A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia
Abstract
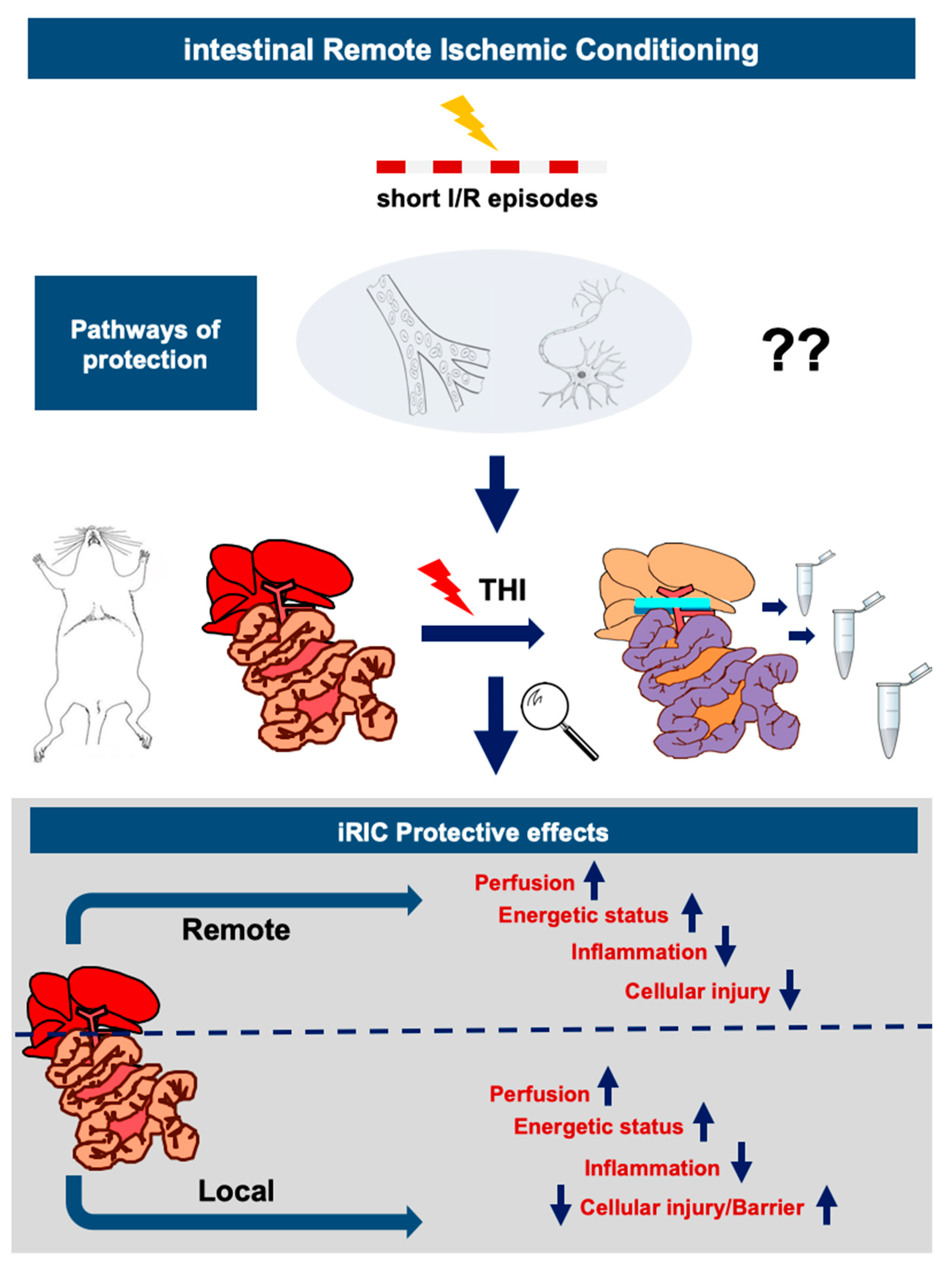
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Technique
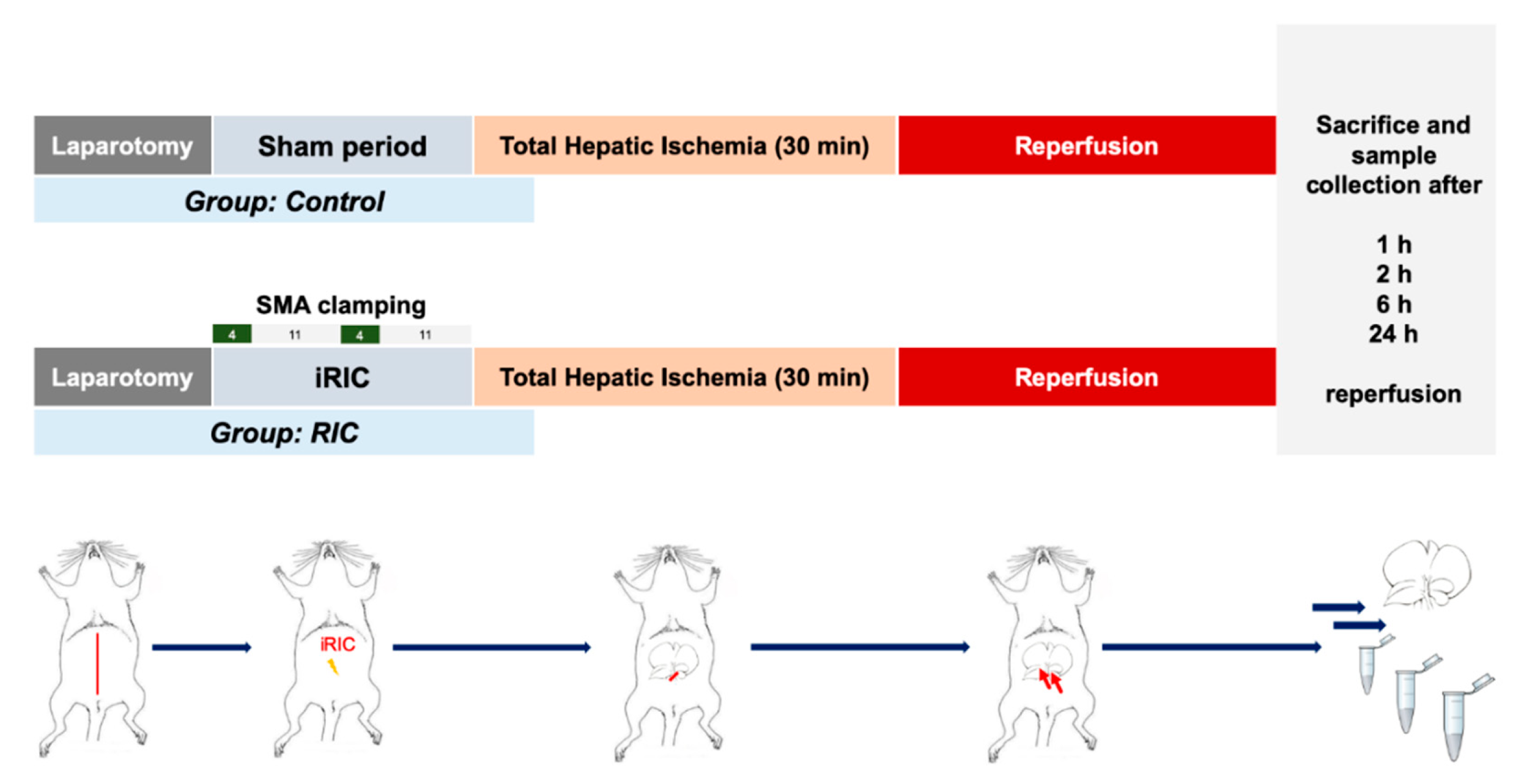
2.3. Experimental Design
2.4. Liver and Ileum Perfusion
2.5. Biochemical Analysis and Serum Cytokines
2.6. Tissue Adenosine Triphosphate Concentration
2.7. Transmission Electron Microscopy
2.8. Bioluminescent Assessment of Bacterial Translocation
2.9. Statistical Analysis
3. Results
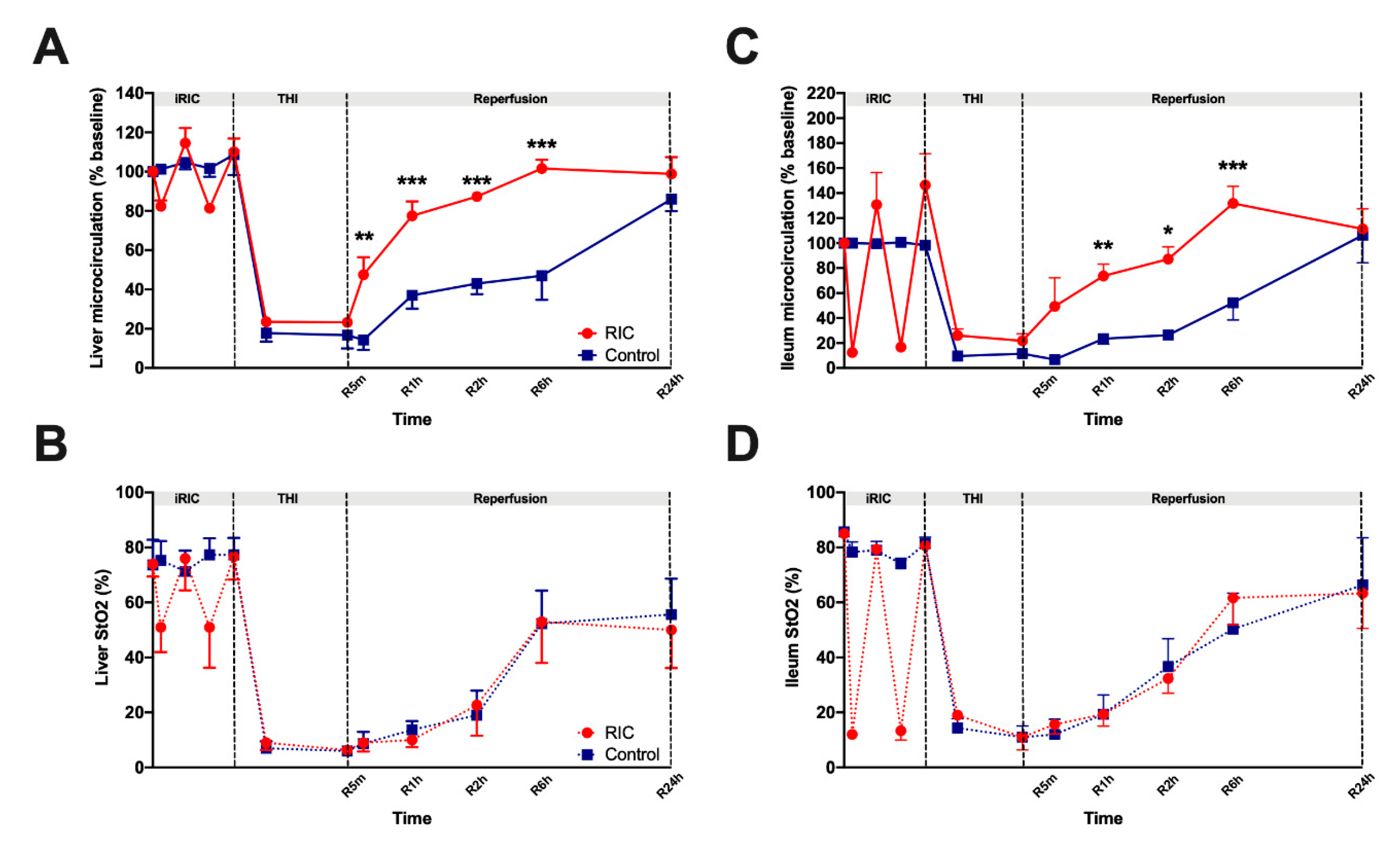
3.1. Liver and Ileum Microcirculation
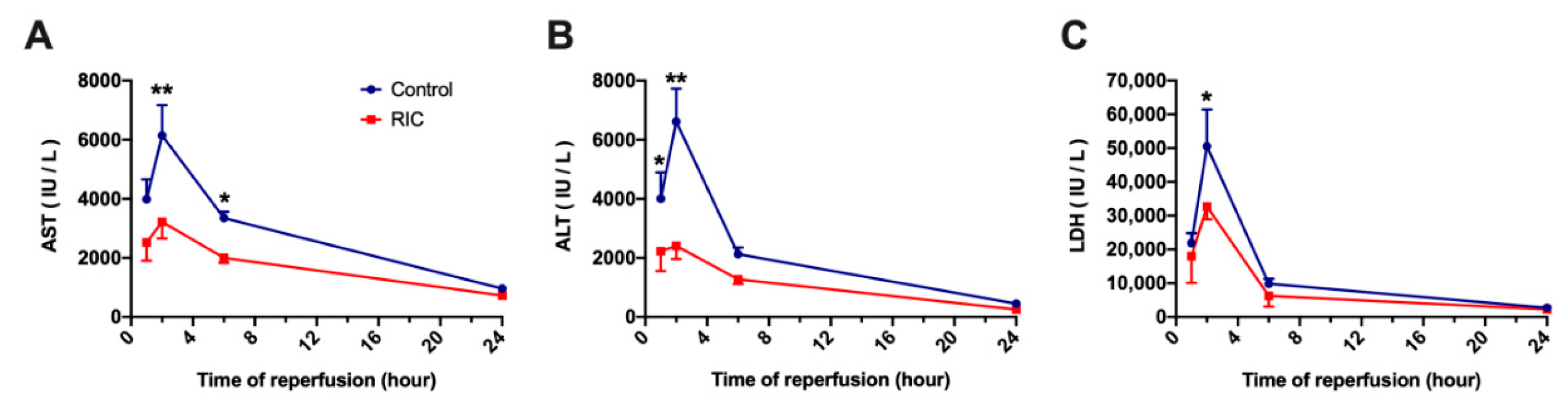
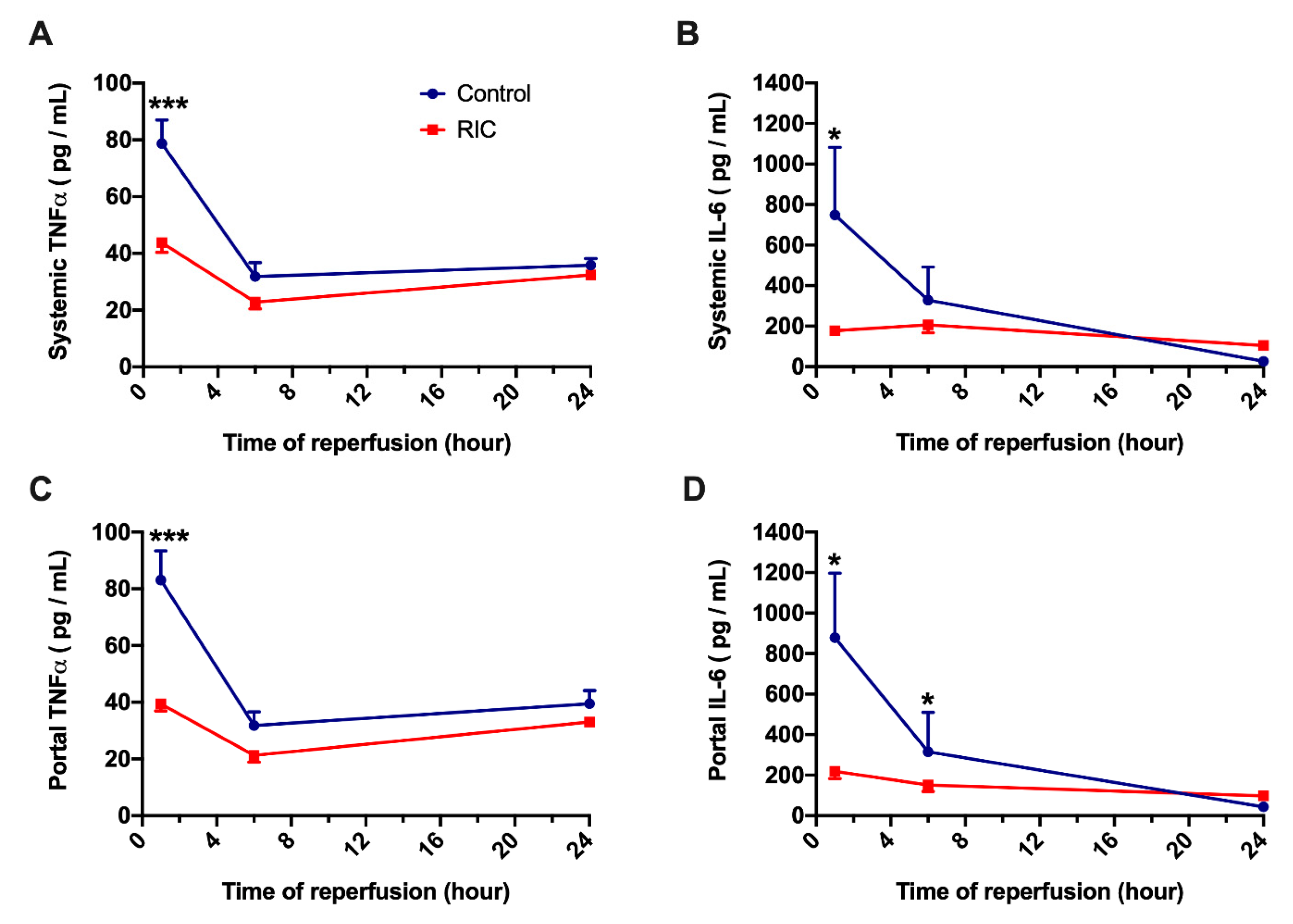
3.2. Biochemical Analysis and Serum Cytokines
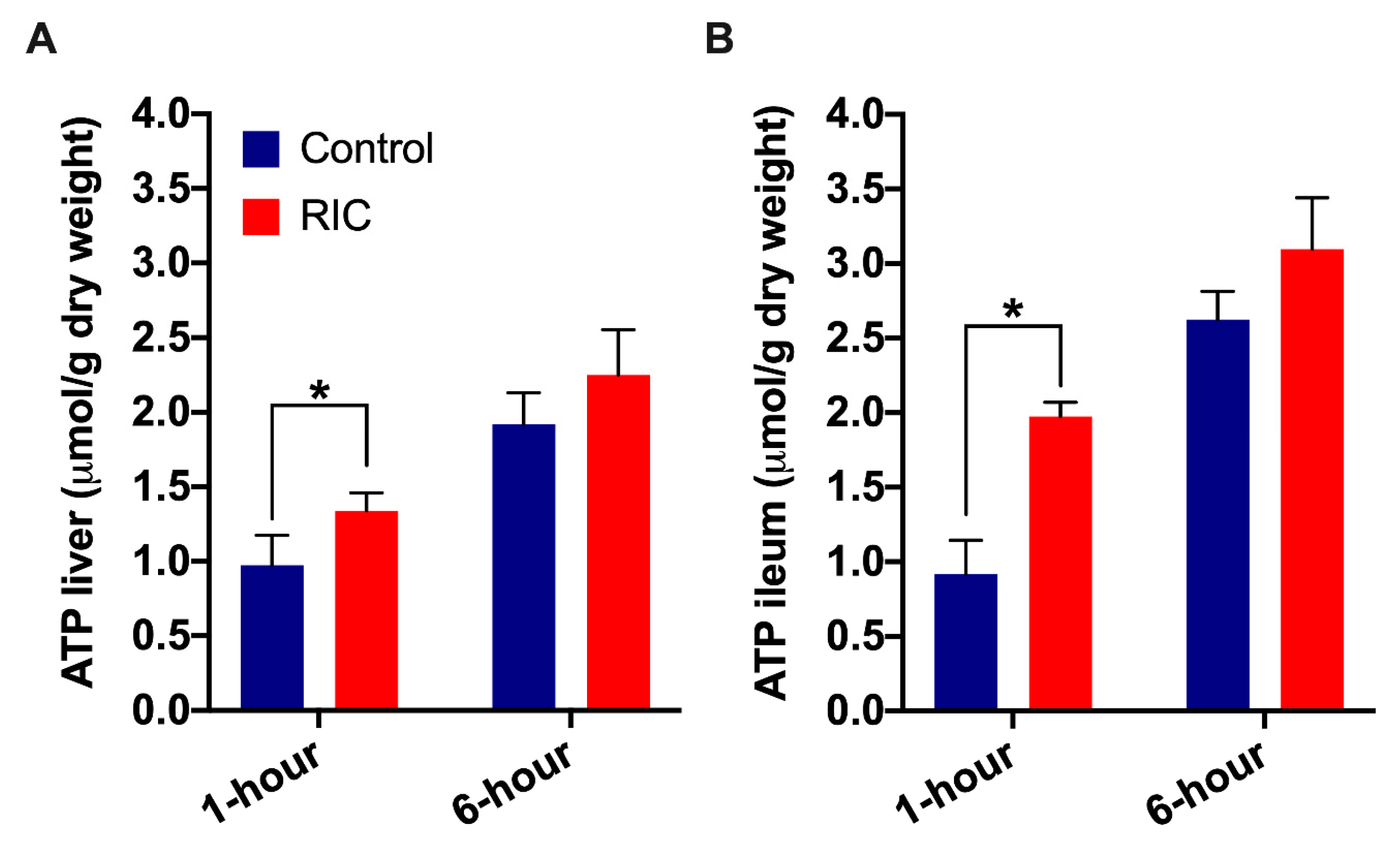
3.3. Tissue Adenosine Triphosphate Concentration
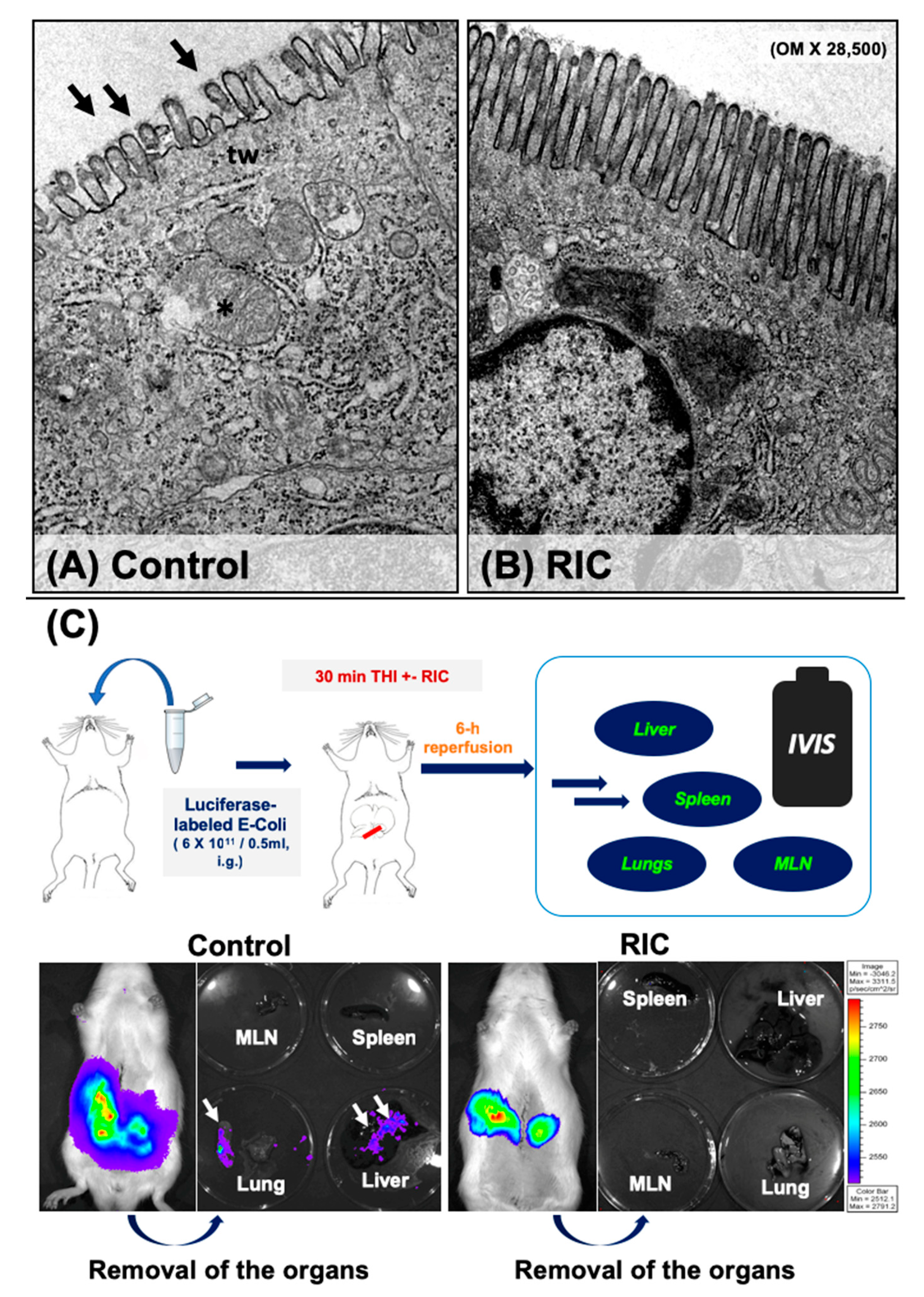
3.4. Intestinal Barrier and Bacterial Translocation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czigany, Z.; Lurje, I.; Tolba, R.H.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs-New kids on the block. Liver Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pringle, J.H.V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann. Surg. 1908, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pereyra, L.H.; Simmons, R.L.; Najarian, J.S. Factors determining successful liver preservation for transplantation. Ann. Surg. 1975, 181, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pereyra, L.H.; Simmons, R.L.; Najarian, J.S. Protection of the ischemic liver by donor pretreatment before transplantation. Am. J. Surg. 1975, 129, 513–517. [Google Scholar] [CrossRef]
- Czigany, Z.; Iwasaki, J.; Yagi, S.; Nagai, K.; Szijarto, A.; Uemoto, S.; Tolba, R.H. Improving Research Practice in Rat Orthotopic and Partial Orthotopic Liver Transplantation: A Review, Recommendation, and Publication Guide. Eur. Surg. Res. 2015, 55, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Scherer, M.N.; Pratschke, J.; Guba, M.; Nadalin, S.; Mehrabi, A.; Berlakovich, G.; Rogiers, X.; Pirenne, J.; Lerut, J.; et al. Technical Aspects of Orthotopic Liver Transplantation-a Survey-Based Study Within the Eurotransplant, Swisstransplant, Scandiatransplant, and British Transplantation Society Networks. J. Gastrointest. Surg. 2018. [Google Scholar] [CrossRef]
- Czigany, Z.; Schoning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Fronek, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic preconditioning protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef]
- Przyklenk, K.; Whittaker, P. Remote Ischemic Preconditioning: Current Knowledge, Unresolved Questions, and Future Priorities. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 255–259. [Google Scholar] [CrossRef]
- Szijarto, A.; Czigany, Z.; Turoczi, Z.; Harsanyi, L. Remote ischemic preconditioning—A simple, low-risk method to decrease ischemic reperfusion injury: Models, protocols and mechanistic background. A review. J. Surg. Res. 2012, 178, 797–806. [Google Scholar] [CrossRef]
- Czigany, Z.; Bleilevens, C.; Beckers, C.; Stoppe, C.; Mohring, M.; Fulop, A.; Szijarto, A.; Lurje, G.; Neumann, U.P.; Tolba, R.H. Limb remote ischemic conditioning of the recipient protects the liver in a rat model of arterialized orthotopic liver transplantation. PLoS ONE 2018, 13, e0195507. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Turoczi, Z.; Bulhardt, O.; Hegedus, V.; Lotz, G.; Rakonczay, Z.; Balla, Z.; Harsanyi, L.; Szijarto, A. Remote ischemic conditioning: Short-term effects on rat liver ischemic-reperfusion injury. Orv. Hetil. 2012, 153, 1579–1587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Czigany, Z.; Turoczi, Z.; Kleiner, D.; Lotz, G.; Homeyer, A.; Harsanyi, L.; Szijarto, A. Neural elements behind the hepatoprotection of remote perconditioning. J. Surg. Res. 2015, 193, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Turoczi, Z.; Onody, P.; Harsanyi, L.; Lotz, G.; Hegedus, V.; Szijarto, A. Remote ischemic perconditioning protects the liver from ischemia-reperfusion injury. J. Surg. Res. 2013, 185, 605–613. [Google Scholar] [CrossRef]
- Emontzpohl, C.; Stoppe, C.; Theissen, A.; Beckers, C.; Neumann, U.P.; Lurje, G.; Ju, C.; Bernhagen, J.; Tolba, R.H.; Czigany, Z. The Role of Macrophage Migration Inhibitory Factor in Remote Ischemic Conditioning Induced Hepatoprotection in A Rodent Model of Liver Transplantation. Shock 2018. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, J.; Jiang, L.; Zhang, J.; Chen, S.; Wang, L.; Zhou, Y.; Xie, H.; Zhou, L.; Zheng, S. Protective effect of remote limb ischemic perconditioning on the liver grafts of rats with a novel model. PLoS ONE 2015, 10, e0121972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kageyama, S.; Hata, K.; Tanaka, H.; Hirao, H.; Kubota, T.; Okamura, Y.; Iwaisako, K.; Takada, Y.; Uemoto, S. Intestinal ischemic preconditioning ameliorates hepatic ischemia/reperfusion injury in rats: Role of heme oxygenase 1 in the second window of protection. Liver Transplant. 2015, 21, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, M.; Shimada, M.; Morine, Y.; Yoshizumi, T.; Imura, S.; Ikegami, T.; Mori, H.; Arakawa, Y. Beneficial effects of follistatin in hepatic ischemia-reperfusion injuries in rats. Dig. Dis. Sci. 2011, 56, 1075–1081. [Google Scholar] [CrossRef]
- Yao, X.M.; Chen, H.; Li, Y. Protective effect of bicyclol on liver injury induced by hepatic warm ischemia/reperfusion in rats. Hepatol. Res. 2009, 39, 833–842. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kanzler, S.; Rix, A.; Czigany, Z.; Tanaka, H.; Fukushima, K.; Kogel, B.; Pawlowsky, K.; Tolba, R.H. Recommendation for severity assessment following liver resection and liver transplantation in rats: Part I. Lab. Anim. 2016, 50, 459–467. [Google Scholar] [CrossRef]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Tolba, R.H.; Wei, L.; Doorschodt, B.M.; Buttner, R.; Yamamoto, Y.; Minor, T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transplant. 2007, 13, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tolba, R.H.; Akbar, S.; Muller, A.; Glatzel, U.; Minor, T. Experimental liver preservation with Celsior: A novel alternative to University of Wisconsin and histidine-tryptophan-alpha-ketoglutarate solutions? Eur. Surg. Res. 2000, 32, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Hachenberg, A.; Tolba, R.; Pauleit, D.; Akbar, S. Fibrinolytic preflush upon liver retrieval from non-heart beating donors to enhance postpreservation viability and energetic recovery upon reperfusion. Transplantation 2001, 71, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.K.; Yagi, S.; Doorschodt, B.; Nagai, K.; Afify, M.; Uemoto, S.; Tolba, R. Impact of venous systemic oxygen persufflation supplemented with nitric oxide gas on cold-stored, warm ischemia-damaged experimental liver grafts. Liver Transplant. 2012, 18, 219–225. [Google Scholar] [CrossRef]
- Moinard, C.; Butel, M.J.; Bureau, M.F.; Choisy, C.; Waligora-Dupriet, A.J.; Moulis, J.; Marc, J.; Cynober, L.; Charrueau, C. In vivo bioluminescent imaging of a new model of infectious complications in head-injury rats. J. Neurotrauma 2012, 29, 335–342. [Google Scholar] [CrossRef]
- Foucault, M.L.; Thomas, L.; Goussard, S.; Branchini, B.R.; Grillot-Courvalin, C. In vivo bioluminescence imaging for the study of intestinal colonization by Escherichia coli in mice. Appl. Environ. Microbiol. 2010, 76, 264–274. [Google Scholar] [CrossRef]
- Chang, M.H.; Cirillo, S.L.; Cirillo, J.D. Using luciferase to image bacterial infections in mice. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Suff, N.; Karda, R.; Diaz, J.A.; Ng, J.; Baruteau, J.; Perocheau, D.; Tangney, M.; Taylor, P.W.; Peebles, D.; Buckley, S.M.K.; et al. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am. J. Pathol. 2018, 188, 2164–2176. [Google Scholar] [CrossRef]
- McLeod, S.L.; Iansavichene, A.; Cheskes, S. Remote Ischemic Perconditioning to Reduce Reperfusion Injury During Acute ST-Segment-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Bromage, D.I.; Pickard, J.M.; Rossello, X.; Ziff, O.J.; Burke, N.; Yellon, D.M.; Davidson, S.M. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: A systematic review and meta-analysis. Cardiovasc. Res. 2017, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.W.; Pang, W.F.; Szeto, C.C. Remote ischaemic pre-conditioning for the prevention of acute kidney injury. Nephrology 2016, 21, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Tapuria, N.; Fuller, B.; Davidson, B.; Seifalian, A. Liver ischemia/reperfusion injury: Processes in inflammatory networks—A review. Liver Transplant. 2010, 16, 1016–1032. [Google Scholar] [CrossRef]
- Kraemer, R.; Lorenzen, J.; Kabbani, M.; Herold, C.; Busche, M.; Vogt, P.M.; Knobloch, K. Acute effects of remote ischemic preconditioning on cutaneous microcirculation—A controlled prospective cohort study. BMC Surg. 2011, 11, 32. [Google Scholar] [CrossRef]
- Kono, Y.; Fukuda, S.; Hanatani, A.; Nakanishi, K.; Otsuka, K.; Taguchi, H.; Shimada, K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des. Dev. Ther. 2014, 8, 1175–1181. [Google Scholar] [CrossRef]
- Liu, Z.; Lai, C.H.; Zhang, X.; Luo, J.; Huang, X.; Qi, X.; Wang, W.; Zhong, Z.; Xiaoli, F.; Li, L.; et al. Simvastatin ameliorates total liver ischemia/reperfusion injury via KLF2-mediated mechanism in rats. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 171–178. [Google Scholar] [CrossRef]
- Jaeschke, H.; Lemasters, J.J. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003, 125, 1246–1257. [Google Scholar] [CrossRef]
- You, L.; Pan, Y.Y.; An, M.Y.; Chen, W.H.; Zhang, Y.; Wu, Y.N.; Li, Y.; Sun, K.; Yin, Y.Q.; Lou, J.S. The Cardioprotective Effects of Remote Ischemic Conditioning in a Rat Model of Acute Myocardial Infarction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Gedik, N.; Kirca, M.; Stoian, L.; Frey, U.; Zandi, A.; Thielmann, M.; Jakob, H.; Peters, J.; Kamler, M.; et al. Mitochondrial and Contractile Function of Human Right Atrial Tissue in Response to Remote Ischemic Conditioning. J. Am. Heart Assoc. 2018, 7, e009540. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, J.; Zhu, J.; Liu, L.X.; Rebecchi, M.; Hu, S.M.; Wang, C. Sevoflurane post-conditioning protects isolated rat hearts against ischemia-reperfusion injury via activation of the ERK1/2 pathway. Acta Pharmacol. Sin. 2014, 35, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Garcia, N.; Gallardo-Perez, J.; Carreno-Fuentes, L.; Rodriguez-Enriquez, S.; Marin-Hernandez, A.; Zazueta, C. Post-conditioning preserves glycolytic ATP during early reperfusion: A survival mechanism for the reperfused heart. Cell. Physiol. Biochem. 2008, 22, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Jimenez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.R.; Luo, F.W.; Liu, X.M.; Tian, X.F.; Zhou, Y. Fish oil alleviates liver injury induced by intestinal ischemia/reperfusion via AMPK/SIRT-1/autophagy pathway. World J. Gastroenterol. 2018, 24, 833–843. [Google Scholar] [CrossRef]
- Jing, H.; Shen, G.; Wang, G.; Zhang, F.; Li, Y.; Luo, F.; Yao, J.; Tian, X.F. MG132 alleviates liver injury induced by intestinal ischemia/reperfusion in rats: Involvement of the AhR and NFkappaB pathways. J. Surg. Res. 2012, 176, 63–73. [Google Scholar] [CrossRef]
- Taylor, C.T.; Dzus, A.L.; Colgan, S.P. Autocrine regulation of epithelial permeability by hypoxia: Role for polarized release of tumor necrosis factor alpha. Gastroenterology 1998, 114, 657–668. [Google Scholar] [CrossRef]
- Holland, J.; Carey, M.; Hughes, N.; Sweeney, K.; Byrne, P.J.; Healy, M.; Ravi, N.; Reynolds, J.V. Intraoperative splanchnic hypoperfusion, increased intestinal permeability, down-regulation of monocyte class II major histocompatibility complex expression, exaggerated acute phase response, and sepsis. Am. J. Surg. 2005, 190, 393–400. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Werner, J.H.; Moulton, M.J.; Agrawal, D.K. Current Modalities and Mechanisms Underlying Cardioprotection by Ischemic Conditioning. J. Cardiovasc. Transl. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Schulz, R. Remote preconditioning. J. Mol. Cell. Cardiol. 2002, 34, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Botker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.M.; Tao, L.D.; Zhang, J.; Zhang, P.J.; Liu, X.; Chen, G.F.; Zhu, Y.J. Intestinal ischemic preconditioning reduces liver ischemia reperfusion injury in rats. Mol. Med. Rep. 2016, 13, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
| Author | Species and Strain | Model | Sample Size | RIC Protocol | Time Points | Short Summary |
|---|---|---|---|---|---|---|
| Czigany et al. (present study) | Rat, Wistar, outbred, male | 30 min THI | 50 rats, 25 RIC | Remote preconditioning, 2 × 4 min of ischemia and 11 min of reperfusion by clamping the SMA directly before THI | 1, 2, 6, 24 h | iRIC is a feasible technique which could potently reduce hepatocellular injury and preserve intestinal barrier integrity following THI |
| Kageyama et al. [17] | Rat, Wistar, outbred, male | 30 min THI | 36 rats, 18 RIC | Second window remote preconditioning, 2 × 4 min of ischemia and 11 min of reperfusion by clamping the SMA 48 h before THI | 2, 6, 24, 240 h | iRIC remarkably attenuates hepatic IRI in the second window of protection after 48-h, presumably by HO-1 induction in hepatocytes |
| Xue et al. [18] | Rat, Sprague-Dawley, outbred, male | 30 min, 70% partial ischemia | 15 rats, 5 RIC | Remote preconditioning, 2 × 10 min of ischemia and 10 min of reperfusion by clamping the SMA directly before THI | 3 h | iRIC provided protection against hepatic IRI by modulating apoptosis and inflammation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czigany, Z.; Hata, K.; Lai, W.; Schwandt, T.; Yamamoto, Y.; Uemoto, S.; Tolba, R.H. A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia. J. Clin. Med. 2019, 8, 1546. https://doi.org/10.3390/jcm8101546
Czigany Z, Hata K, Lai W, Schwandt T, Yamamoto Y, Uemoto S, Tolba RH. A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia. Journal of Clinical Medicine. 2019; 8(10):1546. https://doi.org/10.3390/jcm8101546
Chicago/Turabian StyleCzigany, Zoltan, Koichiro Hata, Wei Lai, Timo Schwandt, Yuzo Yamamoto, Shinji Uemoto, and Rene H. Tolba. 2019. "A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia" Journal of Clinical Medicine 8, no. 10: 1546. https://doi.org/10.3390/jcm8101546
APA StyleCzigany, Z., Hata, K., Lai, W., Schwandt, T., Yamamoto, Y., Uemoto, S., & Tolba, R. H. (2019). A Dual Protective Effect of Intestinal Remote Ischemic Conditioning in a Rat Model of Total Hepatic Ischemia. Journal of Clinical Medicine, 8(10), 1546. https://doi.org/10.3390/jcm8101546






