Steinbrecher, A.; Pischon, T.; Burmester, G.R.; Buttgereit, F.; Detert, J.; et al. Increased Periodontal Attachment Loss in Patients With Systemic Sclerosis. J. Periodontol. 2016, 87, 763–771. [Google Scholar] [CrossRef]
- Leung, W.K.; Chu, C.H.; Mok, M.Y.; Yeung, K.S.; Ng, S.K. Periodontal status of adults with systemic sclerosis: Case-control study. J. Periodontol. 2011, 82, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.; Eaton, K.A.; Moles, D.R. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J. Clin. Periodontol. 2009, 36, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; De Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S219–S229. [Google Scholar] [CrossRef]
- Jagadish, R.; Mehta, D.S.; Jagadish, P. Oral and periodontal manifestations associated with systemic sclerosis: A case series and review. J. Indian Soc. Periodontol. 2012, 16, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Oral Manifestations of Systemic Sclerosis: Toward the Identification of New Prognostic Markers. Available online: https://ichgcp.net/clinical-trials-registry/NCT02371005 (accessed on 21 March 2019).
- Matsuda, S.; Memida, T.; Mizuno, N.; Ogawa, I.; Ouhara, K.; Kajiya, M.; Fujita, T.; Sugiyama, E.; Kurihara, H. Reparative bone-like tissue formation in the tooth of a systemic sclerosis patient. Int. Endod. J. 2018, 51, 1059–1066. [Google Scholar] [CrossRef]
- Seifert, M.H.; Steigerwald, J.C.; Cliff, M.M. Bone resorption of the mandible in progressive systemic sclerosis. Arthritis Rheum. 1975, 18, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Minoux, M.; Manière, M.-C.; Martin, T.; Schmittbuhl, M. Previously undescribed pulpal and periodontal ligament calcifications in systemic sclerosis: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e47–e51. [Google Scholar] [CrossRef]
| Parameter | No (%) or Mean ± SD |
|---|---|
| Demographics | |
| Female | 31 (72) |
| Age | 43.95 (11.36) |
| Smoking status | 10 (23.25) |
| SSc-related measures | |
| Diffuse cutaneous SSc | 29 (67.74) |
| Disease duration | 8.7 (4.5) |
| Modified RODNAN (0–51) | 18 (10.1) |
| Facial skin score (0–3) | 1.5 (0.7) |
| Serology | |
| Anti-SCL70 | 23 (53.48) |
| Anti-centromere B | 10 (23.25) |
| Dental issues | |
| Missing teeth per subject | 9.3 (4.5) |
| Teeth with periodontal disease | 7.2 (3.4) |
| PDL space widening | |
| Patients with uniform PDL space widening | 20 (46.51) |
| Apical PDL space widening (mm) | 0.35 (0.16) |
| Erosions | |
| Patients with mandibular erosions | 10 (23.25) |
| Number of subjects with condylar erosions | 8 (18.60) |
| Parameter | PDL Widening | Erosions | ||
|---|---|---|---|---|
| RR | p | RR | p | |
| Demographics | 1.1 | p > 0.05 | 1.2 | p > 0.05 |
| Female | 1.43 | p > 0.05 | 1.1 | p > 0.05 |
| Age | ||||
| Smoking status | 1.06 | p > 0.05 | 1.06 | p > 0.05 |
| SSc-related measures | ||||
| Diffuse cutaneous SSc | 1.25 | p > 0.05 | 1.02 | p > 0.05 |
| Disease duration | 2.36 * | p < 0.05 | 0.98 | p > 0.05 |
| Modified Rodnan (0–51) | 3.12 * | p < 0.05 | 1.3 | p > 0.05 |
| Facial skin score (0–3) | 2.71 * | p < 0.05 | 1.02 | p > 0.05 |
| SSc activity | 1.21 | p > 0.05 | 1.17 | p > 0.05 |
| SSc severity | 3.09 * | p < 0.05 | 0.92 | p > 0.05 |
| Interdental distance | 1.21 | p > 0.05 | 3.51 * | p < 0.05 |
| Dental issues | ||||
| Missing teeth per subject | 1.02 | p > 0.05 | 0.87 | p > 0.05 |
| Teeth with periodontal disease | 1.15 | p > 0.05 | 1.12 | p > 0.05 |
| Parameter | RR | 95% CI |
|---|---|---|
| Gender | 2.17 | 0.91–14.28 |
| Age | 1.00 * | 0.89–1.73 |
| Smoking status | 5.31 | 4.27–9.12 |
| SSc duration SSc subtype | 3.26 * 4.51 * | 1.20–7.53 2.39–8.14 |
| RODNAN skin score SSc severity SSc activity Anti–topoisomerase 1 | 0.93 * 1.25 * 2.36 5.22 * | 0.89–3.76 1.58–3.89 1.02–3.41 2.3–7.67 |
| Parameter | RR | 95% CI |
|---|---|---|
| Gender | 2.17 | 0.91–14.28 |
| Age | 1.00 | 0.89–1.73 |
| Smoking status | 5.31 | 4.27–9.12 |
| SSc duration SSc subtype | 3.26 * 4.51* | 1.20–7.53 2.39–8.14 |
| RODNAN skin score SSc severity SSc activity Anti-topoisomerase 1 Number of teeth with periodontal disease | 0.93 * 1.25 * 2.32 5.19 * 1.19 | 0.89–3.76 1.58–3.89 1.45–3.22 2.24–7.32 0.87–1.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordache, C.; Antohe, M.-E.; Chirieac, R.; Ancuța, E.; Țănculescu, O.; Ancuța, C. Volumetric Cone Beam Computed Tomography for the Assessment of Oral Manifestations in Systemic Sclerosis: Data from an EUSTAR Cohort. J. Clin. Med. 2019, 8, 1620. https://doi.org/10.3390/jcm8101620
Iordache C, Antohe M-E, Chirieac R, Ancuța E, Țănculescu O, Ancuța C. Volumetric Cone Beam Computed Tomography for the Assessment of Oral Manifestations in Systemic Sclerosis: Data from an EUSTAR Cohort. Journal of Clinical Medicine. 2019; 8(10):1620. https://doi.org/10.3390/jcm8101620
Chicago/Turabian StyleIordache, Cristina, Magda-Ecaterina Antohe, Rodica Chirieac, Eugen Ancuța, Oana Țănculescu, and Codrina Ancuța. 2019. "Volumetric Cone Beam Computed Tomography for the Assessment of Oral Manifestations in Systemic Sclerosis: Data from an EUSTAR Cohort" Journal of Clinical Medicine 8, no. 10: 1620. https://doi.org/10.3390/jcm8101620
APA StyleIordache, C., Antohe, M.-E., Chirieac, R., Ancuța, E., Țănculescu, O., & Ancuța, C. (2019). Volumetric Cone Beam Computed Tomography for the Assessment of Oral Manifestations in Systemic Sclerosis: Data from an EUSTAR Cohort. Journal of Clinical Medicine, 8(10), 1620. https://doi.org/10.3390/jcm8101620





