Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders
Abstract
1. Introduction
2. Material & Methods
2.1. Participants
2.2. Clinical Assessments
2.3. Eye-Tracking Paradigm
2.4. Analyses Strategy
3. Results
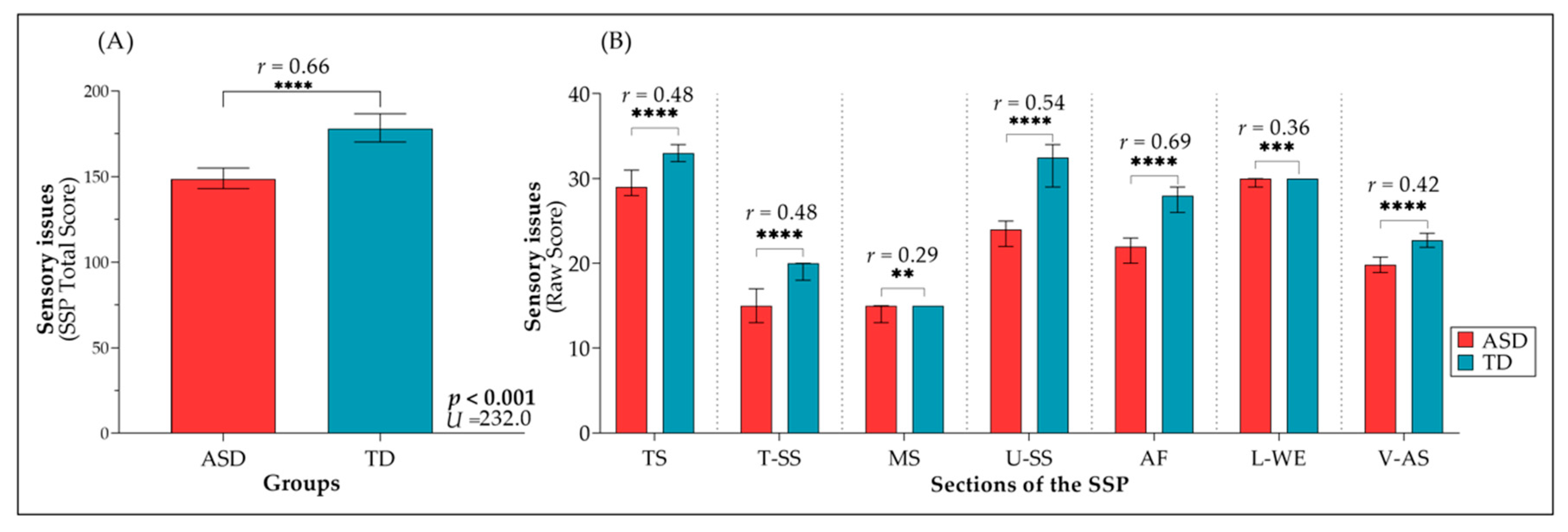
3.1. Description of The Study Sample
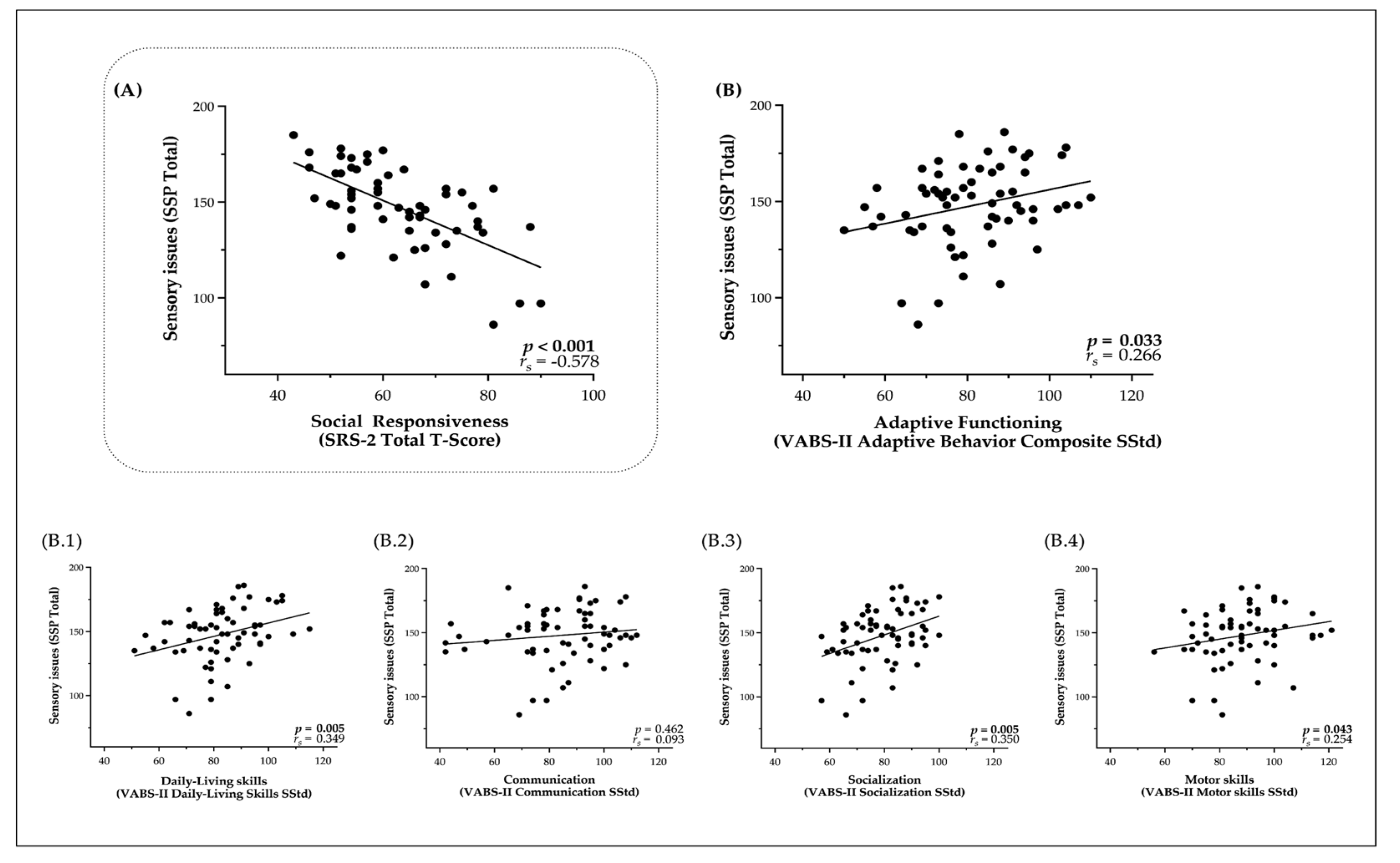
3.2. Relationship of Sensory Processing Issues with Social Difficulties and Adaptive Functioning
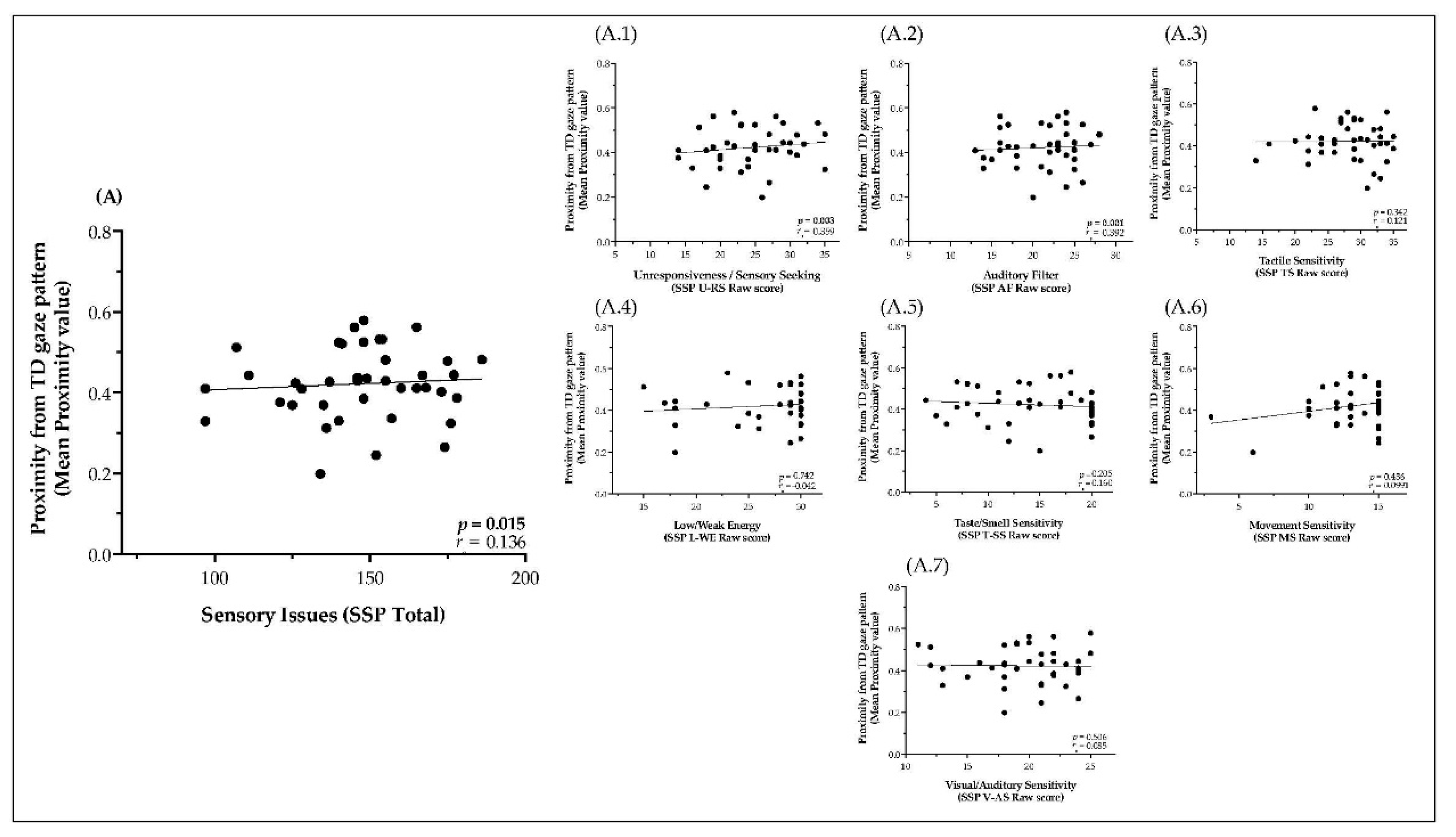
3.3. Relationship of Sensory Processing Issues with Dynamic Visual Exploration of Social Scenes
4. Discussion
4.1. Relation between Sensory Issues and Social Skills
4.2. Relationship Between Sensory Issues and Adaptive Functioning
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Association Publishing: Philadelphia, PA, USA, 2013; ISBN 978-0-89042-557-2. [Google Scholar]
- Klintwall, L.; Eldevik, S.; Eikeseth, S. Narrowing the gap: Effects of intervention on developmental trajectories in autism. Autism Int. J. Res. Pract. 2015, 19, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Rogers, S.; Munson, J.; Smith, M.; Winter, J.; Greenson, J.; Donaldson, A.; Varley, J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics 2010, 125, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Fein, D.; Barton, M.; Eigsti, I.-M.; Kelley, E.; Naigles, L.; Schultz, R.T.; Stevens, M.; Helt, M.; Orinstein, A.; Rosenthal, M.; et al. Optimal Outcome in Individuals with a History of Autism. J. Child Psychol. Psychiatry 2013, 54, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Woynaroski, T.G.; Nowell, S.; Brown, L.T.; Dubay, M.; Crais, E.R.; Watson, L.R. Cascading effects of attention disengagement and sensory seeking on social symptoms in a community sample of infants at-risk for a future diagnosis of autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L. Autistic disturbances of affective contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Baranek, G.T.; Watson, L.R.; Boyd, B.A.; Poe, M.D.; David, F.J.; McGuire, L. Hyporesponsiveness to Social and Nonsocial Sensory Stimuli in Children with Autism, Children with Developmental Delays, and Typically Developing Children. Dev. Psychopathol. 2013, 25, 307–320. [Google Scholar] [CrossRef]
- Baranek, G.T.; Boyd, B.A.; Poe, M.D.; David, F.J.; Watson, L.R. Hyperresponsive Sensory Patterns in Young Children with Autism, Developmental Delay, and Typical Development. Am. J. Ment. Retard. 2007, 112, 233–245. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Damiano-Goodwin, C.R.; Woynaroski, T.G.; Simon, D.M.; Ibañez, L.V.; Murias, M.; Kirby, A.; Newsom, C.R.; Wallace, M.T.; Stone, W.L.; Cascio, C.J. Developmental sequelae and neurophysiologic substrates of sensory seeking in infant siblings of children with autism spectrum disorder. Dev. Cogn. Neurosci. 2018, 29, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.V.; Little, L.M.; Schultz, B.; Baranek, G.T. Observational Characterization of Sensory Interests, Repetitions, and Seeking Behaviors. Am. J. Occup. Ther. 2015, 69, 6903220010p1-9. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr. Res. 2011, 69, 48R–54R. [Google Scholar] [PubMed]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef] [PubMed]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [PubMed]
- Klin, A.; Saulnier, C.A.; Sparrow, S.S.; Cicchetti, D.V.; Volkmar, F.R.; Lord, C. Social and Communication Abilities and Disabilities in Higher Functioning Individuals with Autism Spectrum Disorders: The Vineland and the ADOS. J. Autism Dev. Disord. 2007, 37, 748–759. [Google Scholar] [CrossRef]
- Gliga, T.; Jones, E.; Bedford, R.; Charman, T.; Johnson, M. From early markers to neuro-developmental mechanisms of autism. Dev. Rev. 2014, 34, 189–207. [Google Scholar] [CrossRef]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M.; Baron-Cohen, S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef]
- Robertson, A.E.; Simmons, D.R. The relationship between sensory sensitivity and autistic traits in the general population. J. Autism Dev. Disord. 2013, 43, 775–784. [Google Scholar] [CrossRef]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Gliga, T.; Bedford, R.; Charman, T.; Johnson, M.H.; Baron-Cohen, S. Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms. Curr. Biol. 2015, 25, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Turner-Brown, L.M.; Baranek, G.T.; Reznick, J.S.; Watson, L.R.; Crais, E.R. The First Year Inventory: A longitudinal follow-up of 12-month-old to 3-year-old children. Autism Int. J. Res. Pract. 2013, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Gori, S.; Federici, A.; DeVita, M.; Carna, S.; Sali, M.E.; Molteni, M.; Casartelli, L.; Facoetti, A. Weak surround suppression of the attentional focus characterizes visual selection in the ventral stream in autism. NeuroImage Clin. 2018, 18, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, L.; Riva, M.; Villa, L.; Borgatti, R. Insights from perceptual, sensory, and motor functioning in autism and cerebellar primary disturbances: Are there reliable markers for these disorders? Neurosci. Biobehav. Rev. 2018, 95, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Chita-Tegmark, M. Attention Allocation in ASD: A Review and Meta-analysis of Eye-Tracking Studies. Rev. J. Autism Dev. Disord. 2016, 3, 209–223. [Google Scholar] [CrossRef]
- Frazier, T.W.; Strauss, M.; Klingemier, E.W.; Zetzer, E.E.; Hardan, A.Y.; Eng, C.; Youngstrom, E.A. A Meta-Analysis of Gaze Differences to Social and Nonsocial Information Between Individuals with and Without Autism. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Hilton, C.L.; Harper, J.D.; Kueker, R.H.; Lang, A.R.; Abbacchi, A.M.; Todorov, A.; LaVesser, P.D. Sensory Responsiveness as a Predictor of Social Severity in Children with High Functioning Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Kirby, A.V.; Watson, L.R.; Sideris, J.; Bulluck, J.; Baranek, G.T. Sensory features as predictors of adaptive behaviors: A comparative longitudinal study of children with autism spectrum disorder and other developmental disabilities. Res. Dev. Disabil. 2018, 81, 103–112. [Google Scholar] [CrossRef]
- Dellapiazza, F.; ELENA Study Group; Michelon, C.; Oreve, M.-J.; Robel, L.; Schoenberger, M.; Chatel, C.; Vesperini, S.; Maffre, T.; Schmidt, R.; et al. The Impact of Atypical Sensory Processing on Adaptive Functioning and Maladaptive Behaviors in Autism Spectrum Disorder During Childhood: Results from the ELENA Cohort. J. Autism Dev. Disord. 2019, 49, 1–11. [Google Scholar] [CrossRef]
- Ausderau, K.K.; Sideris, J.; Little, L.M.; Furlong, M.; Bulluck, J.C.; Baranek, G.T. Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Res. 2016, 9, 1316–1327. [Google Scholar] [CrossRef]
- Franchini, M.; De Wilde, H.W.; Glaser, B.; Eliez, S.; Schaer, M.; Gentaz, E. Brief Report: A Preference for Biological Motion Predicts a Reduction in Symptom Severity 1 Year Later in Preschoolers with Autism Spectrum Disorders. Front. Psychol. 2016, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Zöller, D.; Gentaz, E.; Glaser, B.; De Wilde, H.W.; Kojovic, N.; Eliez, S.; Schaer, M. Early Adaptive Functioning Trajectories in Preschoolers with Autism Spectrum Disorders. J. Pediatr. Psychol. 2018, 43, 800–813. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, D.N.; Miller, L.J.; Shyu, V.; Dunn, W. Overview of the short sensory profile (SSP). In The Sensory Profile; Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; DiLavore, P.C.; Gotham, K.; Guthrie, W.; Luyster, R.J.; Risi, S.; Rutter, M. Western Psychological Services (Firm) Autism Diagnostic Observation schedule: ADOS-2; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Hus, V.; Gotham, K.; Lord, C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. J. Autism Dev. Disord. 2014, 44, 2400–2412. [Google Scholar] [CrossRef] [PubMed]
- Esler, A.N.; Bal, V.H.; Guthrie, W.; Wetherby, A.; Weismer, S.E.; Lord, C. The Autism Diagnostic Observation Schedule, Toddler Module: Standardized Severity Scores. J. Autism Dev. Disord. 2015, 45, 2704–2720. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W. Sensory Profile: User’s Manual; Psychological Corp.: San Antonio, TX, USA, 1999; ISBN 978-0-7616-3801-8. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale (SRS); Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Sparrow, S.S.; Balla, D.; Cicchetti, D.V. Vineland II: Vineland Adaptative Behavior Scales: Survey Forms Manual: A Revision of the Vineland Social Maturity Scale by Edgar A. Doll; Pearson: London, UK, 2005. [Google Scholar]
- Howlin, P.; Savage, S.; Moss, P.; Tempier, A.; Rutter, M. Cognitive and language skills in adults with autism: A 40-year follow-up. J. Child Psychol. Psychiatry 2014, 55, 49–58. [Google Scholar] [CrossRef]
- The Tobii I-VT fixation filter. Available online: https://stemedhub.org/resources/2173/download/Tobii_WhitePaper_TobiiIVTFixationFilter.pdf (accessed on 22 August 2019).
- Sperdin, H.F.; Coito, A.; Kojovic, N.; Rihs, T.A.; Jan, R.K.; Franchini, M.; Plomp, G.; Vulliemoz, S.; Eliez, S.; Michel, C.M.; et al. Early alterations of social brain networks in young children with autism. eLife 2018, 7, e31670. [Google Scholar] [CrossRef]
- Kojovic, N.; Franchini, M.; Zöller, D.; Rihs, T.A.; Jan, R.K.; Sperdin, H.F.; Schaer, M. Quantifying the dynamic of visual exploration of complex social scenes in children with autism spectrum disorders without any a-priori: A data-driven eye-tracking approach. Manuscscript in preparation.
- Botev, Z.I.; Grotowski, J.F.; Kroese, D.P. Kernel density estimation via diffusion. Ann. Stat. 2010, 38, 2916–2957. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS; SAGE Publications: Thousand Oaks, CA, USA, 2009; ISBN 978-1-84787-907-3. [Google Scholar]
- Sacrey, L.-A.R.; Bryson, S.E.; Zwaigenbaum, L. Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: A longitudinal study from 6 to 36 months of age. Behav. Brain Res. 2013, 256, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Meltzoff, A.N.; Osterling, J.; Rinaldi, J.; Brown, E. Children with Autism Fail to Orient to Naturally Occurring Social Stimuli. J. Autism Dev. Disord. 1998, 28, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Jones, E.J.; Merkle, K.; Venema, K.; Lowy, R.; Faja, S.; Kamara, D.; Murias, M.; Greenson, J.; Winter, J.; et al. Early Behavioral Intervention Is Associated with Normalized Brain Activity in Young Children with Autism. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The Social Motivation Theory of Autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Glod, M.; Riby, D.M.; Honey, E.; Rodgers, J. Psychological Correlates of Sensory Processing Patterns in Individuals with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2015, 2, 199–221. [Google Scholar] [CrossRef]
- Boyd, B.A.; Baranek, G.T.; Sideris, J.; Poe, M.D.; Watson, L.R.; Patten, E.; Miller, H. Sensory Features and Repetitive Behaviors in Children with Autism and Developmental Delays. Autism Res. 2010, 3, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Wodka, E.L.; Mostofsky, S.H.; Puts, N.A.J. Autism spectrum disorder in the scope of tactile processing. Dev. Cogn. Neurosci. 2018, 29, 140–150. [Google Scholar] [CrossRef]
- Schultz, R.T. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005, 23, 125–141. [Google Scholar] [CrossRef]
- Elison, J.T.; Paterson, S.J.; Wolff, J.J.; Reznick, J.S.; Sasson, N.J.; Gu, H.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; Evans, A.C.; et al. White Matter Microstructure and Atypical Visual Orienting in 7-Month-Olds at Risk for Autism. Am. J. Psychiatry 2013, 170, 899–908. [Google Scholar] [CrossRef]
- Landry, R.; Bryson, S.E. Impaired disengagement of attention in young children with autism. J. Child Psychol. Psychiatry 2004, 45, 1115–1122. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, M.; Duchesne, X.M.; Laugeson, E.A.; Kennedy, D.P.; Adolphs, R.; Zhao, Q. Atypical visual saliency in autism spectrum disorder quantified through model-based eye tracking. Neuron 2015, 88, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, S.; Chen, B.; Hakimi, S.; Paul, L.K.; Zhao, Q.; Adolphs, R. Revealing the world of autism through the lens of a camera. Curr. Biol. 2016, 26, R909–R910. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; DSM-IV-TR®; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Farley, M.A.; McMahon, W.M.; Fombonne, E.; Jenson, W.R.; Miller, J.; Gardner, M.; Block, H.; Pingree, C.B.; Ritvo, E.R.; Ritvo, R.A.; et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res. 2009, 2, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Mouga, S.; Almeida, J.; Café, C.; Duque, F.; Oliveira, G. Adaptive profiles in autism and other neurodevelopmental disorders. J. Autism Dev. Disord. 2015, 45, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, E.; Couture, M.; McKinley, P.; Reid, G.; Fombonne, E.; Gisel, E. Sensori-motor and Daily Living Skills of Preschool Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 231–241. [Google Scholar] [CrossRef]
- Baker, A.E.Z.; Lane, A.; Angley, M.T.; Young, R.L. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. J. Autism Dev. Disord. 2008, 38, 867–875. [Google Scholar] [CrossRef] [PubMed]

| Measures | ASD (n = 68) | TD (n = 36) | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p value * | |
| Age | 4.0 (0.8) | 3.9 (0.7) | p = 0.7692 |
| Best Estimate IQ | 78.3 (26.2) | 113 (13.4) | p < 0.0011 |
| ADOS-CSS | 7.1 (1.7) | 1.0 (0.2) | p < 0.0012 |
| SSP Total | 147.7 (20.5) | 176.6 (11.5) | p < 0.0012 |
| SRS-2 Total | 63.7 (11.6) | 43.2 (5.2) | p < 0.0012 |
| VABS-II Adaptive Behavior Composite | 81.0 (13.4) | 98.5 (11.9) | p < 0.0011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojovic, N.; Ben Hadid, L.; Franchini, M.; Schaer, M. Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders. J. Clin. Med. 2019, 8, 1508. https://doi.org/10.3390/jcm8101508
Kojovic N, Ben Hadid L, Franchini M, Schaer M. Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders. Journal of Clinical Medicine. 2019; 8(10):1508. https://doi.org/10.3390/jcm8101508
Chicago/Turabian StyleKojovic, Nada, Lylia Ben Hadid, Martina Franchini, and Marie Schaer. 2019. "Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders" Journal of Clinical Medicine 8, no. 10: 1508. https://doi.org/10.3390/jcm8101508
APA StyleKojovic, N., Ben Hadid, L., Franchini, M., & Schaer, M. (2019). Sensory Processing Issues and Their Association with Social Difficulties in Children with Autism Spectrum Disorders. Journal of Clinical Medicine, 8(10), 1508. https://doi.org/10.3390/jcm8101508




