Has The Time Arrived to Refine The Indications of Immunosuppressive Therapy and Prognosis in IgA Nephropathy?
Abstract
1. Introduction
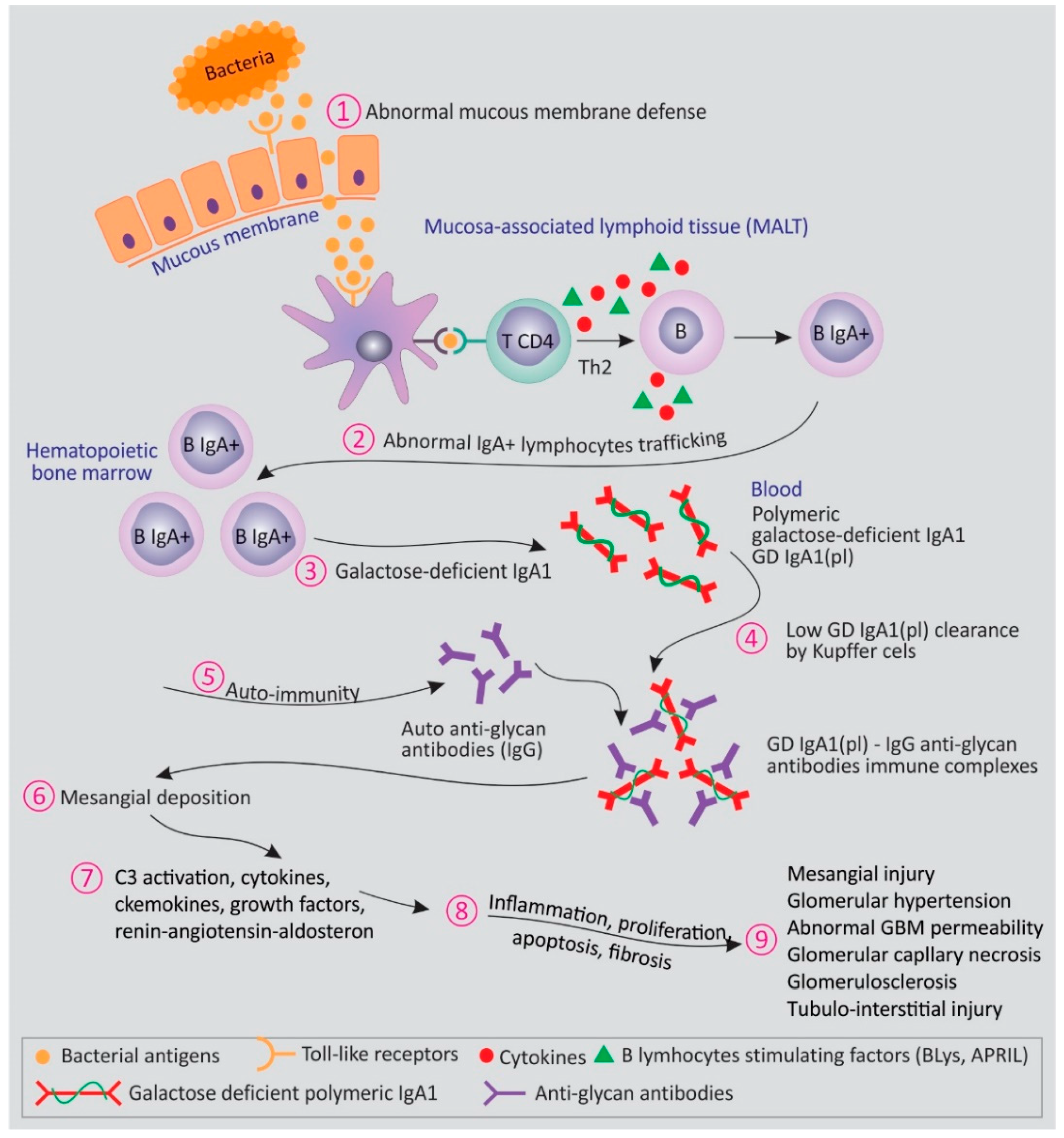
2. Transitioning from Pathogenesis of IgA Nephropathy to Prognosis and Treatment of IgA Nephropathy
2.1. Traditional Risk Factors for Disease Progression
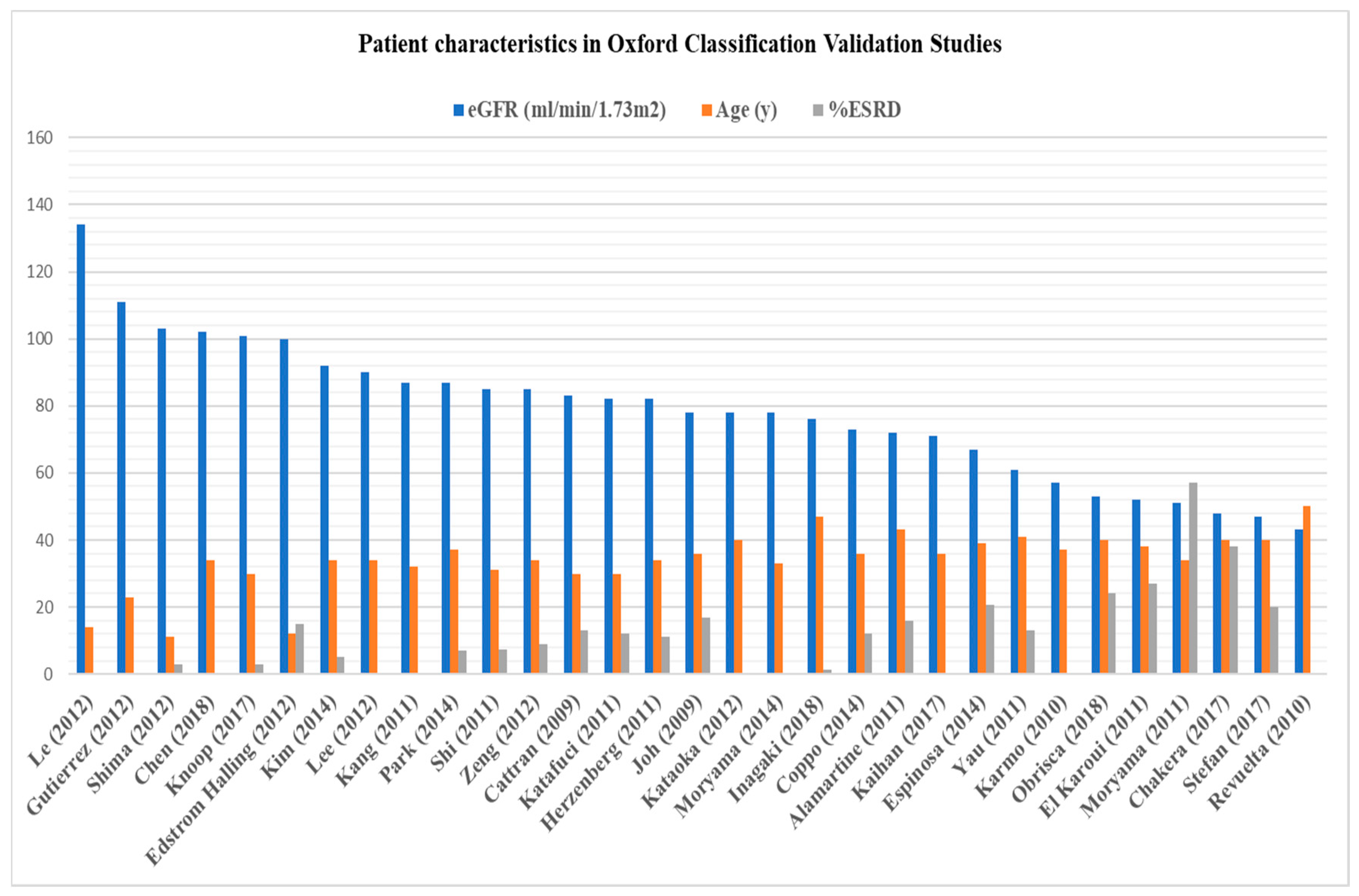
2.2. Oxford Classification of IgA Nephropathy: Where Do We Stand Today?
2.3. Novel and Emerging Tests
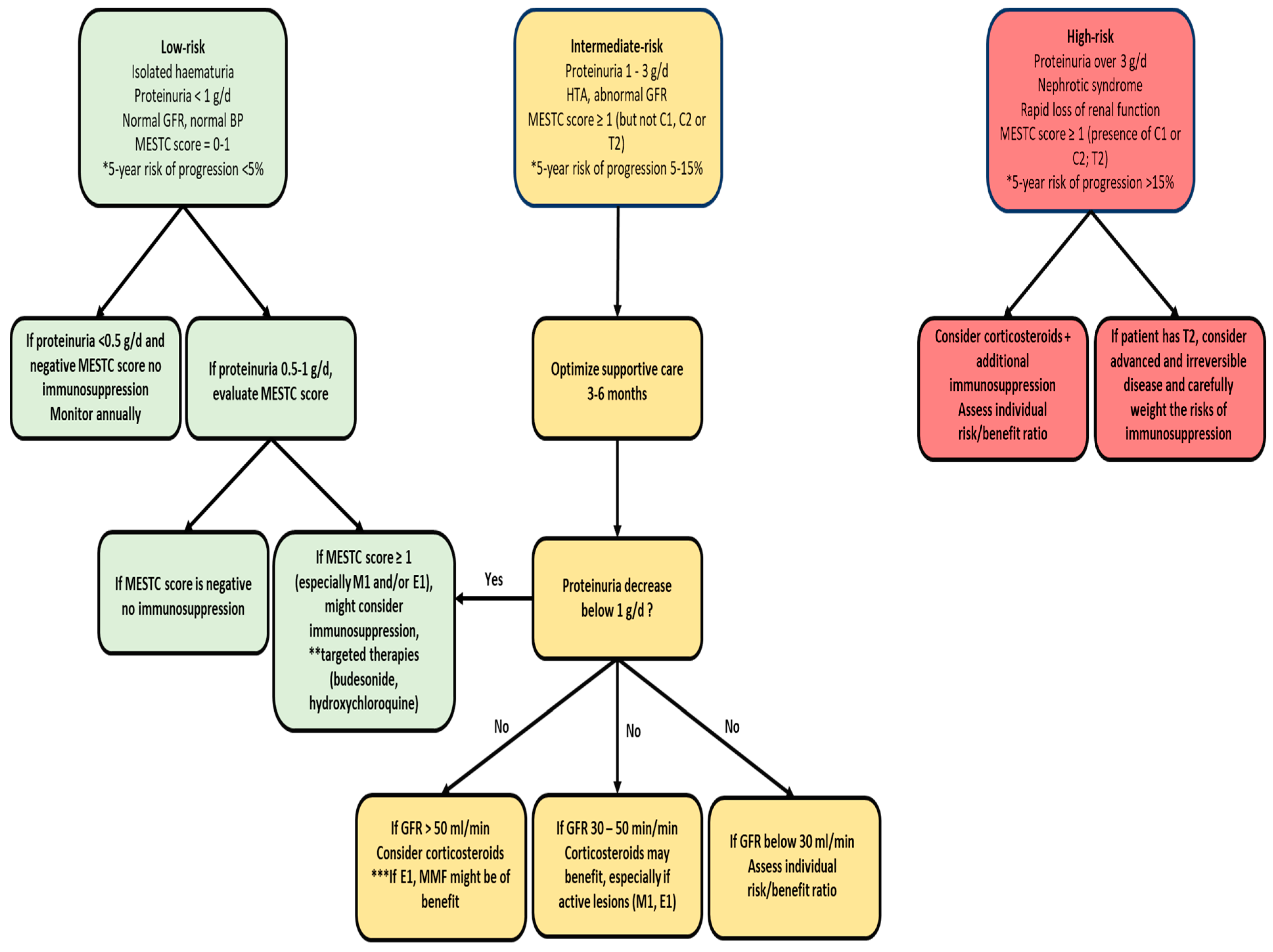
3. Treatment of IgA Nephropathy
3.1. Corticosteroids
3.2. Immunosuppressants
3.3. Future Directions
4. Conclusions
Author Contributions
Conflicts of Interest
Appendix A
| (A) | ||||||
| Cattran (2009) [42] | Joh (2009) [43] | Karmo (2010) [50] | Revuelta (2010) [50] | Lee (2010) [50] | El Karoui (2011) [44] | |
| No. of pts. | 265 | 233 | 62 | 62 | 181 | 128 |
| Ethnicity | Multicountry | Japan | Brazil | Spain | Korea | France |
| Follow-up (mos.) | 69 | 127 | 57 | N/A | 12 | 44 |
| Age (y) | 30 (4–73) | 36 (18–70) | 37 ± 13.6 | 50 | 37 (18–77) | 38.7 (18–78) |
| Serum Creatinine (mg/dL) | N/A | N/A | N/A | 1.66 | N/A | N/A |
| eGFR (mL/min/1.73m2) | 83 ± 36 | 78 | 57 ± 34 | 43 | N/A | 52.1 |
| 24-h Proteinuria (g/d) | 1.7 (0.5–18.5) | 1.3 | 2.9 ± 2.6 | 1.54 | N/A | 2.43 |
| RAS blockade (%) | 74% | 78% | N/A | N/A | N/A | 99% |
| IS therapy (%) | 29% | 35% | N/A | N/A | N/A | 0.8% |
| Lesions (%) | ||||||
| M1 | 80 | N/A | 79 | 97 | 52.5 | 33 |
| E1 | 42 | N/A | 25 | 48 | N/A | 25 |
| S1 | N/A | N/A | N/A | 40 | 48 | 69 |
| T1/T2 | N/A | N/A | N/A | 40/3 | 35 (T1 + 2) | 26/23 |
| C1/C2 | 45 (any crescents) | N/A | 35 (any crescents) | N/A/11 | N/A | 24.2 (any crescents) |
| Outcome | ||||||
| ESRD (%) | 13 | 17 | N/A | N/A | N/A | 27 |
| Composite endpoint(%) 1 | 22 | 25 | N/A | 22 | N/A | 32 |
| Lesions associated with outcomes | M, S, T | N/A | S, T | E, T | T | M, E, S, T |
| IS bias | Yes | N/A | N/A | N/A | N/A | No |
| Observations | Children and adults | Liver disease, Henoch–Schonlein purpura | ||||
| (B) | ||||||
| Kang (2011) [51] | Shi (2011) [52] | Katafuci (2011) [53] | Moryama (2011) [54] | Alamartine (2011) [55] | Herzenberg (2011) [56] | |
| No. of pts. | 197 | 410 | 702 | 42 | 183 | 187 |
| Ethnicity | Korean | Chinese | Japanese | Japanese | France | North America |
| Follow-up (mos.) | 56.8 | 38 | 62 | N/A | 77 | 53 |
| Age (y) | 32.4 ± 12 | 31 ± 10.8 | 30 (8 to 82) | 34.2 ± 12.6 | 43 ± 16 | 34 (18–45) |
| Serum creatinine (mg/dL) | 1.09 ± 0.72 | N/A | N/A | 1.3 ± 0.73 | N/A | N/A |
| eGFR (mL/min/1.73m2) | 87.1 ± 29.2 | 85.8 ± 28.1 | 82 ± 35 | 51.1 ± 24.6 | 72 ± 32 | 82 ± 37 |
| 24-h Proteinuria (g/d) | 2.07 ± 2.81 | 1.7 (0.5–21.8) | 0.85 (0–17) | 5.7 ± 2.5 | 1.24 ± 1.5 | 1.7 (1–2.9) |
| RAS blockade (%) | 82.7% | 86% | 37% | 55% | 65% | 87% |
| IS therapy (%) | 38.1% | 42.7% (steroids) 20% (other IS) | 32% (steroids only) | 64% | 31% | 41% |
| Lesions (%) | ||||||
| M1 | 26 | 56 | 12 | 60 | 21 | N/A |
| E1 | 11 | 57 | 42 | 43 | 14 | N/A |
| S1 | 56 | 75 | 79 | 83 | 54 | N/A |
| T1/T2 | 26/7.6 | 14/8 | 18/12 | 40/26 | 20/10 | N/A |
| C1/C2 | N/A | 60 (any crescents) | 63 (any crescents) | N/A | 5 (any crescents) | N/A |
| Outcome | ||||||
| ESRD (%) | N/A | 7.3 | 12 | 57 | 16 | 11 |
| Composite endpoint(%) 1 | 8 | 7.3 | 12 | 57 | 20 | 14 |
| Lesions associated with outcomes | T | S, T | S, T, C | T | None | E, S, T |
| IS bias | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | Children and adults | |||||
| (C) | ||||||
| Yau (2011) [57] | Edstrom Halling (2012) [58] | Kataoka (2012) [59] | Le (2012) [60] | Gutierrez (2012) [61] | Nasri (2012) [62] | |
| No. of pts. | 54 | 99 | 43 | 218 | 141 | 102 |
| Ethnicity | US | Sweden | Japan | China | Spain | Iran |
| Follow-up (mos.) | 70 | 156 | 120 | 56 | 108 | N/A |
| Age (y) | 41 ± 15 | 12 ± 3.6 | 40 ± 10 | 14 (2–17) | 23.7 ± 14.8 | 37± 13 |
| Serum creatinine (mg/dL) | 1.5 ± 0.8 | N/A | N/A | N/A | 0.8 ± 0.2 | 1.65 ± 1.61 |
| eGFR (mL/min/1.73 m2) | 61 ± 24 | 100 ± 31 | 78 ± 17 | 134 ± 42 | 111 ± 31 | N/A |
| 24-h Proteinuria (g/d) | 2 ± 1.6 | 2 | 1.8 ± 1.5 | 1.5 (0.5–8) | 0.2 (0.1–0.4) | 1.79 ± 1.36 |
| RAS blockade (%) | 78% | 35% | 58% | 61% | 41.8% | N/A |
| IS therapy (%) | 35% | 11% | 51% | 56% | None | N/A |
| Lesions (%) | ||||||
| M1 | 72 | 30 | 81 | 45 | 32.6 | 66 |
| E1 | 20 | 10 | 53 | 23 | 8.5 | 32 |
| S1 | 81 | 23 | 81 | 62 | 15.6 | 67 |
| T1/T2 | 13/22 | 12/3 | N/A | 6/1 | 5/0 | 30/19 |
| C1/C2 | 19 (any crescents) | 18 (any crescents) | 53 (any crescents) | 44 (any crescents) | N/A | 23 (any crescents) |
| Outcome | ||||||
| ESRD (%) | 13 | 15 | 0 | N/A | 0 | N/A |
| Composite endpoint (%) 1 | 19 | 18 | 37 | 12 | 0.7 | N/A |
| Lesions associated with outcomes | T | None | M | T | S | Not done |
| IS bias | Yes | Yes | Yes | Yes | No | N/A |
| Observations | Pediatric cohort | Pediatric cohort | eGFR ≥ 60 mL/min and proteinuria below 0.5 g/d | |||
| (D) | ||||||
| Zeng (2012) [63] | Lee (2012) [64] | Shima (2012) [65] | Tanaka (2013) [66] | Espinosa (2014) [84] | Kim (2014) [46] | |
| No. of pts. | 1026 | 69 | 161 | 698 | 283 | 61 |
| Ethnicity | China | Korea | Japan | Japan | Spain | Korea |
| Follow-up (mos.) | 53 | 85 | 54 | 56.4 | 72 | 49.3 |
| Age (y) | 34 (18–73) | 34 (27–45) | 11.7 (3.6–19.4) | 36.1 ± 15.4 | 39.1 ± 17.2 | 34.1 ± 16.4 |
| Serum creatinine (mg/dL) | N/A | 0.9 ± 0.3 | N/A | N/A | N/A | 0.92 ± 0.21 |
| eGFR (mL/min/1.73m2) | 85 ± 32 | 90 ± 38 | 103 ± 30 | N/A | 67 ± 36 | 92.6 ± 22.4 |
| 24-h Proteinuria (g/d) | 1.3 | 1.2 (0.4–1.9) | 0.7 | N/A | 1.9 (1–3) | 1.69 ± 2.27 |
| RAS blockade (%) | 89% | 90% | N/A | N/A | 79% | 53% |
| IS therapy (%) | 31% | 18% | 16% | N/A | 35% | 61% |
| Lesions (%) | ||||||
| M1 | 43 | 60 | 36 | 12 | 65 | 15 |
| E1 | 11 | 32 | 58 | 35 | 22 | 15 |
| S1 | 83 | 80 | 9 | 70 | 20 | 34 |
| T1/T2 | 24/2.4 | 25/12 | 0.6/0 | 14/7 | 32/28 | 12/2 |
| C1/C2 | 48/2.4 | N/A | 52 (any crescents) | N/A | N/A | 53 (any crescents) |
| Outcome | ||||||
| ESRD (%) | 8.8 | N/A | 3 | 10.5 | 20.5 | 5 |
| Composite endpoint (%) 1 | 15.5 | 23 | 4.3 | 10.5 | 20.5 | 21 |
| Lesions associated with outcomes | M, T | E, T | M, T | M, S, T | T | E, T |
| IS bias | Yes | Yes | Yes | N/A | Yes | Yes |
| Observations | Pediatric cohort | Henoch–Schonlein purpura cohort | ||||
| (E) | ||||||
| Park (2014) [67] | Moryama (2014) [68] | Coppo (2014) [69] | Kaihan (2017) [70] | Chakera (2017) [45] | Stefan (2017) [48] | |
| No. of pts. | 500 | 1012 | 1147 | 86 | 147 | 121 |
| Ethnicity | Korea | Japan | Multicountry | Japan | UK | Romania |
| Follow-up (mos.) | 68 | 95 | 56 | 81 | 82 | 59.7 |
| Age (y) | 37.1 ± 12 | 33 ± 12 | 36 ± 16 | 36 (24–46) | 39.9 ± 14.5 | 40.1 (37.8-42.4) |
| Serum creatinine (mg/dL) | 1.04 ± 0.37 | 0.89 ± 0.42 | N/A | 0.9 (0.7–1.1) | N/A | N/A |
| eGFR (mL/min/1.73m2) | 87.3 ± 28.5 | 78.5 ± 26.2 | 73 ± 30 | 71 (52–92) | 48.7 ± 24.7 | 47 (43–50.4) |
| 24-h Proteinuria (g/d) | 1.45 ± 1.76 | 1.19 ± 1.61 | 1.3 (0.6–2.6) | 1.2 (0.7–1.8) | N/A | 2 (1.7–2.3) |
| RAS blockade (%) | 77.6% | 28.9% | 86% | 84% | 100% | 98% |
| IS therapy (%) | 11% | 40% | 46% | 66% | None | 49% |
| Lesions (%) | ||||||
| M1 | 41 | 47.6 | 28 | 21 | 30 | 72 |
| E1 | 9.6 | 44.3 | 11 | 41 | 20 | 23 |
| S1 | 41.8 | 74.6 | 70 | 67 | 70 | 71 |
| T1/T2 | 10.6/7.2 | 23/5.8 | 17.4/3.6 | 14 (T1+T2) | 26/12 | 79 (T over 25%) |
| C1/C2 | N/A | N/A | 11 (any crescents | 45 (any crescents) | 11 (any crescents) | 31 (any crescents) |
| Outcome | ||||||
| ESRD (%) | 7 | N/A | 12 | 0 | 38.1 | 20 |
| Composite endpoint (%) 1 | 17.4 | N/A | 16 | 15 | N/A | 28 |
| Lesions associated with outcomes | T | None | M, S, T | T | E, T | S, C |
| IS bias | Yes | Yes | Yes | Yes | No | Yes |
| Observations | Children and adults | |||||
| (F) | ||||||
| Knoop (2017) [7] | Chen (2018) [71] | Obrisca (2018) [72] | Inagaki (2018) [47] | |||
| No. of pts. | 145 | 506 | 106 | 74 | ||
| Ethnicity | Norway | China | Romania | Japan | ||
| Follow-up (mos.) | 264 | 50 | 23 | 68 | ||
| Age (y) | 30.1 ± 11.2 | 34.7 ± 9.5 | 40.5 ± 12.5 | 47.8 ± 17.4 | ||
| Serum creatinine (mg/dL) | 0.95 ± 0.17 | 0.9 ± 0.13 | 1.97 ± 1.1 | N/A | ||
| eGFR (mL/min/1.73m2) | 101 ± 18.8 | 102.1 ± 19.8 | 53 ± 30 | 76 ± 25 | ||
| 24-h Proteinuria (g/d) | 0.3 (0–0.5) | 0.56 ± 0.26 | 2.7 ± 2.5 | 1.4 (0.7–2.3) | ||
| RAS blockade (%) | 38.6% | 80% | 88% | 75% | ||
| IS therapy (%) | None | 13.6% (steroid only) | 75% | 82% | ||
| Lesions (%) | ||||||
| M1 | 12.3 | 30.6 | 84 | 6.8 | ||
| E1 | 10.7 | 5.6 | 23 | 51 | ||
| S1 | 23.8 | 69.6 | 56 | 51 | ||
| T1/T2 | 0/0 | 14.8/1.5 | 23/19 | 18.9/5.4 | ||
| C1/C2 | 6.9 (any crescents) | 18.4/0 | 14/8 | 47.3/23 | ||
| Outcome | ||||||
| ESRD (%) | 2.8 | 0 | 24 | 1.3 | ||
| Composite endpoint (%) 1 | 18.6 | 10.6 | 33 | 18.9 | ||
| Lesions associated with outcomes | None | None | T | E | ||
| IS bias | No | No | Yes | Yes | ||
| Observations | eGFR ≥ 60 mL/min and Proteinuria below 1 g/d | eGFR ≥ 60 mL/min and Proteinuria below 1 g/d | Henoch–Schonlein purpura cohort | |||
References
- Coppo, R. Treatment of IgA nephropathy: Recent advances and prospects. Nephrol. Ther. 2018, 14, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, A.; Franssen, C.F.; de Vries, C.S. The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol. Dial. Transplant. 2011, 26, 414–430. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.M.; Hogan, S.L.; Thompson, B.D.; Coppo, R.; Fogo, A.B.; Jennette, J.C. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol. Dial. Transplant. 2018, 33, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Obrișcă, B.; Ștefan, G.; Gherghiceanu, M.; Mandache, E.; Ismail, G.; Stancu, S.; Mircescu, G.; Boitan, B.; Ion, I. ‘Associated’ or ‘ Secondary’ IgA nephropathy? An outcome analysis. PLoS ONE 2019, 14, e0221014. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin. Nephrol. 2004, 24, 179–196. [Google Scholar] [CrossRef]
- Geddes, C.C.; Rauta, V.; Gronhagen-Riska, C.; Bartosik, L.P.; Jardine, A.G.; Ibels, L.S.; Cattran, D.C.; Pei, Y. A tricontinental view of IgA nephropathy. Nephrol. Dial. Transplant. 2003, 18, 1541–1548. [Google Scholar] [CrossRef]
- Knoop, T.; Vikse, B.E.; Mwakimonga, A.; Leh, S.; Bjørneklett, R. Long-term outcome in 145 patients with assumed benign immunoglobulin A nephropathy. Nephrol. Dial. Transpl. 2017, 32, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Magistroni, R.; D’Agati, V.D.; Appel, G.B.; Kiryluk, K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int. 2015, 88, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.D. Pathology of IgA nephropathy. Nat. Rev. Nephrol. 2014, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. CJASN Glomerular Disease Education Series: IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Canetta, P.A.; Kiryluk, K.; Appel, G.B. Glomerular diseases: Emerging tests and therapies for IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012, 2, 139–274. [Google Scholar]
- Floege, J.; Feehally, J. Treatment of IgA nephropathy and Henoch-Schonlein nephritis. Nat. Rev. Nephrol. 2013, 9, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, H.; Chen, Y.; Li, G.; Jiang, L.; Singh, A.K.; Wang, H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am. J. Kidney Dis. 2009, 53, 26–92. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Torres, D.D.; Rossini, M.; Pesce, F.; Schena, F.P. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transpl. 2009, 24, 3694–3701. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Andrulli, S.; Del Vecchio, L.; Melis, P.; Fogazzi, G.B.; Altieri, P.; Locatelli, F.; Ponticelli, C. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J. Am. Soc. Nephrol. 2004, 15, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tesar, V.; Troyanov, S.; Bellur, S.; Verhave, J.C.; Cook, H.T.; Feehally, J.; Coppo, R.; Roberts, I.S.D.; Cattran, D. Corticosteroids in IgA nephropathy: A retrospective analysis from the VALIGA study. J. Am. Soc. Nephrol. 2015, 26, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Kuhlmann, U.; Panzer, U.; Peters, H.; Mertens, P.R.; et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N. Engl. J. Med. 2015, 373, 2225–2236. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, H.; Wong, M.G.; Jardine, M.J.; Hladunewich, M.; Jha, V.; Cattran, D.; Monaghan, H.; Zhao, M.; Barbour, S.; et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING Randomized Clinical Trial. JAMA 2017, 318, 432–442. [Google Scholar] [CrossRef]
- Rauen, T.; Eitner, F.; Fitzner, C.; Floege, J. Con: STOP immunosuppression in IgA nephropathy. Nephrol. Dial. Transplant. 2016, 31, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C. Pro: STOP immunosuppression in IgA nephropathy? Nephrol. Dial. Transpl. 2016, 31, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J. Moderator’s view: Treatment of IgA nephropathy—Getting comfortable with uncertainty. Nephrol. Dial. Transpl. 2016, 31, 1776–1780. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012, 8, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Maillard, N.; Wyatt, R.J.; Julian, B.A.; Kiryluk, K.; Gharavi, A.; Fremeaux-Bacchi, V.; Novak, J. Current understanding of the role of complement in IgA nephropathy. J. Am. Soc. Nephrol. JASN 2015, 26, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Julian, B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhang, H. Insights into the Role of Mucosal Immunity in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2018, 13, 1584–1586. [Google Scholar] [CrossRef]
- Rops, A.L.; Jansen, E.; van der Schaaf, A.; Pieterse, E.; Rother, N.; Hofstra, J.; van Spriel, A.B.; Dijkman, H.B.P.M.; van de Logt, A.-E.; Wetzels, J.; et al. Interleukin-6 is essential for glomerular immunoglobulin A deposition and the development of renal pathology in Cd37-deficient mice. Kidney Int. 2018, 93, 1356–1366. [Google Scholar] [CrossRef]
- Nguyen, C.; König, K.; Tam, F.W.; Hopfer, H.; Molyneux, K.; Binet, F.I.; Kim, M.J. Higher serum galactose-deficient immunoglobulin A1 concentration is associated with stronger mesangial cellular inflammatory response and more severe histologic findings in immunoglobulin A nephropathy. Clin. Kidney J. 2019, 12, 232–238. [Google Scholar] [CrossRef]
- Leung, J.C.; Lai, K.N.; Tang, S.C. Role of Mesangial-Podocytic-Tubular Cross-Talk in IgA Nephropathy. Semin. Nephrol. 2018, 38, 485–495. [Google Scholar] [CrossRef]
- Coppo, R. The Gut-Renal Connection in IgA Nephropathy. Semin. Nephrol. 2018, 38, 504–512. [Google Scholar] [CrossRef]
- Coppo, R. Clinical and histological risk factors for progression of IgA nephropathy: An update in children, young and adult patients. J. Nephrol. 2017, 30, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Reich, H.N. Risk Stratification of Patients With IgA Nephropathy. Am. J. Kidney Dis. 2012, 59, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.N.; Troyanov, S.; Scholey, J.W.; Cattran, D.C. Remission of proteinuria improves prognosis in IgA nephropathy. J. Am. Soc. Nephrol. 2007, 18, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Reich, H.N.; Beanlands, H.J.; Miller, J.A.; Scholey, J.W.; Troyanov, S. The impact of sex in primary glomerulonephritis. Nephrol. Dial. Transplant. 2008, 23, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Obrisca, B.; Ismail, G.; Jurubita, R.; Baston, C.; Andronesi, A.; Mircescu, G. Antiphospholipase A2 Receptor Autoantibodies: A Step Forward in the Management of Primary Membranous Nephropathy. Biomed. Res. Int. 2015, 2015, 249740. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Carroll, K.; Inker, L.A.; Floege, J.; Perkovic, V.; Boyer-Suavet, S.; Gillespie, B.S.; Major, R.W.; Schimpf, J.I.; Barratt, J.; et al. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2019, 14, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, A.M.; Gutiérrez, E.; Yuste, C.; Cavero, T.; Mérida, E.; Rodríguez, P.; Moreno, J.A.; Morales, E.; Moreno, J.A.; Praga, M.; et al. Remission of Hematuria Improves Renal Survival in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 10, 3089–3099. [Google Scholar] [CrossRef]
- Roberts, I.S.D. Oxford classification of immunoglobulin A nephropathy: An update. Curr. Opin. Nephrol. Hypertens. 2013, 22, 281–286. [Google Scholar] [CrossRef]
- Soares, M.F.S.; Roberts, I.S.D. Histologic Classification of IgA Nephropathy: Past, Present, and Future. Semin. Nephrol. 2018, 38, 477–484. [Google Scholar] [CrossRef]
- Obrișcă, B.; Jurubiță, R.; Andronesi, A.; Sorohan, B.; Achim, C.; Bobeica, R.; Ismail, G.; Gherghiceanu, M.; Mandache, E. Histological predictors of renal outcome in lupus nephritis: The importance of tubulointerstitial lesions and scoring of glomerular lesions. Lupus 2018, 27, 1455–1463. [Google Scholar] [CrossRef]
- Network, I.N.; Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Bruijn, J.A.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar]
- Network, I.N.; Roberts, I.S.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Bruijn, J.A.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int. 2009, 76, 546–556. [Google Scholar]
- Lv, J.; Shi, S.; Xu, D.; Zhang, H.; Troyanov, S.; Cattran, D.C.; Wang, H. Evaluation of the Oxford Classification of IgA nephropathy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2013, 62, 891–899. [Google Scholar] [CrossRef] [PubMed]
- El Karoui, K.; Hill, G.S.; Karras, A.; Moulonguet, L.; Caudwell, V.; Loupy, A.; Nochy, D.; Bruneval, P.; Jacquot, C. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 2011, 79, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Chakera, A.; MacEwen, C.; Bellur, S.S.; Chompuk, L.O.; Lunn, D.; Roberts, I.S. Prognostic value of endocapillary hypercellularity in IgA nephropathy patients with no immunosuppression. J. Nephrol. 2016, 29, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lim, B.J.; Bae, Y.S.; Kwon, Y.E.; Kim, Y.L.; Nam, K.H.; Lee, M.J.; Park, K.S.; An, S.Y.; Koo, H.M.; et al. Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch-Schönlein purpura nephritis in adults. Mod. Pathol. 2014, 27, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Kaihan, A.B.; Hachiya, A.; Ozeki, T.; Ando, M.; Kato, S.; Maruyama, S.; Yasuda, Y. Clinical impact of endocapillary proliferation according to the Oxford classification among adults with Henoch-Schönlein purpura nephritis: A multicenter retrospective cohort study. BMC Nephrol. 2018, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Ştefan, G.; Ismail, G.; Stancu, S.; Zugravu, A.; Andronesi, A.; Mandache, E.; Mircescu, G. Validation study of Oxford Classification of IgA Nephropathy: The significance of extracapillary hypercellularity and mesangial IgG immunostaining. Pathol. Int. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Liu, J.; Duan, S.; Chen, P.; Cai, G.; Wang, Y.; Tang, L.; Chen, X.; Liu, S.; Zhou, J.; Wu, D.; et al. Development and validation of a prognostic nomogram for IgA nephropathy. Oncotarget 2017, 8, 94371–94381. [Google Scholar] [CrossRef]
- Carmo, L.; Marques, I.; Repizo, L.; Jorge, L.; Costalonga, E.; Malheiros, D. Oxford Classification of IgA Nephropathy: Outcomes Predictors. Kidney Week Abstracts 2010. J. Am. Soc. Nephrol. 2010, 21, 1–1040. [Google Scholar]
- Kang, S.H.; Choi, S.R.; Park, H.S.; Lee, J.Y.; Sun, I.O.; Hwang, H.S.; Choi, Y.J.; Chung, B.H.; Park, C.W.; Yang, C.W.; et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2012, 27, 252–258. [Google Scholar] [CrossRef]
- Shi, S.F.; Wang, S.X.; Jiang, L.; Ji-Cheng, L.V.; Liu, L.J.; Chen, Y.Q.; Wang, H.Y.; Zhu, S.N.; Liu, G.; Zou, W.Z.; et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: Validation of the oxford classification. Clin. J. Am. Soc. Nephrol. 2011, 6, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Katafuchi, R.; Ninomiya, T.; Nagata, M.; Mitsuiki, K.; Hirakata, H. Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin. J. Am. Soc. Nephrol. 2011, 6, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Nakayama, K.; Iwasaki, C.; Ochi, A.; Tsuruta, Y.; Itabashi, M.; Nitta, K.; Tsukada, M.; Takei, T.; Uchida, K. Severity of nephrotic IgA nephropathy according to the Oxford classification. Int. Urol. Nephrol. 2012, 44, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Alamartine, E.; Sauron, C.; Laurent, B.; Sury, A.; Seffert, A.; Mariat, C. The use of the oxford classification of IgA nephropathy to predict renal survival. Clin. J. Am. Soc. Nephrol. 2011, 6, 2384–2388. [Google Scholar] [CrossRef]
- Herzenberg, A.M.; Fogo, A.B.; Reich, H.N.; Troyanov, S.; Bavbek, N.; Massat, A.E.; Cattran, D.C.; Hunley, T.E.; Julian, B.A.; Fervenza, F.C.; et al. Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011, 80, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Korbet, S.M.; Schwartz, M.M.; Cimbaluk, D.J. The Oxford Classification of IgA Nephropathy: A Retrospective Analysis. Am. J. Nephrol. 2011, 34, 435–444. [Google Scholar] [CrossRef]
- Edström Halling, S.; Söderberg, M.P.; Berg, U.B. Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification). Nephrol. Dial. Transpl. 2012, 27, 715–722. [Google Scholar] [CrossRef]
- Kataoka, H.; Ohara, M.; Shibui, K.; Sato, M.; Suzuki, T.; Amemiya, N.; Nitta, K.; Watanabe, Y.; Honda, K.; Mochizuki, T. Overweight and obesity accelerate the progression of IgA nephropathy: Prognostic utility of a combination of BMI and histopathological parameters. Clin. Exp. Nephrol. 2012, 16, 706–712. [Google Scholar] [CrossRef]
- Le, W.; Zeng, C.H.; Liu, Z.; Liu, D.; Yang, Q.; Lin, R.X.; Xu, H.; Xia, Z.K.; Fan, Z.M.; Zhu, G.; et al. Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC Nephrol. 2012, 13, 158. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Zamora, I.; Ballarín, J.A.; Arce, Y.; Jiménez, S.; Quereda, C.; García, A.; Olea, T.; Segarra, A.; Bernis, C.; et al. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J. Am. Soc. Nephrol. 2012, 23, 1753–1760. [Google Scholar] [CrossRef]
- Nasri, H.; Mortazavi, M.; Ghorbani, A.; Shahbazian, H.; Kheiri, S.; Baradaran, A.; Madihi, Y. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. J. Nephropathol. 2012, 1, 31–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, C.H.; Le, W.; Ni, Z.; Zhang, M.; Miao, L.; Luo, P.; Chen, N.; Wang, R.; Lv, Z.; Chen, J.; et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am. J. Kidney Dis. 2012, 60, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yi, S.H.; Seo, M.S.; Hyun, J.N.; Jeon, J.S.; Noh, H.; Kwon, S.H.; Han, D.C.; Hwang, S.D.; Jin, S.Y. Validation of the Oxford classification of IgA nephropathy: A single-center study in Korean adults. Korean J. Intern. Med. 2012, 27, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Shima, Y.; Nakanishi, K.; Hama, T.; Mukaiyama, H.; Togawa, H.; Hashimura, Y.; Yoshikawa, N.; Kaito, H.; Sako, M.; IijimaM, K. Validity of the Oxford classification of IgA nephropathy in children. Pediatric Nephrol. 2012, 27, 783–792. [Google Scholar] [CrossRef]
- Tanaka, S.; Ninomiya, T.; Katafuchi, R.; Masutani, K.; Tsuchimoto, A.; Noguchi, H.; Kitazono, T.; Hirakata, H.; Tsurauya, K. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2013, 8, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Han, S.H.; Kie, J.H.; Nam, K.H.; Lee, M.J.; Lim, B.J.; Doh, F.M.; Kwon, Y.E.; Kim, Y.L.; Kim, C.H.; et al. Comparison of the Haas and the Oxford classifications for prediction of renal outcome in patients with IgA nephropathy. Hum. Pathol. 2014, 45, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Tanaka, K.; Iwasaki, C.; Oshima, Y.; Ochi, A.; Kataoka, H.; Nitta, K.; Itabashi, M.; Takei, T.; Uchida, K. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS ONE 2014, 9, e91756. [Google Scholar] [CrossRef]
- Coppo, R.; Troyanov, S.; Bellur, S.; Cattran, D.; Cook, H.T.; Feehally, J.; Lunberg, S.; Morando, L.; Camilla, R.; Tesar, V.; et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014, 86, 828–836. [Google Scholar] [CrossRef]
- Kaihan, A.B.; Yasuda, Y.; Katsuno, T.; Kato, S.; Imaizumi, T.; Ozeki, T.; Maruyama, S.; Hishida, M.; Nagata, T.; Ando, M.; et al. The Japanese histologic classification and T-score in the Oxford Classification system could predict renal outcome in Japanese IgA nephropathy patients. Clin. Exp. Nephrol. 2017, 21, 986–994. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Duan, S.; Chen, P.; Tang, L.; Zhang, L.; Chen, X.; Feng, Z.; Cai, G.; Wu, J.; et al. Clinicopathological features to predict progression of IgA nephropathy with mild proteinuria. Kidney Blood Press. Res. 2018, 43, 318–328. [Google Scholar] [CrossRef]
- Obrisca, B.; Jurubita, R.; Sorohan, B.; Rusu, E.; Bobeica, R.; Andronesi, A.; Ismail, G. Endocapillary and extracapillary hypercellularity provide different outcomes in IgA nephropathy. Nephrol. Dial. Transplant. 2018, 33, i87. [Google Scholar] [CrossRef]
- Haas, M.; Verhave, J.C.; Liu, Z.H.; Alpers, C.E.; Barratt, J.; Becker, J.U.; Pani, A.; Cattran, D.; Cook, H.T.; Coppo, R.; et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J. Am. Soc. Nephrol. 2017, 28, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Barratt, J.; Cattran, D.C.; Cook, H.T.; Coppo, R.; Haas, M.; Feehally, J.; Liu, Z.H.; Yuzawa, Y.; Zhang, H.; et al. Oxford classification of IgA nephropathy 2016: An update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017, 91, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Hotta, O.; Furuta, T.; Chiba, S.; Tomioka, S.; Taguma, Y. Regression of IgA nephropathy: A repeat biopsy study. Am. J. Kidney Dis. 2002, 39, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.H.; Liang, S.S.; Chen, H.M.; Le, W.B.; Jiang, S.; Zeng, C.H.; Liu, Z.H.; Zhou, M.L. Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: A repeat-biopsy based observation. J. Nephrol. 2015, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, H.; Medjeral-Thomas, N.; Galliford, J.; Griffith, M.; Levy, J.; Lightstone, L.; Cairns, T.; Palmer, A.; Roufosse, C.; Pusey, C.; et al. Mycophenolate mofetil therapy in immunoglobulin A nephropathy: Histological changes after treatment. Nephrol. Dial. Transplant. 2017, 32, i123–i128. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Le, W.B.; Chen, N.; Wang, W.M.; Liu, Z.S.; Liu, D.; Zeng, C.H.; Chen, J.H.; Tian, J.; Hu, Z.X.; et al. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: A randomized controlled trial. Am. J. Kidney Dis. 2017, 69, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Jullien, P.; Laurent, B.; Berthoux, F.; Masson, I.; Dinic, M.; Claisse, G.; Maillard, N.; Thibaudin, D.; Mariat, C.; Alamartine, E. Repeat renal biopsy improves the Oxford classification-based prediction of immunoglobulin A nephropathy outcome. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef]
- Schimpf, J.I.; Klein, T.; Fitzner, C.; Eitner, F.; Porubsky, S.; Hilgers, R.D.; Rauen, T.; Floege, J.; Groene, H.J. Renal outcomes of STOP-IgAN trial patients in relation to baseline histology (MEST-C scores). BMC Nephrol. 2018, 19, 328. [Google Scholar] [CrossRef]
- Coppo, R.; D’Arrigo, G.; Tripepi, G.; Russo, M.L.; Roberts, I.S.; Bellur, S.; Maixnerova, D.; Cattran, D.; Cook, T.H.; Feehally, J.; et al. Is there long-term value of pathology scoring in immunoglobulin A nephropathy? A validation study of the Oxford Classification for IgA Nephropathy (VALIGA) update. Nephrol. Dial. Transplant. 2018. [Google Scholar] [CrossRef]
- Bellur, S.S.; Troyanov, S.; Cook, H.T.; Roberts, I.S. Immunostaining findings in IgA nephropathy: Correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol. Dial. Transpl. 2011, 26, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.S.; Andeen, N.K.; Brodsky, S.; Hinton, A.; Nadasdy, T.; Alpers, C.E.; Rovin, B.H.; Blosser, C.; Najafian, B.; Rovin, B.H. Location of glomerular immune deposits, not codeposition of immunoglobulin G, influences definitive renal outcomes in immunoglobulin A nephropathy. Nephrol. Dial. Transplant. 2018, 33, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.; Ortega, R.; Sánchez, M.; Segarra, A.; Salcedo, M.T.; González, F.; Pinedo, F.; Camacho, R.; Valdivia, M.A.; Cabrera, R.; et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yasutake, J.; Makita, Y.; Tanbo, Y.; Yamasaki, K.; Sofue, T.; Suzuki, Y.; Kano, T. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018, 93, 700–705. [Google Scholar] [CrossRef]
- Rizk, D.V.; Saha, M.K.; Hall, S.; Novak, L.; Brown, R.; Huang, Z.Q.; Novak, J.; Fatima, H.; Julian, B.A. Glomerular Immunodeposits of Patients with IgA Nephropathy Are Enriched for IgG Autoantibodies Speci fi c for Galactose-De fi cient IgA1. J. Am. Soc. Nephrol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; Ling, C.; Hall, S.; Reily, C.; Brown, R.; Neprasova, M.; Tesar, V.; Suchanek, M.; Honsova, E.; Zima, T.; et al. Galactose-deficient IgA1 and the corresponding IgG autoantibodies predict IgA nephropathy progression. PLoS ONE 2019, 14, e0212254. [Google Scholar] [CrossRef]
- Chen, P.; Yu, G.; Zhang, X.; Xie, X.; Wang, J.; Shi, S.; Zhang, H.; Liu, L.; Lv, J. Plasma Galactose-Deficient IgA1 and C3 and CKD Progression in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H.; Canzonieri, R.; Schiel, A.; Costales-Collaguazo, C.; Stern, A.; Paulero, M.; Lombi, F.; Rengel, T.; Lotti, A.; Lombi, F.; et al. In IgA nephropathy, glomerulosclerosis is associated with increased urinary CD80 excretion and urokinase-type plasminogen activator receptor-positive podocyturia. Nephron Extra 2017, 7, 52–61. [Google Scholar] [CrossRef]
- Shi, M.; Ouyang, Y.; Yang, M.; Yang, M.; Zhang, X.; Huang, W.; Pan, X.; Wang, W.; Wang, Z.; Zhang, W.; et al. IgA nephropathy susceptibility loci and disease progression. Clin. J. Am. Soc. Nephrol. 2018, 13, 1330–1338. [Google Scholar] [CrossRef]
- Barbour, S.J.; Espino-Hernandez, G.; Reich, H.N.; Coppo, R.; Roberts, I.S.; Feehally, J.; Troyanov, S.; Herzenberg, A.M. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int. 2016, 89, 167–175. [Google Scholar] [CrossRef]
- Xie, J.; Lv, J.; Wang, W.; Li, G.; Liu, Z.; Chen, H.; Yang, M.; Xu, F.; Sun, J.; Zhang, X.; et al. Kidney failure risk prediction equations in IgA nephropathy: A multicenter risk assessment study in Chinese patients. Am. J. Kidney Dis. 2018, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.H.; Suzuki, Y.; Matsuzaki, K.; Reich, H.N.; Reich, H.N.; Cattran, D.C.; Kim, S.J.; et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA 2019, 179, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Jarrick, S.; Lundberg, S.; Welander, A.; Carrero, J.J.; Höijer, J.; Bottai, M.; Ludvigsson, J.F. Mortality in IgA nephropathy: A nationwide population-based cohort study. J. Am. Soc. Nephrol. 2019, 30, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Bolasco, P.; Fogazzi, G.; Andrulli, S.; Altieri, P.; Ponticelli, C.; Locatelli, F. Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 1999, 353, 883–887. [Google Scholar] [CrossRef]
- Shoji, T.; Nakanishi, I.; Suzuki, A.; Hayashi, T.; Togawa, M.; Okada, N.; Tsubakihara, Y.; Imai, E.; Hori, M. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am. J. Kidney Dis. 2000, 35, 194–201. [Google Scholar] [CrossRef]
- Katafuchi, R.; Ikeda, K.; Mizumasa, T.; Tanaka, H.; Ando, T.; Yanase, T.; Fujimi, S.; Masutina, K.; Kubo, M. Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisolone therapy. Am. J. Kidney Dis. 2003, 41, 972–983. [Google Scholar] [CrossRef]
- Koike, M.; Takei, T.; Uchida, K.; Honda, K.; Moriyama, T.; Horita, S.; Nitta, K.; Ogawa, T.; Yoshida, T.; Tsuchiya, K. Clinical assessment of low-dose steroid therapy for patients with IgA nephropathy: A prospective study in a single center. Clin. Exp. Nephrol. 2008, 12, 250–255. [Google Scholar] [CrossRef]
- Hogg, R.J.; Lee, J.; Nardelli, N.; Julian, B.A.; Cattran, D.; Waldo, B.; Fitzgibbons, L.; Wyatt, R.; Jennette, J.C.; Sibley, R.; et al. Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: Report from the Southwest Pediatric Nephrology Study Group. Clin. J. Am. Soc. Nephrol. 2006, 1, 467–474. [Google Scholar] [CrossRef]
- Inker, L.A.; Mondal, H.; Greene, T.; Masaschi, T.; Locatelli, F.; Schena, F.P.; Praga, M.; Katafuchi, R.; Appel, G.B.; Li, P.K.; et al. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am. J. Kidney Dis. 2016, 68, 392–401. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Zhang, W.; He, Q.; Tao, X.; Chen, J. Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. Am. J. Nephrol. 2009, 30, 315–322. [Google Scholar] [CrossRef]
- Lv, J.; Xu, D.; Perkovic, V.; Ma, X.; Johnson, D.W.; Woodward, M.; Levin, A.; Zhang, H.; Wang, H.; TESTING Study Group. Corticosteroid therapy in IgA nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Gutiérrez, E.; González, E.; Morales, E.; Hernández, E. Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J. Am. Soc. Nephrol. 2003, 14, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Peruzzi, L.; Amore, A.; Piccoli, A.; Cochat, P.; Stone, R.; Linné, T.; Kirschstein, M. IgACE: A placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J. Am. Soc. Nephrol. 2007, 18, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Fellström, B.C.; Barratt, J.; Cook, H.; Coppo, R.; Feehally, J.; de Fijter, J.W.; Maes, B.D.; Floege, J.; Hetzel, G.; Jardine, A.G.; et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 2017, 389, 2117–2127. [Google Scholar] [CrossRef]
- Maes, B.D.; Oyen, R.; Claes, K.; Evenepoel, P.; Kuypers, D.; Vanwalleghem, J.; Vanrenterghem, Y.F.C.; Van Damme, B. Mycophenolate mofetil in IgA nephropathy: Results of a 3-year prospective placebo-controlled randomized study. Kidney Int. 2004, 65, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Frisch, G.; Lin, J.; Rosenstock, J.; Markowitz, G.; D’agati, V.; Radhakrishnan, J.; Appel, G.; Preddie, D.; Crew, J.; Valeri, A. Mycophenolate mofetil (MMF) vs. placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol. Dial. Transplant. 2005, 20, 2139–2145. [Google Scholar] [CrossRef]
- Tang, S.C.; Tang, A.W.; Wong, S.S.; Leung, J.C.; Ho, Y.W.; Lai, K.N. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 2010, 77, 543–549. [Google Scholar] [CrossRef]
- Tang, S.; Leung, J.C.; Chan, L.Y.; Lui, Y.U.N.H.O.I.; Tang, C.S.; Kan, C.H.; Lai, K.N.; Ho, Y.I.U.W. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. 2005, 68, 802–812. [Google Scholar] [CrossRef]
- Hogg, R.J.; Bay, R.C.; Jennette, J.C.; Sibley, R.; Kumar, S.; Fervenza, F.C.; Cerda, J.; Appel, G.; Cattran, D.; Fischer, D.; et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am. J. Kidney Dis. 2015, 66, 783–791. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, F.M.; Li, P.K.; Vallance-Owen, J. Vallance-Owen, Cyclosporin treatment of IgA nephropathy: A short term controlled trial. Br. Med J. 1987, 295, 1165–1168. [Google Scholar] [CrossRef]
- Kim, Y.C.; Chin, H.J.; Koo, H.S.; Kim, S. Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: A double-blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. PLoS ONE 2013, 8, e71545. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, X.; Fang, Y.; Ji, J.; Zhang, X.; Yuan, M.; Ding, X.; Liu, C. Comparison of Glucocorticoids Alone and Combined with Cyclosporine A in Patients with IgA Nephropathy: A Prospective Randomized Controlled Trial. Intern. Med. 2014, 53, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Z.C.; Guan, G.J.; Lv, X.A.; Luo, Q. Cyclosporine A combined with medium/low dose prednisone in progressive IgA nephropathy. Kaohsiung J. Med. Sci. 2014, 30, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Andrulli, S.; Pani, A.; Scaini, P.; Del Vecchio, L.; Fogazzi, G.; Procaccini, A.D.; Vogt, B.; De Cristofaro, V.; Allegri, L.; et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J. Am. Soc. Nephrol. 2010, 21, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, R.A.; Canetta, P.A.; Rovin, B.H.; Appel, G.B.; Novak, J.; Nath, K.A.; Erickson, S.; Sethi, S.; Tumlin, J.A.; Mehta, K.; et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J. Am. Soc. Nephrol. 2017, 28, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Ballardie, F.W.; Roberts, I.S.D. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J. Am. Soc. Nephrol. 2002, 13, 142–148. [Google Scholar]
- Rauen, T.; Fitzner, C.; Eitner, F.; Sommerer, C.; Zeier, M.; Otte, B.; Kuhlmann, U.; Panzer, U.; Peters, H.; Benck, U.; et al. Effects of two immunosuppressive treatment protocols for IgA nephropathy. J. Am. Soc. Nephrol. 2018, 29, 317–325. [Google Scholar] [CrossRef]
- Coppo, R. Corticosteroids in IgA Nephropathy: Lessons from Recent Studies. J. Am. Soc. Nephrol. 2017, 26, 25–33. [Google Scholar] [CrossRef]
- Moran, S.M.; Cattran, D.C. Immunoglobulin A nephropathy: Prognosis and management. Nephrol. Dial. Transpl. 2018, 34, 1099–1101. [Google Scholar] [CrossRef]
- Obrisca, B.; Jurubita, R.; Sorohan, B.; Ion, O.; Andronesi, A.; Caceaune, N.; Ismail, G.; Dina, C.; Mircescu, G. The Efficact of Budesonide in the Treatment of Patients with IgA Nephropathy at High Risk of Progression. Nephrol. Dial. Transpl. 2019, 34. [Google Scholar] [CrossRef]
- Liu, L.J.; Yang, Y.Z.; Shi, S.F.; Bao, Y.F.; Yang, C.; Zhu, S.N.; Zhang, H.; Sui, G.L.; Chen, Y.Q.; Lv, J.C. Effects of Hydroxychloroquine on Proteinuria in IgA Nephropathy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2019, 74, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Smerud, H.K.; Bárány, P.; Lindström, K.; Fernström, A.; Sandell, A.; Påhlsson, P.; Fellström, B. New treatment for IgA nephropathy: Enteric budesonide targeted to the ileocecal region ameliorates proteinuria. Nephrol. Dial. Transplant. 2011, 26, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Selvaskandan, H.; Cheung, C.K.; Muto, M.; Barratt, J. New strategies and perspectives on managing IgA nephropathy. Clin. Exp. Nephrol. 2019, 23, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sarcina, C.; Tinelli, C.; Ferrario, F.; Pani, A.; De Silvestri, A.; Scaini, P.; Pozzi, C.; Del Vecchio, L.; Alberghini, E.; Buzzi, L.; et al. Changes in proteinuria and side effects of corticosteroids alone or in combination with azathioprine at different stages of IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Tang, S.C.W. Treatment of IgA Nephropathy: Evolution Over Half a Century. Semin. Nephrol. 2018, 38, 531–540. [Google Scholar] [CrossRef]
| A | ||||||
| Trial | ACE inhibitors | Corticosteroids | ||||
| Praga (2003) [103] | Coppo (2007) [104] | Shoji (2000) [96] | Katafuchi (2003) [97] | Hogg (2006) [99] | Koike (2008) [98] | |
| Number of pts. | 44 | 66 | 21 | 90 | 96 | 48 |
| Serum creatinine (mg/dL) | 0.95 ± 0.2 | - | 0.74 ± 0.22 | 0.91 ± 0.22 | - | 1.04 ± 0.31 |
| eGFR (mL/min/1.73m2) | 100 ± 23 | 112 ± 21 | 106 ± 30 | 90 ± 26 | 114 ± 43 | - |
| Proteinuria (g/day) | 1.7–2 | 1.7 ± 0.7 | 0.75 ± 0.31 | 1.63 ± 1.53 | 1.4–2.2 | 0.93 ± 0.63 |
| RAAS blockade (%) | 52% | 48% | 0% | 2% | 52% | 23% |
| Treatment | Enalapril vs. placebo | Benazepril vs. placebo | Prednisone vs. dipyridamole | Low-dose prednisone vs. dipyridamole | Prednisone vs. omega 3 fatty acids vs. placebo | Low-dose prednisone vs. dipyridamole |
| Progression | ||||||
| Definition | 50% increase in SCr | 30% decrease in ClCr | - | ESRD | 60% decrease in eGFR | - |
| Proportion | 13% vs. 57% | 3.1% vs. 14.7% | - | 6.9% vs. 6.3% | 6% vs. 25% vs. 13% | - |
| ∆GFR/year | - | - | - | - | - | - |
| Remission | ||||||
| Definition | - | Proteinuria below 0.5 g/d | Percentage decrease of proteinuria | Changes in urinary protein excretion from baseline | - | Changes in proteinuria from baseline |
| Proportion | - | 40.6% vs. 8.8% | 41% vs. 0% | −0.84 vs. 0.26 | - | More decrease in steroid group |
| Follow-up (months.) | 76 | 38 | 13 | 65 | 24 | 24 |
| Conclusion | Positive | Positive | Positive | Negative | Negative | Positive |
| B | ||||||
| Trial | Corticosteroids | |||||
| Pozzi (1999) [16,95] | Manno (2009) [15] | Lv (2009) [14] | Lv (2017) [19] TESTING Trial | Fellstrom (2017) [105] NEFIGAN Trial | ||
| Number of pts. | 86 | 97 | 63 | 262 | 149 | |
| Serum creatinine (mg/dL) | 0.9–1.09 | 1.07 ± 0.3 | 1.1 ± 0.3 | 1.55 ± 0.6 | - | |
| eGFR (mL/min/1.73m2) | 87–93 | 99 ± 27 | 101 | 59 ± 25 | 78 ± 25 | |
| Proteinuria (g/day) | 1.8–2 | 1.5–1.7 | 2–2.5 | 2.4 | 1.2 | |
| RAAS blockade (%) | 54% | 100% | 100% | 100% | 100% | |
| Treatment | Corticosteroids vs. supportive treatment | Prednisone + ramipril vs. ramipril | Prednisone + cilazapril vs. cilazapril | Methylprednisolone vs. placebo | Budesonide 16 mg vs. budesonide 8 mg vs. placebo | |
| Progression | ||||||
| Definition | Doubling of SCr | Doubling of SCr or ESRD | 50% increase in SCr | 40% decrease in eGFR/ESRD/death | Percentage change from baseline of eGFR (9 months) | |
| Proportion | 2.3% vs. 30.2% | 4.2% vs. 26.5% | 3% vs. 24.1% | 5.9% vs. 15.9% | 0.6% vs. −0.9% vs. −9.8% | |
| ∆GFR/year | - | −0.56 vs. −6.17 | - | −1.79 vs. −6.95 | - | |
| Remission | ||||||
| Definition | Proteinuria below 0.5 g/d | - | - | Complete/partial remission at 12 months | Changes in urinary protein excretion from baseline (12 months) | |
| Proportion | 26% vs. 5% (after 1 year) | - | - | 52.2% vs. 13.6% | −32% vs. −22% vs. 0.5% | |
| Follow-up (months) | 84 | 96 | 27 | 25 | 12 | |
| Conclusion | Positive | Positive | Positive | Possible renal benefit, excess infectious SAE | Positive | |
| C | ||||||
| Trial | Mycophenolate mofetil | |||||
| Maes (2004) [106] | Frisch (2005) [107] | Tang (2005) [108,109] | Hogg (2015) [110] | Hou (2017) [78] | ||
| Number of pts. | 34 | 32 | 40 | 52 | 176 | |
| Serum Creatinine (mg/dL) | 1.42 ± 0.09 | 2.4 ± 0.96 | 1.59 ± 0.2 | - | 0.93 | |
| eGFR (mL/min/1.73m2) | 71 ± 6 (inulin clearance) | 39 ± 24 | 51 ± 4 | 100 ± 42 | 92 | |
| Proteinuria (g/day) | 1.6 | 2.7 | 1.8 | 1.48 | 2.42 | |
| RAAS blockade (%) | 100% | 100% | 100% | 100% | 24% | |
| Treatment | MMF vs. placebo | MMF vs. placebo | MMF vs. placebo | MMF vs. placebo | MMF + low dose prednisone vs. full-dose prednisone | |
| Progression | ||||||
| Definition | 25% decrease in inulin clearance | 50% increase in SCr/ESRD | Doubling SCr/ESRD | - | ESRD | |
| Proportion | 33% vs. 15.3% | 29% vs. 13% | 15% vs. 50% | - | 0% vs. 2.2% | |
| ∆GFR/year | −4.3 vs. −0.66 | - | −1.12 vs. −3.81 | −7 vs. 2.8 (at 6 months) | - | |
| Remission | ||||||
| Definition | - | Partial remission (50% reduction of proteinuria) | Partial remission (50% reduction of proteinuria) | Complete remission | Complete remission (undetectable proteinuria and stable renal function) | |
| Proportion | - | 17% vs. 13% | 80% vs. 30% | None | 48% vs. 53% | |
| Follow-up (months) | 36 | 24 | 72 | 24 | 12 | |
| Conclusion | Negative | Negative | Positive | Negative | Positive | |
| D | ||||||
| Trial | Calcineurin inhibitors | |||||
| Lai (1987) [111] | Kim (2013) [112] | Liu (2014) [113] | Xu (2014) [114] | |||
| Number of pts. | 19 | 40 | 48 | 96 | ||
| Serum creatinine (mg/dL) | 1.32 | 1.02 ± 0.28 | 1.01 ± 0.27 | 0.99 ± 0.23 | ||
| eGFR (mL/min/1.73m2) | 72 (creatinine clearance) | 82 ± 22 | 80 ± 20 | 76 ± 24 | ||
| Proteinuria (g/day) | 3.35 | 1.3 | 2.88 | 2.04 | ||
| RAAS blockade (%) | 0% | 50% | 100% | 100% | ||
| Treatment | Cyclosporine A vs. placebo | Tacrolimus vs. placebo | Cyclosporin A + medium-dose prednisone vs. full-dose prednisone | Cyclosporin A + medium-dose prednisone vs. full-dose prednisone | ||
| Progression | ||||||
| Definition | - | - | 25% decrease of eGFR | - | ||
| Proportion | - | - | 9% vs. 0% | - | ||
| ∆GFR/year | - | - | - | - | ||
| Remission | ||||||
| Definition | 50% reduction of proteinuria | Percentage decrease of proteinuria | Complete remission | Complete remission | ||
| Proportion | 77% vs. 0% | 52% vs. 17% | 50% vs. 45.8% | 52% vs. 21% | ||
| Follow-up (months) | 7 | 4 | 36 | 12 | ||
| Conclusion | Negative (renal function deterioration in CSA group) | Positive | Positive | Positive | ||
| E | ||||||
| Trial | Azathioprine | Rituximab | Cyclophosphamide/Azathioprine | |||
| Pozzi (2010) [115] | Lafayette (2017) [116] | Ballardie and Roberts (2001) [117] | Rauen (2015) [18] STOP trial | |||
| Number of pts. | 207 | 34 | 38 | 337 (Run-in phase) 162 (Trial phase) | ||
| Serum creatinine (mg/dL) | 1.2 (1–1.6) | 1.4 (0.8–2.4) | - | 1.5 ± 0.6 | ||
| eGFR (mL/min/1.73m2) | 66 (48–87) | 49 (30–122) | - | 61 ± 27 | ||
| Proteinuria (g/day) | 2 (1.5–3) | 2.1 (0.6–5.3) | 4.25 | 2.2 ± 1.8 | ||
| RAAS blockade (%) | 90% | 100% | - | 100% | ||
| Treatment | Corticosteroids vs. corticosteroids + azathioprine | Rituximab vs. placebo | Corticosteroid + cyclophosphamide followed by azathioprine vs. placebo | Pozzi/Ballardie regimen vs. placebo | ||
| Progression | ||||||
| Definition | 50% increase in SCr | 25% decrease of eGFR | 5-year renal survival | eGFR decrease ≥ 15 mL/min/1.73m2 | ||
| Proportion | 11.3% vs. 12.9% | 1/17 vs. 0/17 | 72% vs. 5% | 26% vs. 28% | ||
| ∆GFR/year | - | - | −1.07 vs. −5.12 | −1.4 vs. −1.6 | ||
| Remission | ||||||
| Definition | Percentage decrease of proteinuria | Partial remission | - | Full clinical remission | ||
| Proportion | 49.9% vs. 44.8% | 3/16 vs. 3/15 | - | 17% vs. 5% | ||
| Follow-up (months.) | 59 | 12 | Up to 72 | 36 | ||
| Conclusion | Negative | Negative | Positive | More complete remissions in steroid groups, no difference in renal function decline | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obrișcă, B.; Sinescu, I.; Ismail, G.; Mircescu, G. Has The Time Arrived to Refine The Indications of Immunosuppressive Therapy and Prognosis in IgA Nephropathy? J. Clin. Med. 2019, 8, 1584. https://doi.org/10.3390/jcm8101584
Obrișcă B, Sinescu I, Ismail G, Mircescu G. Has The Time Arrived to Refine The Indications of Immunosuppressive Therapy and Prognosis in IgA Nephropathy? Journal of Clinical Medicine. 2019; 8(10):1584. https://doi.org/10.3390/jcm8101584
Chicago/Turabian StyleObrișcă, Bogdan, Ioanel Sinescu, Gener Ismail, and Gabriel Mircescu. 2019. "Has The Time Arrived to Refine The Indications of Immunosuppressive Therapy and Prognosis in IgA Nephropathy?" Journal of Clinical Medicine 8, no. 10: 1584. https://doi.org/10.3390/jcm8101584
APA StyleObrișcă, B., Sinescu, I., Ismail, G., & Mircescu, G. (2019). Has The Time Arrived to Refine The Indications of Immunosuppressive Therapy and Prognosis in IgA Nephropathy? Journal of Clinical Medicine, 8(10), 1584. https://doi.org/10.3390/jcm8101584





