Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Settings and Participants
2.3. Study Data
2.4. Statistical Analysis
3. Results
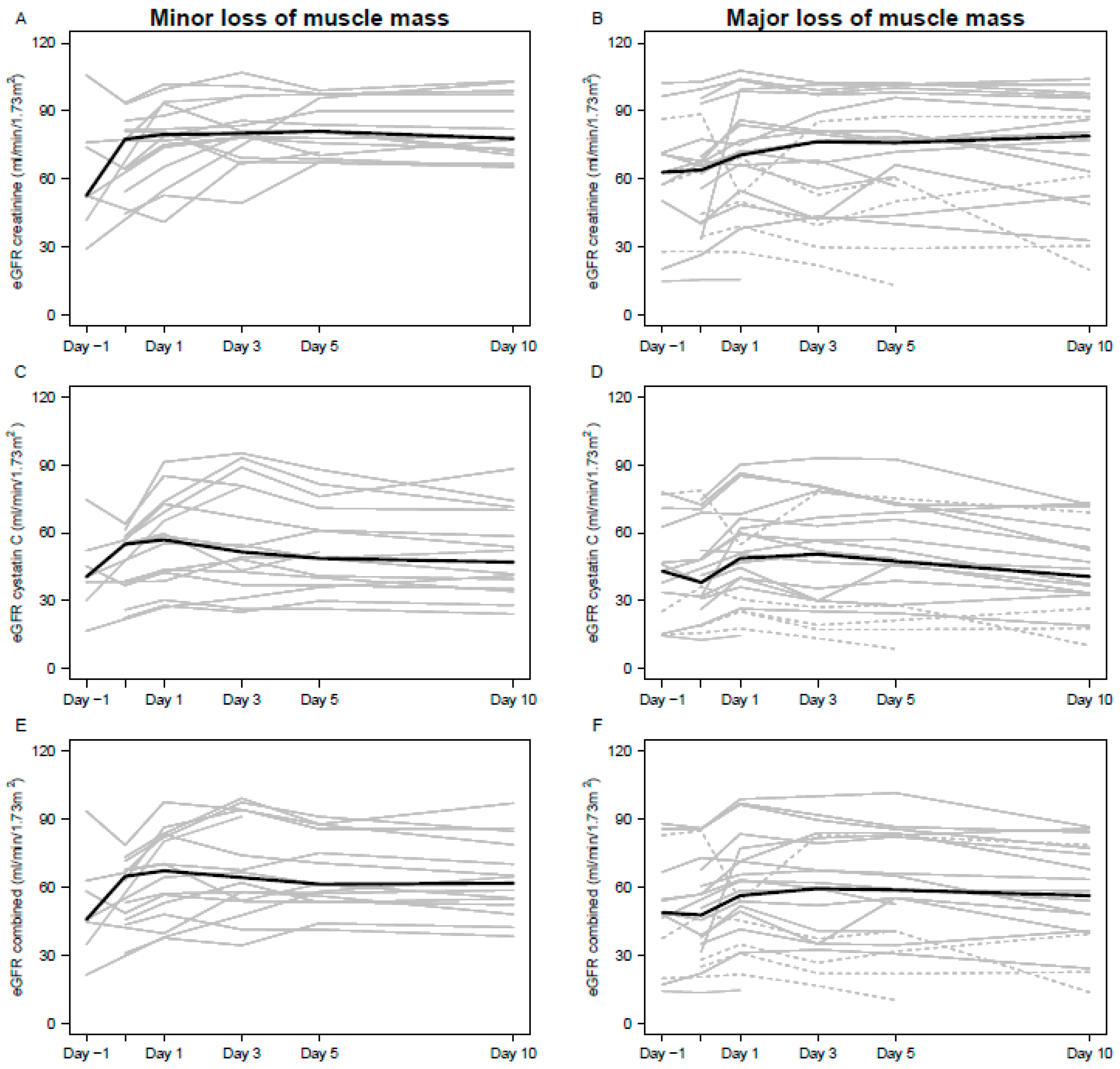
3.1. Impact of Amputation on Kidney Function
3.2. Prescribing Renally-Eliminated Medications
3.3. Kidney Function Estimates During Hospitalization
4. Discussion
4.1. eGFR Differences Before, and After, Amputation
4.2. Choice of eGFR Equation and Biomarker to Amputation Patients
4.3. Choice of eGFR Biomarkers Impact on Dosing Discrepancies
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seyffart, G. Seyffart’s Directory of Drug Dosage in Kidney Disease; Dustri-Verlag Feistle: Oberhaching-Munich, Germany, 2011. [Google Scholar]
- Drug Therapy for the Elderly; Wehling, M., Ed.; Springer: Wien, NY, USA, 2013; ISBN 978-3-7091-0911-3. [Google Scholar]
- Iversen, E.; Bodilsen, A.C.; Klausen, H.; Treldal, C.; Andersen, O.; Houlind, M.; Petersen, J. Kidney function estimates using cystatin C versus creatinine: Impact on medication prescribing in acutely hospitalized elderly patients. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Henriksen, D.P.; Marinakis, C.; Hellebek, A.; Birn, H.; Nybo, M.; Søndergaard, J.; Nymark, A.; Pedersen, C. Drug dosing in patients with renal insufficiency in a hospital setting using electronic prescribing and automated reporting of estimated glomerular filtration rate. Basic Clin. Pharmacol. Toxicol. 2014, 114, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Long, C.L.; Raebel, M.A.; Price, D.W.; Magid, D.J. Compliance with dosing guidelines in patients with chronic kidney disease. Ann. Pharmacother. 2004, 38, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Helldén, A.; Bergman, U.; von Euler, M.; Hentschke, M.; Odar-Cederlöf, I.; Ohlén, G. Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department: A retrospective study. Drugs Aging 2009, 26, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.S.; Petersen, J.; Kirketerp-Møller, K.; Poulsen, I.; Andersen, O. Progression of disease preceding lower extremity amputation in Denmark: A longitudinal registry study of diagnoses, use of medication and healthcare services 14 years prior to amputation. BMJ Open 2017, 7, e016030. [Google Scholar] [CrossRef] [PubMed]
- Neland, M.; Birn, H.; Lise Kamper, A.; A Lagedfoged, S.; Randers, E.; Rehling, M.; Reinholdt, B.; Rossing, P.; Marie Schmidt, I. Kronisk nyresygdom: Analysemetoder og Klinisk evaluering.Rekommandationer for vurdering af glomerulær Filtrationsrate og Albuminuri. Available online: http://www.nephrology.dk/Publikationer/Kronisk%20nyresygdom%202015%20endelig,%2014-08-15.pdf (accessed on 13 January 2019).
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease Clinical Practice Guidelines. Available online: https://www.guidelinecentral.com/summaries/kdigo-2012-clinical-practice-guideline-for-the-evaluation-and-management-of-chronic-kidney-disease/#section-society (accessed on 12 September 2018).
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Guidedance for Industry—Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling; Center for Drug Evaluation and Research: Washington, DC, USA, 2010.
- Cartet-Farnier, E.; Goutelle-Audibert, L.; Maire, P.; De la Gastine, B.; Goutelle, S. Implications of using the MDRD or CKD-EPI equation instead of the Cockcroft-Gault equation for estimating renal function and drug dosage adjustment in elderly patients. Fundam. Clin. Pharmacol. 2017, 31, 110–119. [Google Scholar] [CrossRef]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J.; Bennett, W.M.; Decker, B.S.; Eckardt, K.-U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney disease-a clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [CrossRef]
- Michels, W.M.; Grootendorst, D.C.; Verduijn, M.; Elliott, E.G.; Dekker, F.W.; Krediet, R.T. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 1003–1009. [Google Scholar] [CrossRef]
- Shannon, J.A. The renal excretion of creatinine in man. J. Clin. Investig. 1935, 14, 403–410. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [PubMed]
- Beddhu, S.; Samore, M.H.; Roberts, M.S.; Stoddard, G.J.; Pappas, L.M.; Cheung, A.K. Creatinine Production, Nutrition, and Glomerular Filtration Rate Estimation. J. Am. Soc. Nephrol. 2003, 14, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Abbott, K.C.; Linberg, A.; Little, D.; Fenderson, J.; Olson, S.W. SCr and SCysC concentrations before and after traumatic amputation in male soldiers: A case-control study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 63, 167–170. [Google Scholar] [CrossRef]
- Acute Kidney Injury (AKI). Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 12 September 2018).
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 348–354. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Rule, A.D.; Voskoboev, N.; Baumann, N.A.; Lieske, J.C. Performance of cystatin C- and creatinine-based estimated glomerular filtration rate equations depends on patient characteristics. Clin. Chem. 2015, 61, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- Fan, L.; Levey, A.S.; Gudnason, V.; Eiriksdottir, G.; Andresdottir, M.B.; Gudmundsdottir, H.; Indridason, O.S.; Palsson, R.; Mitchell, G.; Inker, L.A. Comparing GFR Estimating Equations Using Cystatin C and Creatinine in Elderly Individuals. J. Am. Soc. Nephrol. JASN 2015, 26, 1982–1989. [Google Scholar] [CrossRef]
- Raman, M.; Middleton, R.J.; Kalra, P.A.; Green, D. Estimating renal function in old people: An in-depth review. Int. Urol. Nephrol. 2017, 49, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Inker, L.A.; Rossert, J.; Froissart, M.; Rossing, P.; Mauer, M.; Levey, A.S. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 1195–1203. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G.; et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.M.; Warkentin, K.D.; Chilibeck, P.D.; Magnus, C.R.A. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J. Strength Cond. Res. 2010, 24, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, A.C.; Klausen, H.H.; Petersen, J.; Beyer, N.; Andersen, O.; Jørgensen, L.M.; Juul-Larsen, H.G.; Bandholm, T. Prediction of Mobility Limitations after Hospitalization in Older Medical Patients by Simple Measures of Physical Performance Obtained at Admission to the Emergency Department. PLoS ONE 2016, 11, e0154350. [Google Scholar] [CrossRef] [PubMed]
- Alley, D.E.; Shardell, M.D.; Peters, K.W.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 559–566. [Google Scholar] [CrossRef]
- Peters, B.J.; Rule, A.D.; Kashani, K.B.; Lieske, J.C.; Mara, K.C.; Dierkhising, R.A.; Barreto, E.F. Impact of Serum Cystatin C-Based Glomerular Filtration Rate Estimates on Drug Dose Selection in Hospitalized Patients. Pharmacotherapy 2018, 38, 1068–1073. [Google Scholar] [CrossRef]
- Wang, C.H.; Rubinsky, A.D.; Minichiello, T.; Shlipak, M.G.; Price, E.L. Creatinine Versus Cystatin C: Differing Estimates of Renal Function in Hospitalized Veterans Receiving Anticoagulants. J. Gen. Intern. Med. 2018, 33, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Benraad, C.; Wetzels, J.; Rikkert, M.O.; Kramers, C. Clinical Relevance of Differences in Glomerular Filtration Rate Estimations in Frail Older People by Creatinine- vs. Cystatin C-Based Formulae. Drugs Aging 2017, 34, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.A.; Willey, J.Z.; Moon, Y.P.; Elkind, M.S.V.; Sacco, R.L.; Wolf, M.; Cheung, K.; Wright, C.B.; Mohan, S. Creatinine- versus cystatin C-based renal function assessment in the Northern Manhattan Study. PLoS ONE 2018, 13, e0206839. [Google Scholar] [CrossRef]
- Stern, J.R.; Wong, C.K.; Yerovinkina, M.; Spindler, S.J.; See, A.S.; Panjaki, S.; Loven, S.L.; D’Andrea, R.F.; Nowygrod, R. A Meta-analysis of Long-term Mortality and Associated Risk Factors following Lower Extremity Amputation. Ann. Vasc. Surg. 2017, 42, 322–327. [Google Scholar] [CrossRef]
- Nelson, M.T.; Greenblatt, D.Y.; Soma, G.; Rajimanickam, V.; Greenberg, C.C.; Kent, K.C. Preoperative factors predict mortality after major lower-extremity amputation. Surgery 2012, 152, 685–694; discussion 694–696. [Google Scholar] [CrossRef]
- Legrand, H.; Werner, K.; Christensson, A.; Pihlsgård, M.; Elmståhl, S. Prevalence and determinants of differences in cystatin C and creatinine-based estimated glomerular filtration rate in community-dwelling older adults: A cross-sectional study. BMC Nephrol. 2017, 18, 350. [Google Scholar] [CrossRef]
- Inker, L.A.; Levey, A.S.; Coresh, J. Estimated Glomerular Filtration Rate from a Panel of Filtration Markers-Hope for Increased Accuracy Beyond Measured Glomerular Filtration Rate? Adv. Chronic Kidney Dis. 2018, 25, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Katz, R.; Cushman, M.; Sarnak, M.J.; Stehman-Breen, C.; Psaty, B.M.; Siscovick, D.; Tracy, R.P.; Newman, A.; Fried, L. Cystatin-C and inflammatory markers in the ambulatory elderly. Am. J. Med. 2005, 118, 1416.e25–1416.e31. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, N.; Koenig, W.; Kaski, J.C. Cystatin C and cardiovascular risk. Clin. Chem. 2009, 55, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.A.; Anderson, G.D. Methods of Estimating Kidney Function for Drug Dosing in Special Populations. Clin. Pharmacokinet. 2018, 57, 943–976. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of the Pharmacokinetics of Medicinal Products in Patients with decreased Renal Function | European Medicines Agency. Available online: https://www.ema.europa.eu/en/evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function (accessed on 30 December 2018).
- Steubl, D.; Inker, L.A. How best to estimate glomerular filtration rate? Novel filtration markers and their application. Curr. Opin. Nephrol. Hypertens. 2018, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mårtensson, J.; Groth, S.; Rehling, M.; Gref, M. Chromium-51-EDTA clearance in adults with a single-plasma sample. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998, 39, 2131–2137. [Google Scholar]
- Luis-Lima, S.; Gaspari, F.; Negrín-Mena, N.; Carrara, F.; Díaz-Martín, L.; Jiménez-Sosa, A.; González-Rinne, F.; Torres, A.; Porrini, E. Iohexol plasma clearance simplified by dried blood spot testing. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2017. [Google Scholar] [CrossRef]
- Dörks, M.; Allers, K.; Schmiemann, G.; Herget-Rosenthal, S.; Hoffmann, F. Inappropriate Medication in Non-Hospitalized Patients with Renal Insufficiency: A Systematic Review. J. Am. Geriatr. Soc. 2017, 65, 853–862. [Google Scholar] [CrossRef]
- Schmidt-Mende, K.; Wettermark, B.; Andersen, M.; Elsevier, M.; Carrero, J.-J.; Shemeikka, T.; Hasselström, J. Prevalence of renally inappropriate medicines in older people with renal impairment—A cross-sectional register-based study in a large primary care population. Basic Clin. Pharmacol. Toxicol. 2018. [Google Scholar] [CrossRef]
- Sönnerstam, E.; Sjölander, M.; Gustafsson, M. Inappropriate Prescription and Renal Function Among Older Patients with Cognitive Impairment. Drugs Aging 2016, 33, 889–899. [Google Scholar] [CrossRef]
- Gheewala, P.A.; Peterson, G.M.; Curtain, C.M.; Nishtala, P.S.; Hannan, P.J.; Castelino, R.L. Impact of the pharmacist medication review services on drug-related problems and potentially inappropriate prescribing of renally cleared medications in residents of aged care facilities. Drugs Aging 2014, 31, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.J.; Joel, S.P.; Slevin, M.L. Morphine intoxication in renal failure: The role of morphine-6-glucuronide. Br. Med. J. Clin. Res. Ed. 1986, 292, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Houlind, M.; Petersen, K.; Palm, H.; Jørgensen, L.; Aakjær, M.; Christrup, L.; Petersen, J.; Andersen, O.; Treldal, C.; Houlind, M.B.; et al. Creatinine-Based Renal Function Estimates and Dosage of Postoperative Pain Management for Elderly Acute Hip Fracture Patients. Pharmaceuticals 2018, 11, 88. [Google Scholar] [CrossRef] [PubMed]
| Minor Muscle Loss | Major Muscle Loss |
|---|---|
| Amputation at or below knee or Transfemoral amputation with a prior transtibial amputation on the same leg | Transfemoral amputation with no prior amputations on the same leg |
| Characteristics | All Patients (n = 38) | Patients with a Minor Loss of Muscle (n = 22) | Patients with a Major Loss of Muscle (n = 16) |
|---|---|---|---|
| Age, years, median (range) | 75 (53–95) | 74 (53–89) | 78 (60–95) |
| Age ≥ 80 years, n (%) | 14 (37) | 6 (27) | 8 (50) |
| Sex, female, n (%) | 11 (29) | 4 (18) | 7 (44) |
| Weight, kg, median (range) | 67 (43–112) | 77 (43–112) | 60 (46–92) |
| BMI a, kg/m2, median (range) | 22 (15–34) | 23 (15–34) | 22 (17–31) |
| BMI ≤ 18.5 kg/m2, n (%) | 5 (13) | 2 (9) | 3 (19) |
| Kidney function b | |||
| Serum creatinine, mg/dL, median (range) | 1.1 (0.5–3.8) | 1.1 (0.5–3.8) | 0.9 (0.6–2.1) |
| Serum cystatin C, mg/L, median (range) | 1.5 (0.9–3.8) | 1.5 (0.9–3.8) | 1.4 (1.1–3.0) |
| Smoking, current, n (%) | 17 (45) | 11 (50) | 6 (38) |
| TSH, ng/mL, median (range) c | 1.7 (0.1–26.3) | 1.7 (0.4–8.8) | 1.7 (0–26.3) |
| CRP d, mg/dL, median (range) | 88 (14–240) | 68 (14–240) | 98 (23–220) |
| Handgrip strength e, kg, median (range) | 22 (4–40) | 24 (9–38) | 18 (4–40) |
| Low handgrip strength f, n (%) | 21 (57) | 11 (52) | 10 (63) |
| Comorbidities | |||
| Diabetes, n (%) | 17 (45) | 10 (45) | 7 (44) |
| Atherosclerosis, n (%) | 29 (76) | 15 (68) | 14 (88) |
| Hypertension, n (%) | 22 (58) | 13 (59) | 9 (56) |
| Raw Data | Mixed Models | |||
|---|---|---|---|---|
| Before, Median (Range) | After, Median (Range) | Mean Difference, (95% CI) | pb | |
| eGFRCreatinine, mL/min/1.73 m2 | 65 (15–103) | 80 (22–107) | 8.5 (5.1; 11.8) | < 0.01 |
| eGFRCystatinC mL/min/1.73 m2 | 38 (13–79) | 51 (13–95) | 6.1 (3.6; 8.6) | < 0.01 |
| eGFRCombined mL/min/1.73 m2 | 48 (13–86) | 62 (16–100) | 7.4 (4.7; 10) | < 0.01 |
| Friedmans test, p a | < 0.01 | < 0.01 | ||
| Active Substance | Patients with Dosing Discrepancies, n (%) | Total Patients Prescribed, n (%) | ||
|---|---|---|---|---|
| CKD-EPI equation | ||||
| Creatinine | Cystatin C | Combined | ||
| Morphine (N02AA01) | 2 (8) | 6 (25) | 3 (13) | 24 (65) |
| Gabapentin (N03AX12) | 3 (15) | 7 (35) | 4 (20) | 20 (54) |
| Simvastatin (C10AA01) | 0 (0) | 1 (7) | 0 (0) | 14 (38) |
| Zopiclone (N05CF01) | 1 (9) | 2 (18) | 1 (9) | 11 (30) |
| Metformin (A10BA02) | 2 (29) | 5 (71) | 3 (43) | 7 (19) |
| Allopurinol (M04AA01) | 0 (0) | 1 (33) | 0 (0) | 3 (8) |
| Hydrochlorothiazide (C03AA03) | 0 (0) | 1 (33) | 0 (0) | 3 (8) |
| Mirtazapine (N06AX11) | 0 (0) | 1 (33) | 0 (0) | 3 (8) |
| Sitagliptin (A10BH01) | 1 (33) | 3 (100) | 1 (33) | 3 (8) |
| Bendroflumethiazide (C03AB01) | 0 (0) | 1 (50) | 0 (0) | 2 (5) |
| Cetirizine (R06AE07) | 1 (50) | 1 (50) | 1 (50) | 2 (5) |
| Ciprofloxacin (J01MA02) | 0 (0) | 0 (0) | 0 (0) | 2 (5) |
| Dabigatran (B01AE07) | 0 (0) | 1 (50) | 0 (0) | 2 (5) |
| Magnesium (A02AA04) | 0 (0) | 1 (50) | 1 (50) | 2 (5) |
| Metoclopramide (A03FA01) | 0 (0) | 1 (50) | 0 (0) | 2 (5) |
| Colchicin (M04AC01) | 0 (0) | 1 (100) | 0 (0) | 1 (3) |
| Venlafaxine (N06AX16) | 1 (100) | 1 (100) | 1 (100) | 1 (3) |
| Total, patients | 4 (11) | 14 (39) | 6 (17) | - |
| Total, patients without potential AKI (n = 31) | 1 (3) | 10 (28) | 3 (8) | - |
| Day −1–Day 0 | Day 0–Day 1 | Day 1–Day 3 | Day 3–Day 5 | Day 5–Day 10 | |
|---|---|---|---|---|---|
| eGFRCreatinine, change, n (%) decrease, n (%) | 2 (6.1) | 5 (13.2) | 6 (16.2) | 6 (16.2) | 4 (12.5) |
| 0 (0.0) | 1 (2.6) | 3 (8.1) | 2 (5.6) | 2 (6.3) | |
| eGFRCystatinC, change, n (%) decrease, n (%) | 2 (6.1) | 8 (21.1) | 7 (18.9) | 3 (8.3) | 6 (18.8) |
| 0 (0.0) | 1 (2.6) | 5 (13.5) | 1 (2.8) | 5 (15.6) | |
| eGFRCombined, change, n (%) decrease, n (%) | 0 (0.0) | 7 (18.4) | 6 (16.2) | 5 (13.9) | 5 (15.6) |
| 0 (0.0) | 1 (2.6) | 4 (10.8) | 3 (8.3) | 4 (12.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aakjær, M.; Houlind, M.B.; Treldal, C.; Ankarfeldt, M.Z.; S. Jensen, P.; Andersen, O.; Iversen, E.; Christrup, L.L.; Petersen, J. Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing. J. Clin. Med. 2019, 8, 89. https://doi.org/10.3390/jcm8010089
Aakjær M, Houlind MB, Treldal C, Ankarfeldt MZ, S. Jensen P, Andersen O, Iversen E, Christrup LL, Petersen J. Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing. Journal of Clinical Medicine. 2019; 8(1):89. https://doi.org/10.3390/jcm8010089
Chicago/Turabian StyleAakjær, Mia, Morten B. Houlind, Charlotte Treldal, Mikkel Z. Ankarfeldt, Pia S. Jensen, Ove Andersen, Esben Iversen, Lona L. Christrup, and Janne Petersen. 2019. "Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing" Journal of Clinical Medicine 8, no. 1: 89. https://doi.org/10.3390/jcm8010089
APA StyleAakjær, M., Houlind, M. B., Treldal, C., Ankarfeldt, M. Z., S. Jensen, P., Andersen, O., Iversen, E., Christrup, L. L., & Petersen, J. (2019). Differences in Kidney Function Estimates Based on Creatinine and/or Cystatin C in Non-Traumatic Amputation Patients and Their Impact on Drug Prescribing. Journal of Clinical Medicine, 8(1), 89. https://doi.org/10.3390/jcm8010089





