Heat Generation at the Implant–Bone Interface by Insertion of Ceramic and Titanium Implants
Abstract
1. Introduction
2. Materials and Methods
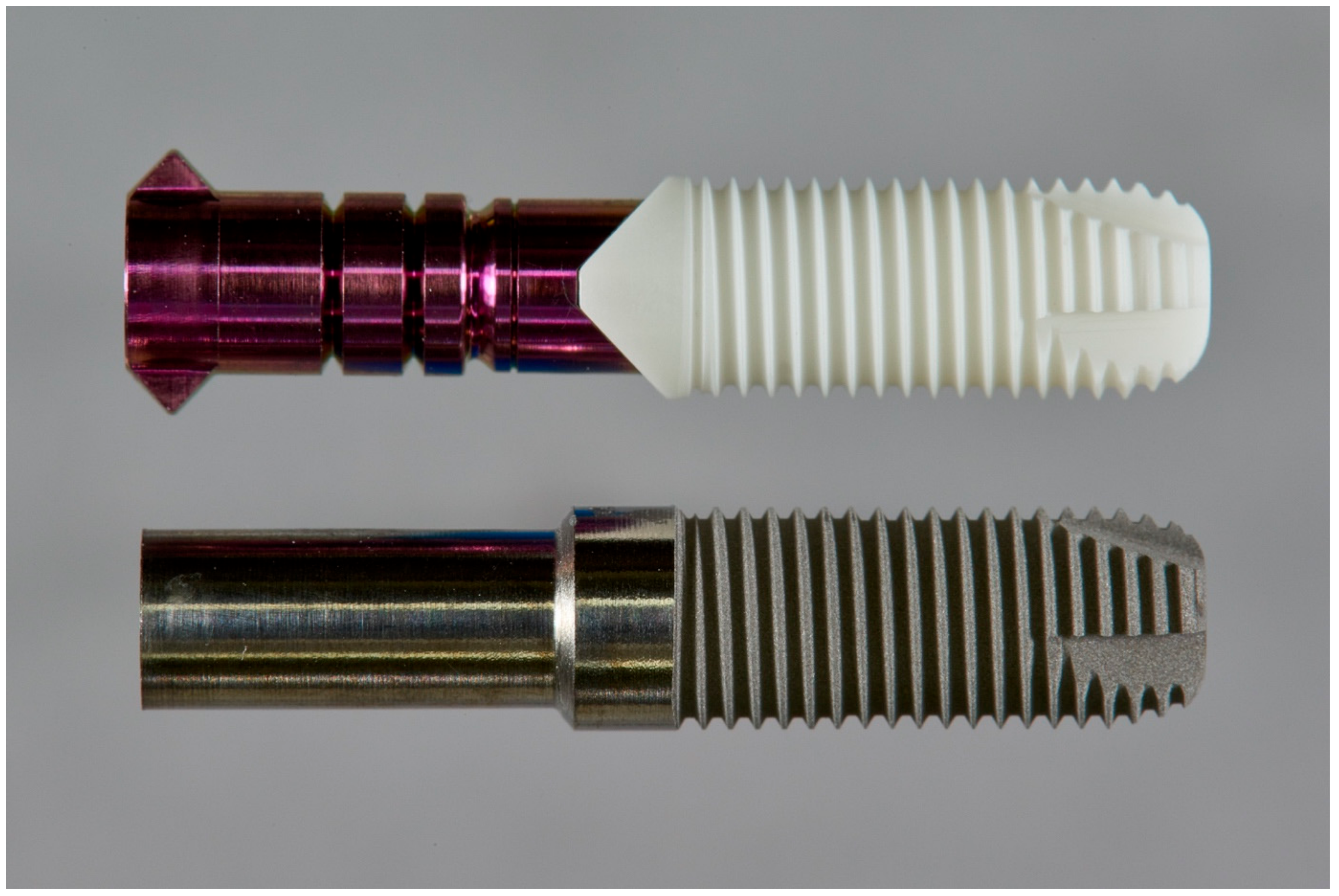
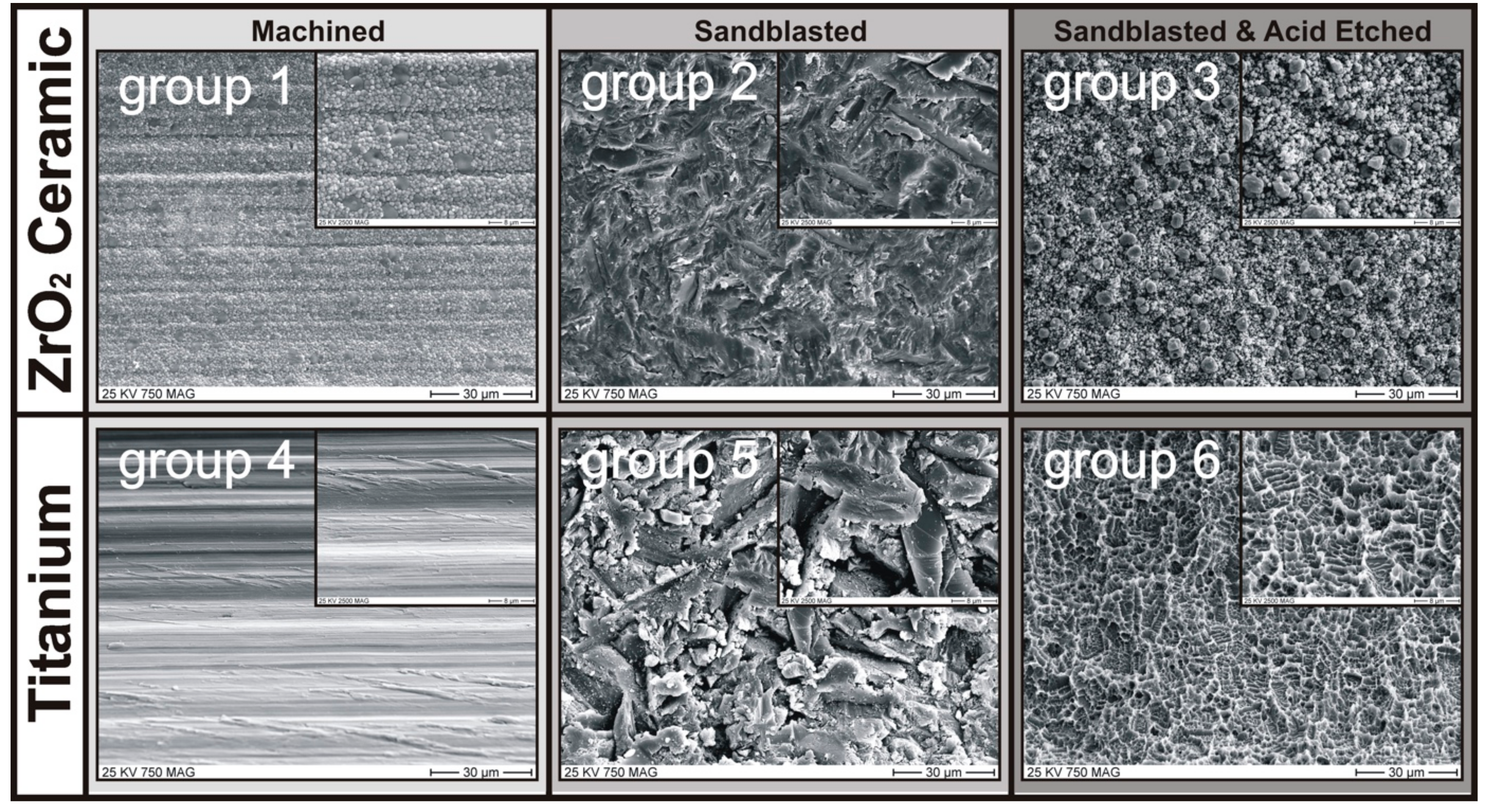
2.1. Implant Specimen and Surface Treatments
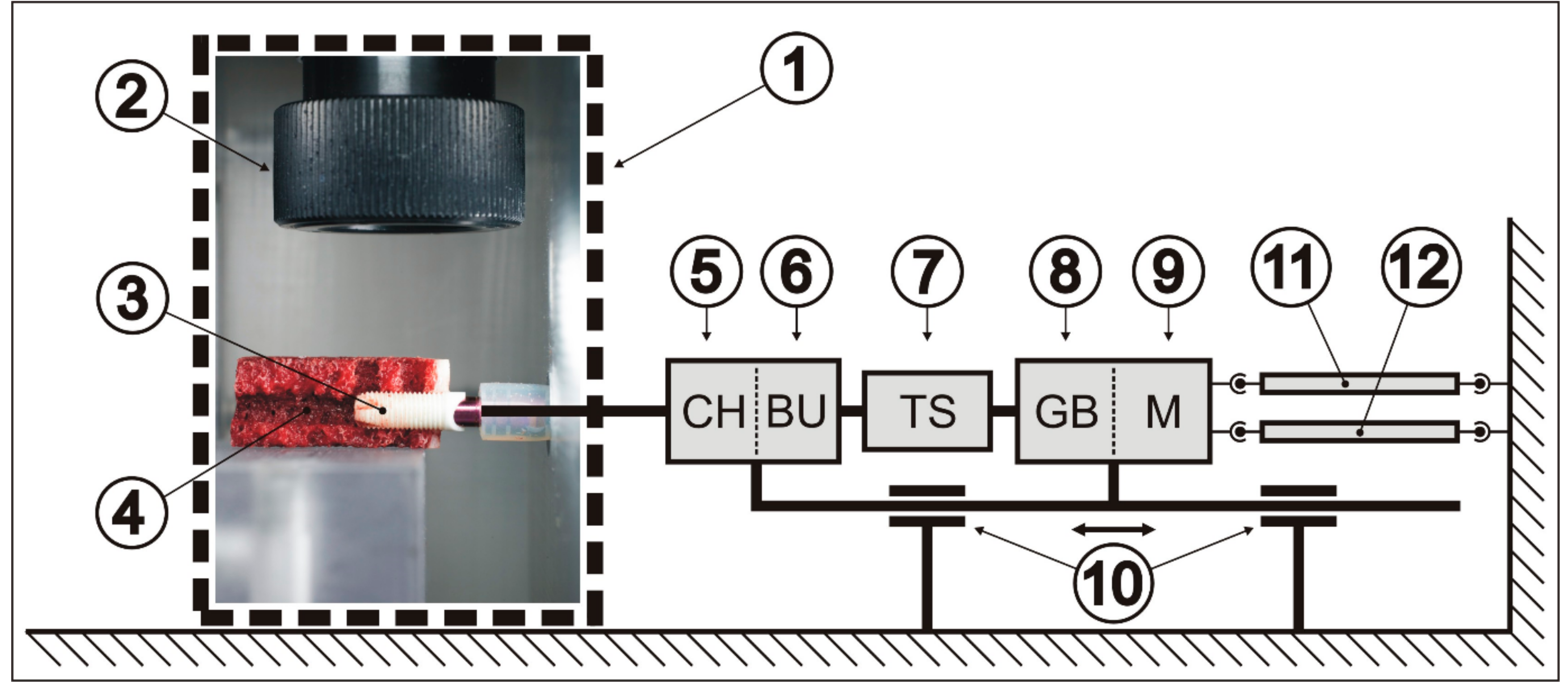
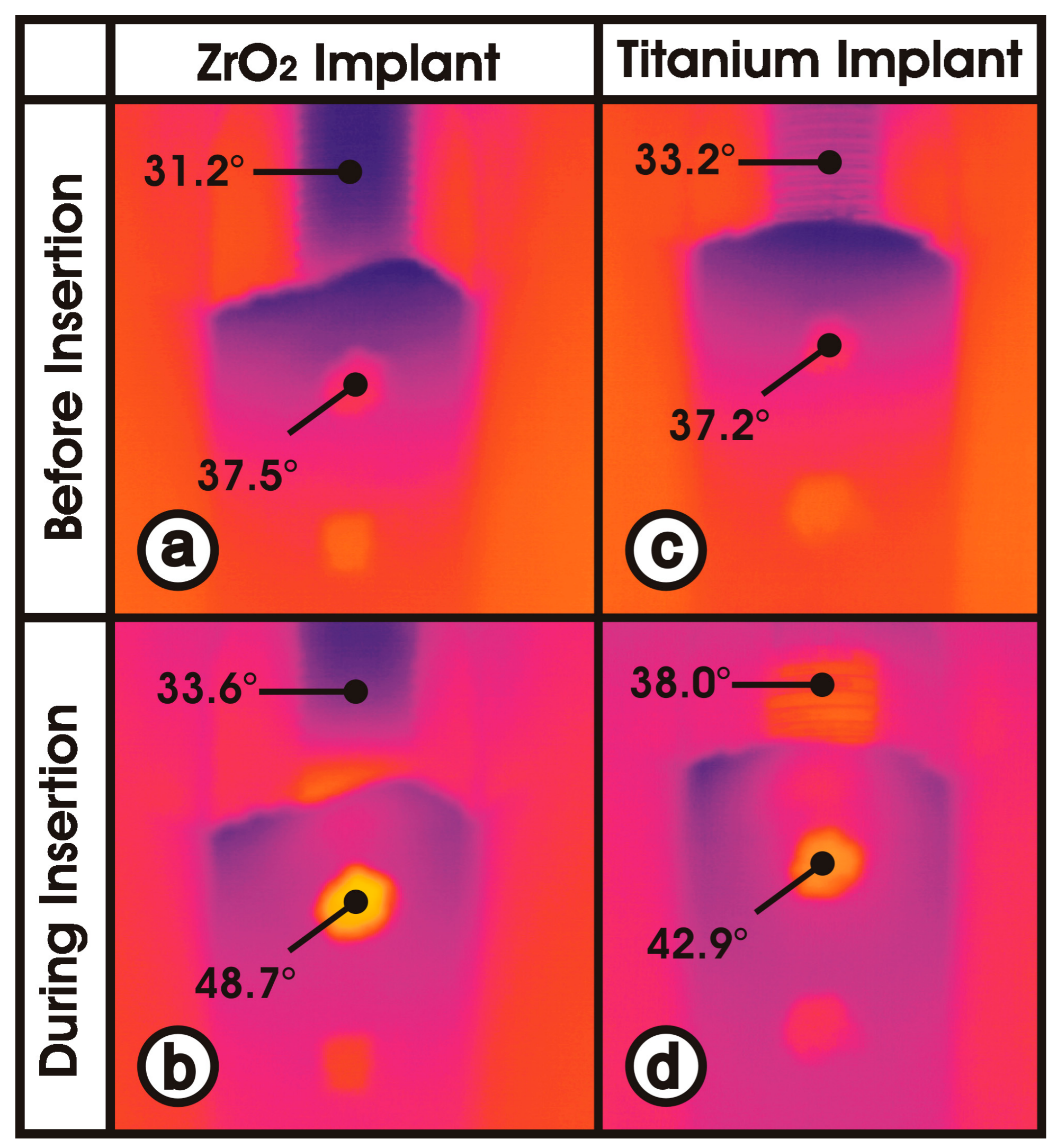
2.2. Measurement of Heat Generation and Dissipation
2.3. Bone Segments
2.4. Experimental Settings
2.5. Experimental Protocol
2.6. Statistical Analysis
3. Results
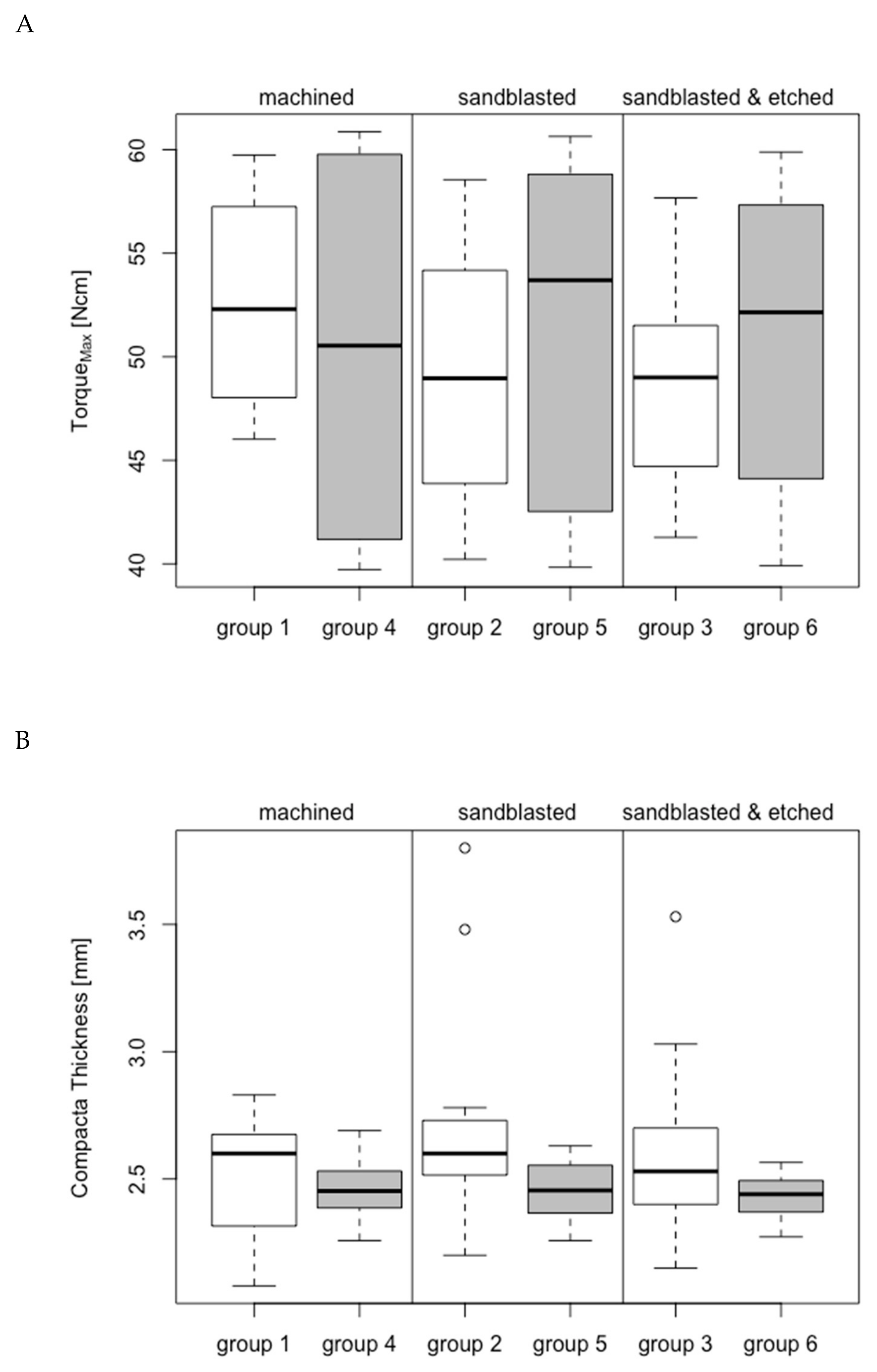
3.1. Group-Related Parameters of Specimens and in Vitro Setup
3.2. Maximum Temperature Increase
3.2.1. Bone Window 1
3.2.2. Bone Window 2
3.3. Overheating Exposure Time
3.3.1. Bone Window 1
3.3.2. Bone Window 2
3.4. Material-Dependent Thermal Energy Propagation
4. Discussion
4.1. Identical Specimen
4.2. In Vitro Setup
4.3. Temperature Increase at Bone Window 1
4.4. Temperature Increase at Bone Window 2
4.5. Differences in Bone Window 1 versus Bone Window 2
4.6. Heat Caused Damage to Peri-implant Bone
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cionca, N.; Müller, N.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: A prospective clinical study. Clin. Oral Implant. Res. 2015, 26, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Jemt, T.; Mölne, J.; Tengvall, P.; Wennerberg, A. On inflammation-immunological balance theory-A critical apprehension of disease concepts around implants: Mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin. Implant Dent. Relat. Res. 2019, 21, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, S.; Jager, B.; Axmann, D.; Gomez-Roman, G.; Groten, M.; Weber, H. Resonance frequency analysis and damping capacity assessment. Part I: An in vitro study on measurement reliability and a method of comparison in the determination of primary dental implant stability. Clin. Oral Implant. Res. 2006, 17, 75–79. [Google Scholar] [CrossRef]
- Garcia-Vives, N.; Andres-Garcia, R.; Rios-Santos, V.; Fernandez-Palacin, A.; Bullon-Fernandez, P.; Herrero-Climent, M.; Herrero-Climent, F. In vitro evaluation of the type of implant bed preparation with osteotomes in bone type IV and its influence on the stability of two implant systems. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 455–460. [Google Scholar]
- Moon, S.H.; Um, H.S.; Lee, J.K.; Chang, B.S.; Lee, M.K. The effect of implant shape and bone preparation on primary stability. J. Periodontal Implant Sci. 2010, 40, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Vora, S.; Bairam, L.; Kim, H.I.; Davis, E.L.; Andreana, S. Piezoelectric vs. conventional implant site preparation: Ex vivo implant primary stability. Clin. Oral Implant. Res. 2012, 23, 433–437. [Google Scholar] [CrossRef]
- Matsuoka, M.; Motoyoshi, M.; Sakaguchi, M.; Shinohara, A.; Shigeede, T.; Saito, Y.; Matsuda, M.; Shimizu, N. Friction heat during self-drilling of an orthodontic miniscrew. Int. J. Oral Maxillofac. Surg. 2011, 40, 191–194. [Google Scholar] [CrossRef]
- Aghvami, M.; Brunski, J.B.; Tulu, U.S.; Chen, C.H.; Helms, J.A. A Thermal and Biological Analysis of Bone Drilling. J. Biomech. Eng. 2018, 140, 101010. [Google Scholar] [CrossRef]
- Sumer, M.; Misir, A.F.; Telcioglu, N.T.; Guler, A.U.; Yenisey, M. Comparison of heat generation during implant drilling using stainless steel and ceramic drills. J. Oral Maxillofac. Surg. 2011, 69, 1350–1354. [Google Scholar] [CrossRef]
- Biyikli, S.; Modest, M.F.; Tarr, R. Measurements of thermal properties for human femora. J. Biomed. Mater. Res. 1986, 20, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P.; Masciotra, L. Effect of 50 to 60°C heating on osseointegration of dental implants in dense bone: An in vivo histological study. Implant Dent. 2014, 23, 516–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flanagan, D. Heat generated during seating of dental implant fixtures. J. Oral Implantol. 2014, 40, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.S.; Hirsch, C. Temperatures measured in human cortical bone when drilling. J. Bone Jt. Surg. 1972, 54, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.S.; Green, C.A.; Goldstein, S.A. The thermal effects of skeletal fixation-pin insertion in bone. J. Bone Jt. Surg. 1984, 66, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Bachus, K.N.; Rondina, M.T.; Hutchinson, D.T. The effects of drilling force on cortical temperatures and their duration: An in vitro study. Med. Eng. Phys. 2000, 22, 685–691. [Google Scholar] [CrossRef]
- Oh, H.J.; Wikesjo, U.M.; Kang, H.S.; Ku, Y.; Eom, T.G.; Koo, K.T. Effect of implant drill characteristics on heat generation in osteotomy sites: A pilot study. Clin. Oral Implant. Res. 2011, 22, 722–726. [Google Scholar] [CrossRef]
- Sener, B.C.; Dergin, G.; Gursoy, B.; Kelesoglu, E.; Slih, I. Effects of irrigation temperature on heat control in vitro at different drilling depths. Clin. Oral Implant. Res. 2009, 20, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Schmelzeisen, H. Thermische Schäden bei der Osteosynthese. OP-Journal 1992, 8, 26–29. (In German) [Google Scholar]
- Eriksson, A.R.; Albrektsson, T.; Albrektsson, B. Heat caused by drilling cortical bone. Temperature measured in vivo in patients and animals. Acta. Orthop. Scand. 1984, 55, 629–631. [Google Scholar] [CrossRef]
- Kunze, K.G.; Hofstetter, H.; Posalaky, I.; Winkler, B. Veränderung der Knochendurchblutung nach Osteotomien und Osteosynthesen. Unfallchirurgie 1981, 7, 169–180. (In German) [Google Scholar] [CrossRef]
- Markovic, A.; Misic, T.; Milicic, B.; Calvo-Guirado, J.L.; Aleksic, Z.; Dinic, A. Heat generation during implant placement in low-density bone: Effect of surgical technique, insertion torque and implant macro design. Clin. Oral Implant. Res. 2013, 24, 798–805. [Google Scholar] [CrossRef]
- Stocchero, M.; Jinno, Y.; Toia, M.; Ahmad, M.; Papia, E.; Yamaguchi, S.; Becktor, J.P. Intraosseous Temperature Change during Installation of Dental Implants with Two Different Surfaces and Different Drilling Protocols: An In Vivo Study in Sheep. J. Clin. Med. 2019, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Ludema, K.C.; Ajayi, L. Friction, Wear, Lubrication: A Textbook in Tribology, 2nd ed.; CRS Press: Boca Raton, FL, USA, 2018; ISBN 9781482210170. [Google Scholar]
- Markovic, A.; Misic, T.; Mancic, D.; Jovanovic, I.; Scepanovic, M.; Jezdic, Z. Real-time thermographic analysis of low-density bone during implant placement: A randomized parallel-group clinical study comparing lateral condensation with bone drilling surgical technique. Clin. Oral Implant. Res. 2014, 25, 910–918. [Google Scholar] [CrossRef]
- Sumer, M.; Keskiner, I.; Mercan, U.; Misir, F.; Cankaya, S. Assessment of heat generation during implant insertion. J. Prosthet. Dent. 2014, 112, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Lundskog, J. Heat and bone tissue. An experimental investigation of the thermal properties of bone and threshold levels for thermal injury. Scand. J. Plast. Reconstr. Surg. 1972, 9, 1–80. [Google Scholar] [PubMed]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Schmelzeisen, H. Der Bohrvorgang in der Kortikalis: Mechanik, Thermometrie, Morphologie; Springer: Berlin/Heidelberg, Germany, 1990. (In German) [Google Scholar]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of temperature on the dental implant osseointegration development in low-density bone: An in vivo histological evaluation. Implant Dent. 2015, 24, 96–100. [Google Scholar] [CrossRef] [PubMed]

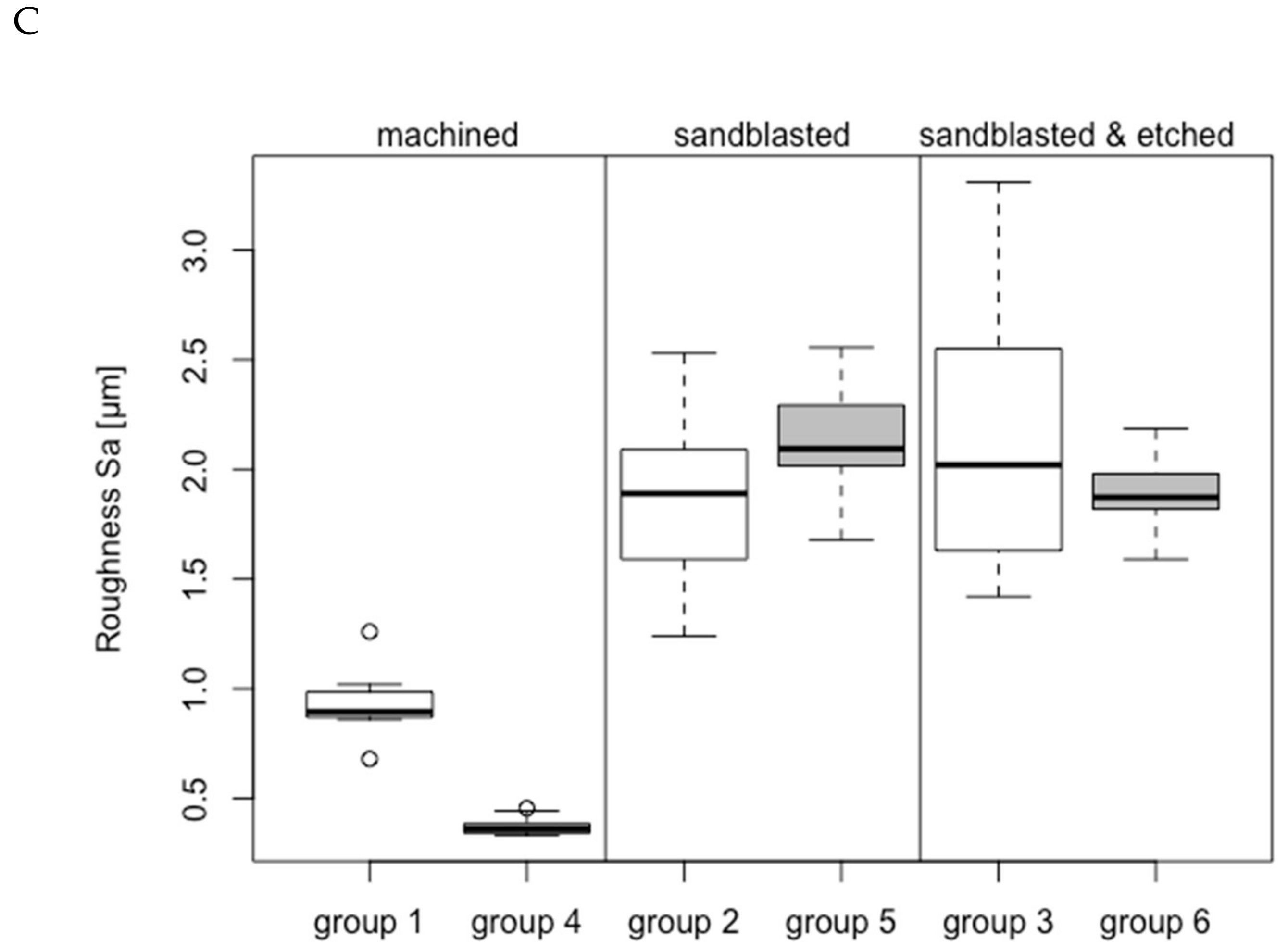
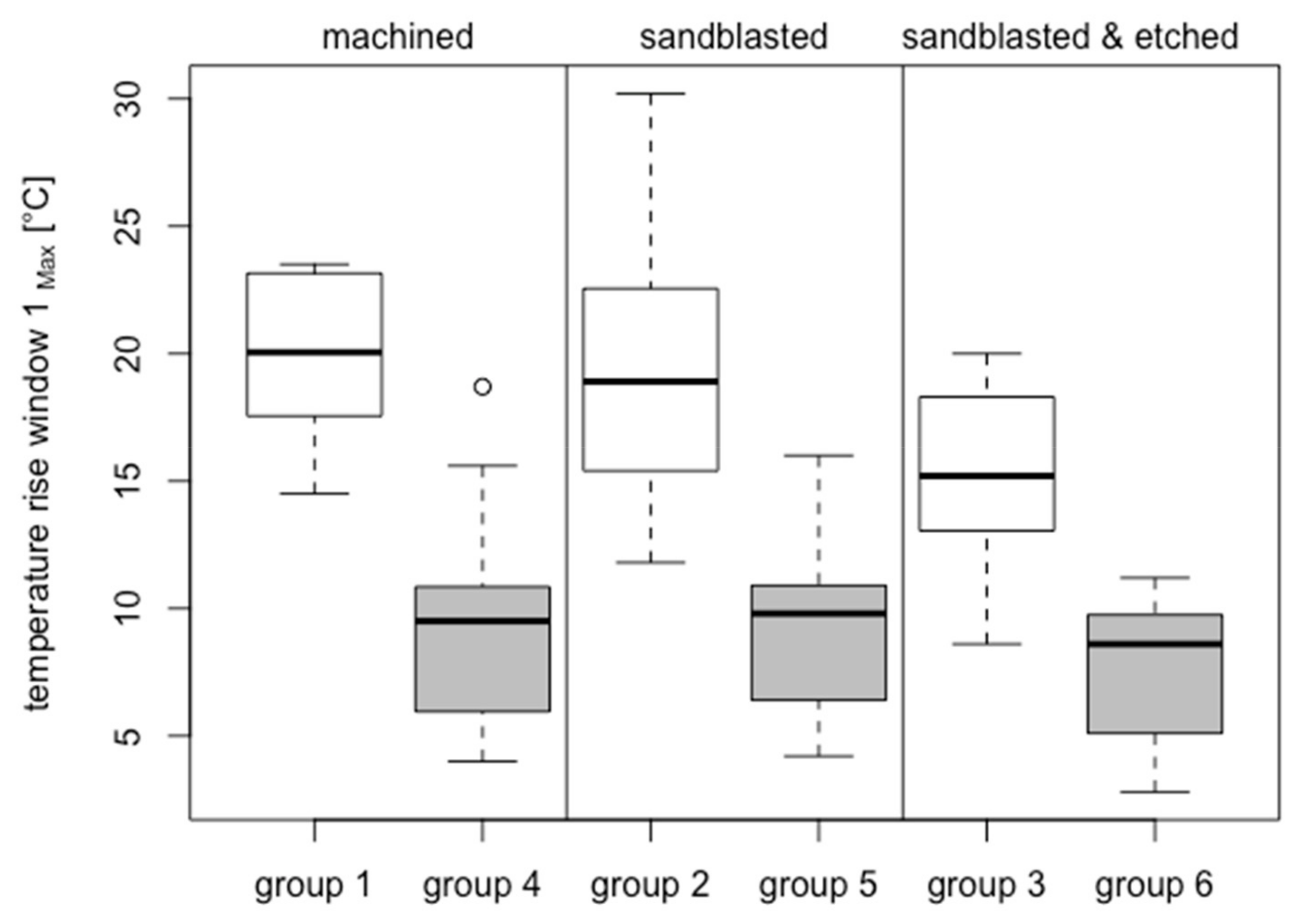
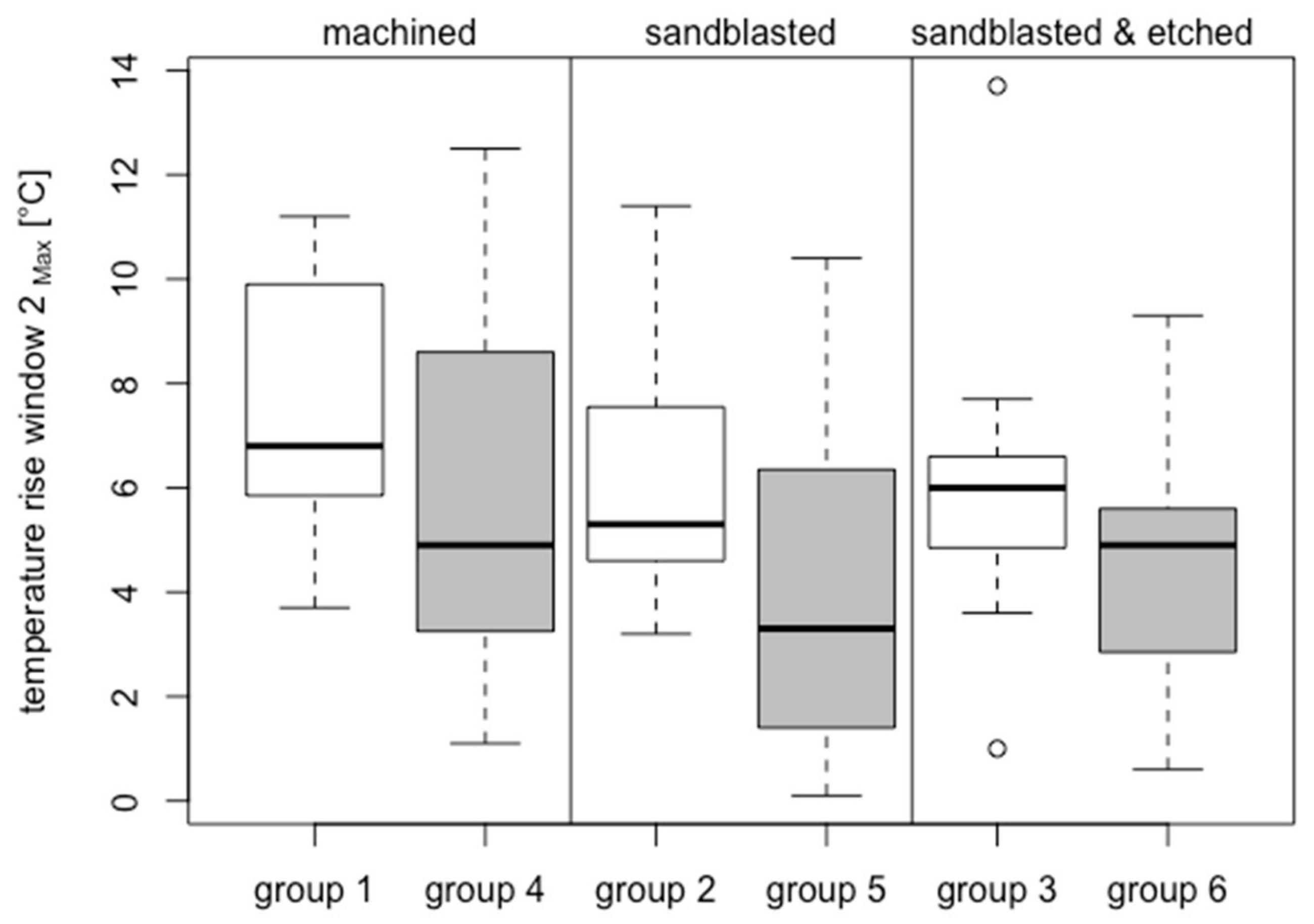
| Group | Analyzed (40–60 Ncm)/Inserted | Material | Surface Treatment | Surface Roughness Sa (µm) | Compacta Thickness (mm) | Insertion Torque (Ncm) | Temperature Increase BW-1 (°C) | ΔT > 10 °C BW-1 | ΔT > 13 °C BW-1 | ΔT > 18 °C BW-1 | Temperature Increase BW-2 (°C) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Time (s) | No. | Time (s) | No. | Time (s) | |||||||||
| 1 | 8/14 | ZrO2 | M | 0.93 ± 0.16 | 2.51 ± 0.25 | 52.61 ± 5.10 | 19.94 ± 3.28 | 8/8 | 39.06 | 8/8 | 25.10 | 5/8 | 11.36 | 7.50 ± 2.64 °C |
| 2 | 11/14 | ZrO2 | S | 1.86 ± 0.38 | 2.75 ± 0.47 | 48.97 ± 6.43 | 19.39 ± 5.73 | 11/11 | 28.10 | 10/11 | 16.93 | 6/11 | 7.98 | 6.14 ± 2.40 °C |
| 3 | 11/14 | ZrO2 | SAE | 2.14 ± 0.62 | 2.61 ± 0.39 | 48.79 ± 5.19 | 15.44 ± 3.63 | 10/11 | 20.37 | 8/11 | 10.19 | 3/11 | 0.43 | 6.07 ± 3.11 °C |
| 4 | 15/22 | Ti Grade 4 | M | 0.37 ± 0.04 | 2.46 ± 0.12 | 50.10 ± 8.69 | 9.33 ± 4.18 | 7/15 | 22.49 | 3/15 | 19.07 | 1/15 | 20.40 | 6.03 ± 3.69 °C |
| 5 | 15/20 | Ti Grade 4 | S | 2.15 ± 0.24 | 2.46 ± 0.12 | 50.99 ± 7.97 | 9.20 ± 3.31 | 6/15 | 23.05 | 2/15 | 15.65 | - | - | 4.33 ± 3.26 °C |
| 6 | 15/21 | Ti Grade 4 | SAE | 1.89 ± 0.18 | 2.42 ± 0.09 | 50.72 ± 7.22 | 7.68 ± 2.68 | 2/15 | 17.45 | - | - | - | - | 4.41 ± 2.47 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zipprich, H.; Weigl, P.; König, E.; Toderas, A.; Balaban, Ü.; Ratka, C. Heat Generation at the Implant–Bone Interface by Insertion of Ceramic and Titanium Implants. J. Clin. Med. 2019, 8, 1541. https://doi.org/10.3390/jcm8101541
Zipprich H, Weigl P, König E, Toderas A, Balaban Ü, Ratka C. Heat Generation at the Implant–Bone Interface by Insertion of Ceramic and Titanium Implants. Journal of Clinical Medicine. 2019; 8(10):1541. https://doi.org/10.3390/jcm8101541
Chicago/Turabian StyleZipprich, Holger, Paul Weigl, Eugenie König, Alexandra Toderas, Ümniye Balaban, and Christoph Ratka. 2019. "Heat Generation at the Implant–Bone Interface by Insertion of Ceramic and Titanium Implants" Journal of Clinical Medicine 8, no. 10: 1541. https://doi.org/10.3390/jcm8101541
APA StyleZipprich, H., Weigl, P., König, E., Toderas, A., Balaban, Ü., & Ratka, C. (2019). Heat Generation at the Implant–Bone Interface by Insertion of Ceramic and Titanium Implants. Journal of Clinical Medicine, 8(10), 1541. https://doi.org/10.3390/jcm8101541





