Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS)
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Clinical Assessment
2.3. At-Home Polysomnography Recording
2.4. HRV Analysis
- (a)
- During quiet wakefulness before sleep and during a stable sleep epoch; that is, a 5 min period during which the sleep stage did not change and without arousal or PLMS. The analysis during waking may suggest the presence of a basal sympathetic hyperactivity as a consequence of chronic PLMS activation during sleep.
- (b)
- The same analysis was performed during stable sleep periods with PLMS, considering the effect of age, sex, PLMS duration, interval, periodicity, density and EMG activity, in order to assess the factor that might play a key role in the increase of sympathetic activity.
2.5. HR Response to Leg Movements
2.6. Analysis of Leg Muscular Activity (EMG)
2.7. Statistical Analyses
3. Results
3.1. Clinical Data and Sleep Parameters
3.2. Muscular EMG Power
3.3. HRV Analysis During Sleep
3.4. Autonomic Response to PLMS
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allen, R.P.; Walters, A.S.; Montplaisir, J.; Hening, W.; Myers, A.; Bell, T.J.; Ferini-Strambi, L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005, 165, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.; Hening, W.A.; Trenkwalder, C.; Walters, A.S.; Montplaisir, J. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003, 4, 101–119. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Finn, L.; Young, T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006, 7, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Bharmal, M.; Calloway, M. Prevalence and disease burden of primary restless legs syndrome: Results of a general population survey in the United States. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.; Shen, W.K.; Sofi, A.; Jahangir, A.; Mori, N.; Tajik, A.J.; Jahangir, A. Frequent periodic leg movement during sleep is associated with left ventricular hypertrophy and adverse cardiovascular outcomes. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2013, 26, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Strus, J.; Ming, X.; Lee, I.A.; Chokroverty, S.; Walters, A.S. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2007, 118, 1923–1930. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Walters, A.S.; Sica, D. The relationship among restless legs syndrome (Willis-Ekbom Disease), hypertension, cardiovascular disease, and cerebrovascular disease. J. Neurol. 2014, 261, 1051–1068. [Google Scholar] [CrossRef]
- Bertisch, S.M.; Muresan, C.; Schoerning, L.; Winkelman, J.W.; Taylor, J.A. Impact of restless legs syndrome on cardiovascular autonomic control. Sleep 2016, 39, 565–571. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Shahar, E.; Sharief, I.; Gottlieb, D.J. Association of restless legs syndrome and cardiovascular disease in the sleep heart health study. Neurology 2008, 70, 35–42. [Google Scholar] [CrossRef]
- Koo, B.B.; Blackwell, T.; Ancoli-Israel, S.; Stone, K.L.; Stefanick, M.L.; Redline, S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: Outcomes of sleep disorders in older men (MrOS) study. Circulation 2011, 124, 1223–1231. [Google Scholar] [CrossRef]
- Li, Y.; Walters, A.S.; Chiuve, S.E.; Rimm, E.B.; Winkelman, J.W.; Gao, X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation 2012, 126, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.C.; Schurks, M.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M.; Berger, K.; Kurth, T. Restless legs syndrome and risk of incident cardiovascular disease in women and men: Prospective cohort study. BMJ Open 2012, 2, e000866. [Google Scholar] [CrossRef] [PubMed]
- Giannini, G.; Zanigni, S.; Melotti, R.; Gogele, M.; Provini, F.; Facheris, M.F.; Cortelli, P.; Pramstaller, P.P. Association between restless legs syndrome and hypertension: A preliminary population-based study in South Tyrol, Italy. Eur. J. Neurol. 2014, 21, 72–78. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Albers, K.B.; Davidson, J.E.; Kushida, C.A.; Leimpeter, A.D.; Nelson, L.M.; Popat, R.; Tanner, C.M.; Bibeau, K.; Quesenberry, C.P. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from kaiser permanente Northern California. Sleep 2015, 38, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Nannapaneni, S.; Ramar, K. Periodic limb movements during sleep and their effect on the cardiovascular system: Is there a final answer? Sleep Med. 2014, 15, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Allen, R.; Hogl, B.; Paulus, W.; Winkelmann, J. Restless legs syndrome associated with major diseases: A systematic review and new concept. Neurology 2016, 86, 1336–1343. [Google Scholar] [CrossRef]
- Sforza, E.; Juony, C.; Ibanez, V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: Implications for arousal mechanisms. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 883–891. [Google Scholar] [CrossRef]
- Pennestri, M.H.; Montplaisir, J.; Colombo, R.; Lavigne, G.; Lanfranchi, P.A. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology 2007, 68, 1213–1218. [Google Scholar] [CrossRef]
- Palma, J.A.; Alegre, M.; Valencia, M.; Artieda, J.; Iriarte, J.; Urrestarazu, E. Basal cardiac autonomic tone is normal in patients with periodic leg movements during sleep. J. Neural Transm. 2014, 121, 385–390. [Google Scholar] [CrossRef]
- Walter, L.M.; Foster, A.M.; Patterson, R.R.; Anderson, V.; Davey, M.J.; Nixon, G.M.; Trinder, J.; Walker, A.M.; Horne, R.S. Cardiovascular variability during periodic leg movements in sleep in children. Sleep 2009, 32, 1093–1099. [Google Scholar] [CrossRef]
- Gosselin, N.; Lanfranchi, P.; Michaud, M.; Fantini, L.; Carrier, J.; Lavigne, G.; Montplaisir, J. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 2188–2195. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the american academy of sleep medicine. J. Clin. Sleep Med. Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.L.; Quan, S.F.; The American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Zucconi, M.; Ferri, R.; Allen, R.; Baier, P.C.; Bruni, O.; Chokroverty, S.; Ferini-Strambi, L.; Fulda, S.; Garcia-Borreguero, D.; Hening, W.A.; et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG). Sleep Med. 2006, 7, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Skeba, P.; Hiranniramol, K.; Earley, C.J.; Allen, R.P. Inter-movement interval as a primary stable measure of periodic limb movements of sleep. Sleep Med. 2016, 17, 138–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferri, R.; Rundo, F.; Zucconi, M.; Manconi, M.; Arico, D.; Bruni, O.; Ferini-Strambi, L.; Fulda, S. Diagnostic accuracy of the standard and alternative periodic leg movement during sleep indices for restless legs syndrome. Sleep Med. 2016, 22, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Pichot, V.; Roche, F.; Celle, S.; Barthelemy, J.C.; Chouchou, F. HRVanalysis: A free software for analyzing cardiac autonomic activity. Front. Physiol. 2016, 7, 557. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology; North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Huang, A.S.; Skeba, P.; Yang, M.S.; Sgambati, F.P.; Earley, C.J.; Allen, R.P. MATPLM1, A MATLAB script for scoring of periodic limb movements: Preliminary validation with visual scoring. Sleep Med. 2015, 16, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; LeBrocq, C.; Dhar, A.; Hening, W.; Rosen, R.; Allen, R.P.; Trenkwalder, C. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003, 4, 121–132. [Google Scholar]
- Brandenberger, G.; Viola, A.U.; Ehrhart, J.; Charloux, A.; Geny, B.; Piquard, F.; Simon, C. Age-related changes in cardiac autonomic control during sleep. J. Sleep Res. 2003, 12, 173–180. [Google Scholar] [CrossRef]
- Izzi, F.; Placidi, F.; Romigi, A.; Lauretti, B.; Marfia, G.A.; Mercuri, N.B.; Marciani, M.G.; Rocchi, C. Is autonomic nervous system involved in restless legs syndrome during wakefulness? Sleep Med. 2014, 15, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Hogl, B.; Kiechl, S.; Willeit, J.; Saletu, M.; Frauscher, B.; Seppi, K.; Muller, J.; Rungger, G.; Gasperi, A.; Wenning, G.; et al. Restless legs syndrome: A community-based study of prevalence, severity, and risk factors. Neurology 2005, 64, 1920–1924. [Google Scholar] [CrossRef] [PubMed]
- Rothdach, A.J.; Trenkwalder, C.; Haberstock, J.; Keil, U.; Berger, K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology 2000, 54, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Yatsu, S.; Kasai, T.; Suda, S.; Matsumoto, H.; Shiroshita, N.; Kato, M.; Kawana, F.; Murata, A.; Kato, T.; Hiki, M.; et al. Impact on clinical outcomes of periodic leg movements during sleep in hospitalized patients following Acute decompensated heart failure. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, I.; Erikh, I.; Nassar, M.; Sprecher, E. Restless legs syndrome in stroke patients. Sleep Med. 2015, 16, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, A.G.; Hess, C.W.; Mathis, J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep 2007, 30, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Rye, D.B.; Hicks, A.; Petursson, H.; Ingason, A.; Thorgeirsson, T.E.; Palsson, S.; Sigmundsson, T.; Sigurdsson, A.P.; Eiriksdottir, I.; et al. A genetic risk factor for periodic limb movements in sleep. N. Engl. J. Med. 2007, 357, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Thireau, J.; Farah, C.; Molinari, N.; Bouilloux, F.; Torreilles, L.; Winkelmann, J.; Scholz, S.; Richard, S.; Dauvilliers, Y.; Marmigere, F. MEIS1 variant as a determinant of autonomic imbalance in Restless Legs Syndrome. Sci. Rep. 2017, 7, 46620. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
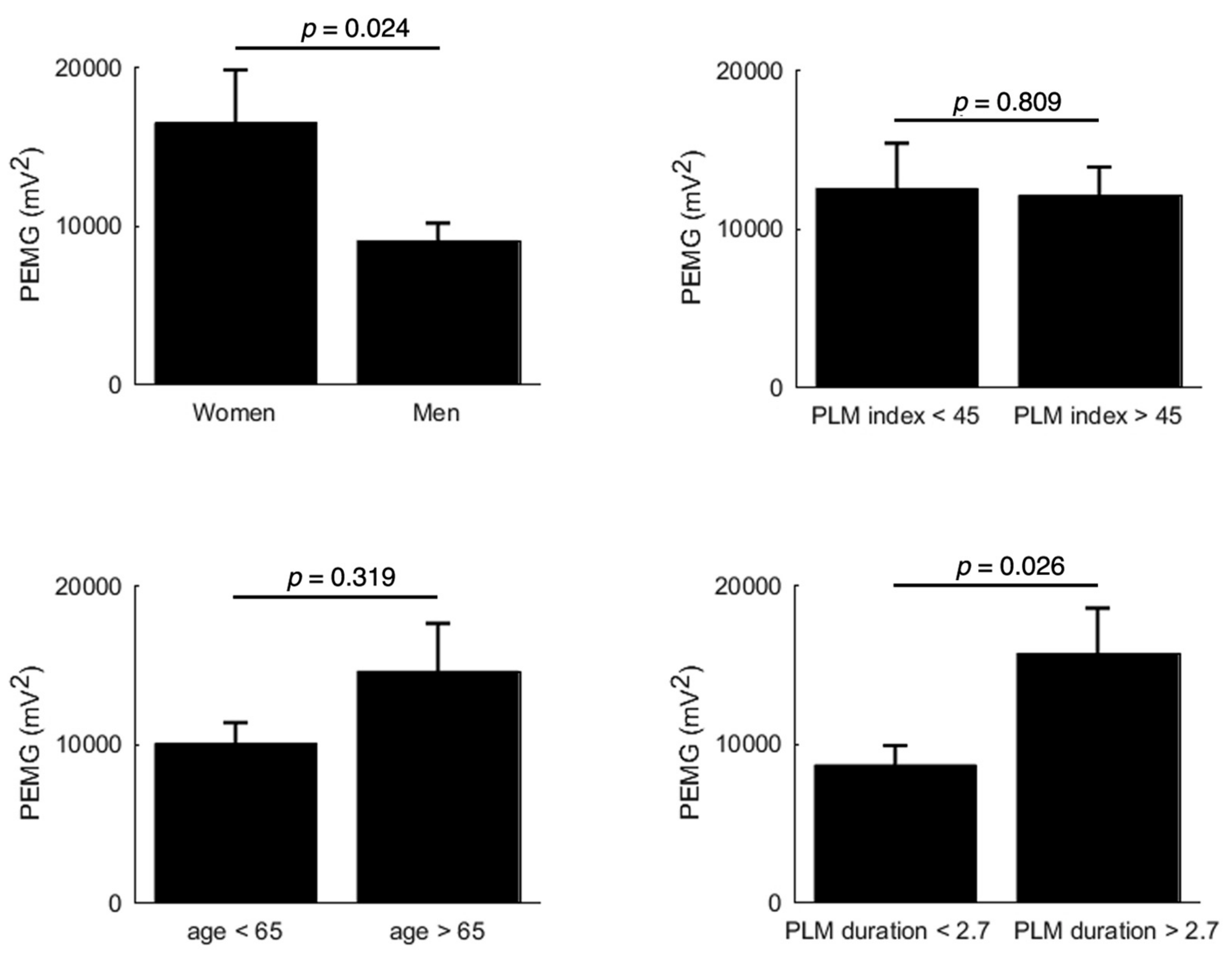
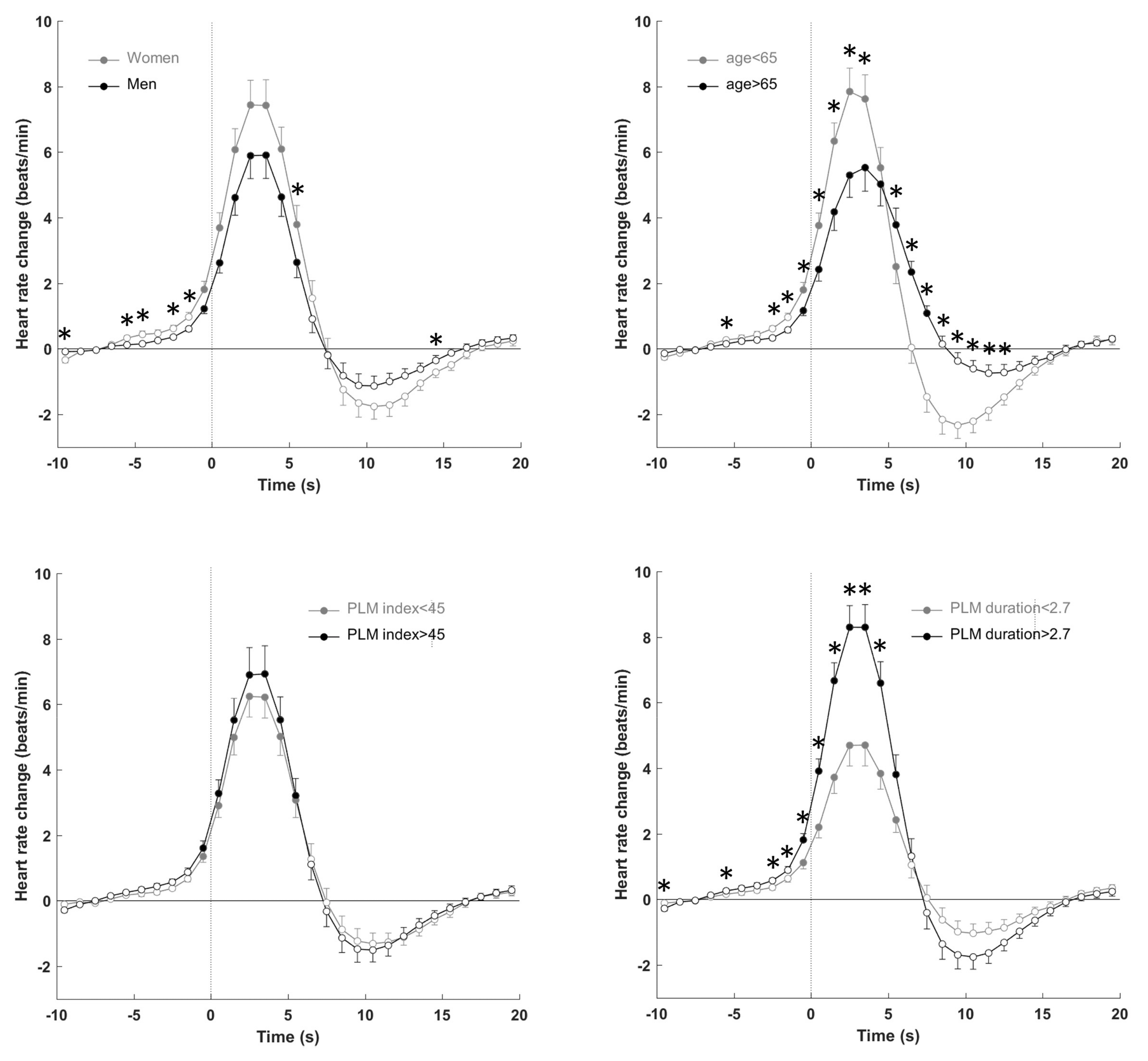
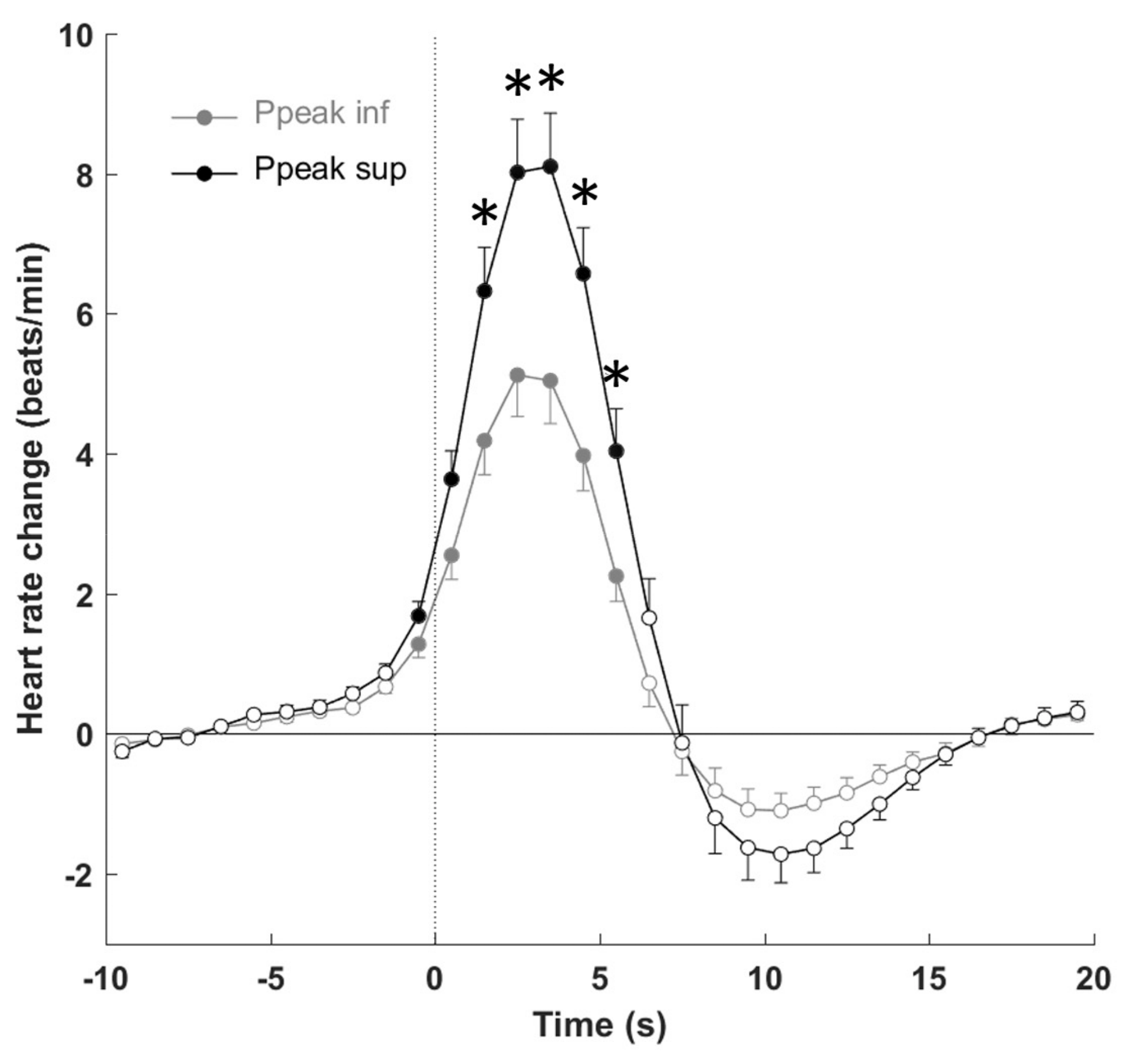
| Variable | All (n = 50) | Women (n = 22) | Men (n = 28) | p |
|---|---|---|---|---|
| Age (y) | 62.6 ± 11.1 | 63.1 ± 10.7 | 62.1 ± 11.6 | 0.77 |
| Total sleep time (min) | 394.6 ± 74.4 | 392.3 ± 59.2 | 396.3 ± 85.2 | 0.85 |
| WASO (min) | 99.2 ± 63.8 | 101.8 ± 56.7 | 97.2 ± 69.9 | 0.80 |
| Awakenings (n) | 25.8 ± 12.0 | 22.3 ± 9.2 | 28.5 ± 13.4 | 0.07 |
| Sleep efficiency (%) | 77.1 ± 12.9 | 77.4 ± 10.5 | 76.9 ± 14.8 | 0.89 |
| Sleep latency (min) | 16.7 ± 28.5 | 15.6 ± 24.3 | 17.7 ± 32.0 | 0.79 |
| REM latency (min) | 123.5 ± 61.8 | 128.7 ± 62.5 | 119.4 ± 62.1 | 0.60 |
| Stage 1 (%) | 12.1 ± 6.5 | 10.2 ± 3.3 | 13.6 ± 7.9 | 0.07 |
| Stage 2 (%) | 53.2 ± 7.8 | 53.3 ± 7.9 | 53.1 ± 7.9 | 0.96 |
| Light sleep (%) | 65.3 ± 8.8 | 63.4 ± 9.1 | 66.7 ± 8.5 | 0.19 |
| SWS (%) | 14.8 ± 6.7 | 16.5 ± 6.4 | 13.6 ± 6.7 | 0.13 |
| REM (%) | 19.8 ± 5.9 | 20.1 ± 6.0 | 19.7 ± 5.8 | 0.80 |
| PLMS index (n/h) | 45.7 ± 24.7 | 45.4 ± 22.1 | 45.9 ± 26.9 | 0.94 |
| PLMS duration (s) | 2.82 ± 0.95 | 3.03 ± 0.73 | 2.66 ± 1.08 | 0.17 |
| PLMS IMI (s) | 23.9 ± 4.5 | 23.7 ± 3.8 | 24.0 ± 5.0 | 0.83 |
| PLMS PI (s) | 0.70 ± 0.13 | 0.71 ± 0.11 | 0.70 ± 0.15 | 0.91 |
| Without PLMS | With PLMS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wake | Light Sleep | SWS | REM | Non-REM | Light Sleep | SWS | REM | Non-REM | |
| HR (bpm) | 74.5 ± 11.8 | 62.1 ± 10.2 +++ | 62.9 ± 9.5 +++ | 60.7 ± 8.8 +++ | 62.2 ± 9.2 +++ | 63.1 ± 9.3 +++ | 62.5 ± 7.9 +++ | 61.9 ± 9.0 +++ | 63.3 ± 9.1 +++ |
| SDNN (ms) | 42.1 ± 16.1 | 35.4 ± 15.1 | 30.0 ± 15.6 +++ | 48.7 ± 19.8 | 33.0 ± 14.0 + | 56.8 ± 27.9 *** | 49.3 ± 26.5 *** | 66.2 ± 30.0 *++ | 55.5 ± 27.0 *** |
| pNN50 (%) | 5.4 ± 8.8 | 10.2 ± 12.5 | 9.9 ± 14.7 | 9.3 ± 11.5 | 9.7 ± 12.1 | 13.3 ± 14.4 + | 10.8 ± 14.0 | 14.1 ± 13.2 | 12.9 ± 14.0 |
| rMSSD (ms) | 23.5 ± 13.6 | 28.1 ± 14.3 | 28.2 ± 19.8 | 29.9 ± 17.5 | 27.5 ± 14.2 | 34.3 ± 19.3 ++ | 31.7 ± 19.5 | 37.7 ± 21.2 + | 34.0 ± 18.9 + |
| Ptot (ms2) | 794 ± 800 | 651 ± 603 | 487 ± 561 ++ | 1077 ± 967 | 583 ± 548 | 1841 ± 1780 ***++ | 1441 ± 1485 *** | 2102 ± 2045 *++ | 1764 ± 1681 ***++ |
| VLF (ms2) | 390 ± 489 | 232 ± 250 + | 141 ± 199 +++ | 530 ± 521 | 197 ± 223 +++ | 573 ± 512 *** | 431 ± 444 *** | 900 ± 816 ++ | 549 ± 477 *** |
| LF (ms2) | 256 ± 219 | 259 ± 306 | 175 ± 260 ++ | 374 ± 364 | 226 ± 270 | 1069 ± 1153 ***+++ | 834 ± 931 ***++ | 931 ± 1182 *++ | 1018 ± 1084 ***+++ |
| HF (ms2) | 78 ± 85 | 130 ± 126 | 143 ± 192 | 110 ± 120 | 131 ± 118 | 165 ± 189 + | 153 ± 214 | 192 ± 218 | 166 ± 191 + |
| LF/HF | 5.67 ± 4.85 | 2.55 ± 1.81 +++ | 1.83 ± 1.73 +++ | 5.56 ± 5.04 | 2.38 ± 1.73 +++ | 8.89 ± 6.57 ***+ | 7.37 ± 5.88 *** | 7.14 ± 4.85 | 8.51 ± 6.47 ***+ |
| Light Sleep | Slow Wave Sleep | REM | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| HR (bpm) | 64.1 ± 8.0 | 62.3 ± 10.3 | 64.4 ± 6.8 | 61.3 ± 8.4 | 62.2 ± 7.3 | 61.5 ± 11.7 |
| SDNN (ms) | 59.3 ± 24.3 | 54.8 ± 30.8 | 48.5 ± 26.5 | 49.8 ± 27.0 | 69.2 ± 29.7 | 61.7 ± 31.8 |
| pNN50 (%) | 14.6 ± 15.7 | 12.2 ± 13.4 | 10.0 ± 17.7 | 11.4 ± 11.4 | 16.0 ± 13.9 | 11.2 ± 12.3 |
| rMSSD (ms) | 35.9 ± 19.4 | 33.0 ± 19.4 | 30.5 ± 23.2 | 32.4 ± 17.2 | 40.3 ± 21.0 | 33.5 ± 22.2 |
| Ptot (ms2) | 1 908 ± 1646 | 1788 ± 1908 | 1413 ± 1646 | 1459 ± 1407 | 2412 ± 2265 | 1620 ± 1653 |
| VLF (ms2) | 629 ± 523 | 528 ± 509 | 456 ± 535 | 414 ± 385 | 1 040 ± 919 | 682 ± 610 |
| LF (ms2) | 1 053 ± 964 | 1082 ± 1300 | 758 ± 820 | 883 ± 1009 | 1062 ± 1303 | 726 ± 1001 |
| HF (ms2) | 187 ± 236 | 148 ± 145 | 175 ± 311 | 139 ± 124 | 226 ± 258 | 139 ± 132 |
| LF/HF | 8.89 ± 6.56 | 8.88 ± 6.70 | 7.56 ± 5.21 | 7.25 ± 6.38 | 6.84 ± 4.48 | 7.61 ± 5.63 |
| Light sleep | Slow wave sleep | REM | ||||
| <65 y (n = 25) | ≥65 y (n = 25) | <65 y (n = 25) | ≥65 y (n = 25) | <65 y (n = 25) | ≥65 y (n = 25) | |
| HR (bpm) | 63.6 ± 9.9 | 62.5 ± 8.9 | 63.2 ± 8.4 | 61.8 ± 7.3 | 63.8 ± 10.1 | 60.1 ± 7.9 |
| SDNN (ms) | 64.8 ± 32.0 | 48.7 ± 20.9 | 56.5 ± 30.7 | 41.8 ± 19.2 | 79.3 ± 34.7 | 54.2 ± 19.5 |
| pNN50 (%) | 15.7 ± 15.6 | 10.8 ± 12.8 | 14.7 ± 16.9 | 6.8 ± 8.9 | 16.4 ± 14.1 | 12.0 ± 12.7 |
| rMSSD (ms) | 38.3 ± 22.1 | 30.3 ± 15.4 | 36.1 ± 22.7 | 27.0 ± 14.6 | 40.0 ± 21.7 | 35.5 ± 21.5 |
| Ptot (ms2) | 2383 ± 2165 | 1298 ± 1084 | 1875 ± 1807 | 986 ± 883 | 2815 ± 2627 | 1449 ± 1056 |
| VLF (ms2) | 680 ± 603 | 465 ± 386 | 520 ± 511 | 337 ± 349 | 1052 ± 857 | 760 ± 787 |
| LF (ms2) | 1469 ± 1409 | 669 ± 630 * | 1137 ± 1127 | 517 ± 528 | 1460 ± 1542 | 445 ± 304 |
| HF (ms2) | 203 ± 219 | 127 ± 149 | 196 ± 266 | 108 ± 134 | 223 ± 270 | 164 ± 164 |
| LF/HF | 9.30 ± 4.96 | 8.47 ± 7.95 | 6.82 ± 3.85 | 7.94 ± 7.52 | 8.55 ± 5.27 | 5.85 ± 4.24 |
| Light Sleep | Slow Wave Sleep | REM | ||||
|---|---|---|---|---|---|---|
| <45/h (n = 25) | ≥45/h (n = 25) | <45/h (n = 25) | ≥45/h (n = 25) | <45/h (n = 25) | ≥45/h (n = 25) | |
| HR (bpm) | 65.3 ± 10.6 | 60.8 ± 7.4 | 62.7 ± 9.1 | 62.3 ± 7.0 | 66.2 ± 9.0 | 58.0 ± 7.3 * |
| SDNN (ms) | 53.9 ± 23.6 | 59.6 ± 31.9 | 48.8 ± 24.9 | 49.7 ± 28.3 | 60.7 ± 22.4 | 71.2 ± 35.9 |
| pNN50 (%) | 13.4 ± 14.2 | 13.2 ± 14.9 | 10.1 ± 11.8 | 11.4 ± 15.8 | 13.1 ± 11.5 | 15.0 ± 15.1 |
| rMSSD (ms) | 33.6 ± 19.1 | 35.0 ± 19.8 | 31.7 ± 19.7 | 31.6 ± 19.8 | 34.0 ± 18.2 | 41.0 ± 24.0 |
| Ptot (ms2) | 1572 ± 1220 | 2110 ± 2198 | 1324 ± 1165 | 1533 ± 1715 | 1554 ± 1005 | 2605 ± 2621 |
| VLF (ms2) | 495 ± 385 | 650 ± 613 | 466 ± 425 | 403 ± 466 | 674 ± 485 | 1107 ± 1010 |
| LF (ms2) | 871 ± 748 | 1267 ± 1440 | 685 ± 661 | 951 ± 1098 | 631 ± 568 | 1206 ± 1525 |
| HF (ms2) | 168 ± 170 | 162 ± 210 | 145 ± 164 | 159 ± 250 | 178 ± 153 | 205 ± 271 |
| LF/HF | 7.95 ± 5.18 | 9.82 ± 7.71 | 6.54 ± 4.84 | 8.01 ± 6.62 | 6.57 ± 4.96 | 7.66 ± 4.90 |
| Light sleep | Slow wave sleep | REM | ||||
| <2.7 s (n = 24) | ≥2.7 s (n = 26) | <2.7 s (n = 24) | ≥2.7 s (n = 26) | <2.7 s (n = 24) | ≥2.7 s (n = 26) | |
| HR (bpm) | 60.8 ± 9.3 | 65.1 ± 9.1 | 61.2 ± 7.3 | 63.9 ± 8.4 | 57.5 ± 9.1 | 64.8 ± 8.0 |
| SDNN (ms) | 48.7 ± 26.1 | 64.1 ± 28.0 * | 42.6 ± 21.2 | 57.1 ± 30.3 | 53.5 ± 20.5 | 74.4 ± 32.9 |
| pNN50 (%) | 9.9 ± 11.5 | 16.4 ± 16.2 | 8.2 ± 10.0 | 13.8 ± 17.4 | 11.1 ± 12.2 | 16.0 ± 13.9 |
| rMSSD (ms) | 29.9 ± 16.4 | 38.4 ± 21.1 | 28.6 ± 15.9 | 35.2 ± 22.9 | 33.6 ± 21.4 | 40.3 ± 21.5 |
| Ptot (ms2) | 1409 ± 1648 | 2239 ± 1835 * | 1055 ± 1110 | 1888 ± 1752 | 1429 ± 1090 | 2535 ± 2415 |
| VLF (ms2) | 468 ± 507 | 669 ± 508 * | 325 ± 346 | 553 ± 518 | 791 ± 846 | 970 ± 820 |
| LF (ms2) | 811 ± 1071 | 1307 ± 1195 * | 595 ± 740 | 1112 ± 1065 | 450 ± 317 | 1239 ± 1428 |
| HF (ms2) | 105 ± 93 | 221 ± 236 | 114 ± 135 | 198 ± 276 | 121 ± 135 | 238 ± 252 |
| LF/HF | 8.44 ± 6.50 | 9.30 ± 6.74 | 6.93 ± 5.21 | 7.87 ± 6.68 | 7.05 ± 4.39 | 7.20 ± 5.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sforza, E.; Roche, F.; Pichot, V. Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS). J. Clin. Med. 2019, 8, 1619. https://doi.org/10.3390/jcm8101619
Sforza E, Roche F, Pichot V. Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS). Journal of Clinical Medicine. 2019; 8(10):1619. https://doi.org/10.3390/jcm8101619
Chicago/Turabian StyleSforza, Emilia, Frédéric Roche, and Vincent Pichot. 2019. "Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS)" Journal of Clinical Medicine 8, no. 10: 1619. https://doi.org/10.3390/jcm8101619
APA StyleSforza, E., Roche, F., & Pichot, V. (2019). Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS). Journal of Clinical Medicine, 8(10), 1619. https://doi.org/10.3390/jcm8101619




