Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Exposure of Interest and Comorbidities
2.3. End Points
2.4. Power Calculations and Statistical Analysis
3. Results
Primary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Bistrian, B.R. Nutrition in critical illness: A current conundrum. F1000Research 2016, 5, 2531. [Google Scholar] [CrossRef] [PubMed]
- Cerri, A.P.; Bellelli, G.; Mazzone, A.; Pittella, F.; Landi, F.; Zambon, A.; Annoni, G. Sarcopenia and malnutrition in acutely ill hospitalized elderly: Prevalence and outcomes. Clin. Nutr. 2015, 34, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J. Protein requirement in critical illness. Appl. Physiol. Nutr. Metab. 2016, 41, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Batt, J.; dos Santos, C.C.; Cameron, J.I.; Herridge, M.S. Intensive care unit-acquired weakness: Clinical phenotypes and molecular mechanisms. Am. J. Respir. Crit. Care Med. 2013, 187, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.R. Parenteral nutrition in the critically ill patient. N. Engl. J. Med. 2009, 361, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Bistrian, B.R. Why critically ill patients are protein deprived. JPEN J. Parenter. Enter. Nutr. 2013, 37, 300–309. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Bistrian, B.R. What is the best nutritional support for critically ill patients? Hepatobiliary Surg. Nutr. 2014, 3, 172–174. [Google Scholar] [CrossRef]
- Heyland, D.K. Should We PERMIT Systematic Underfeeding in All Intensive Care Unit Patients? Integrating the Results of the PERMIT Study in Our Clinical Practice Guidelines. JPEN J. Parenter. Enter. Nutr. 2016, 40, 156–158. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. Intensive Care Med 2009, 35, 1728–1737. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Wang, M.; Day, A.G. The prevalence of iatrogenic underfeeding in the nutritionally ‘at-risk’ critically ill patient: Results of an international, multicenter, prospective study. Clin. Nutr. 2015, 34, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Simpson, F.; Sweetman, E.A.; Finfer, S.R.; Cooper, D.J.; Heighes, P.T.; Davies, A.R.; O’Leary, M.; Solano, T.; Peake, S.; et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA 2013, 309, 2130–2138. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Singer, P.; Anbar, R.; Cohen, J.; Shapiro, H.; Shalita-Chesner, M.; Lev, S.; Grozovski, E.; Theilla, M.; Frishman, S.; Madar, Z. The tight calorie control study (TICACOS): A prospective, randomized, controlled pilot study of nutritional support in critically ill patients. Intensive Care Med. 2011, 37, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Oudemans-van Straaten, H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Nicolo, M.; Heyland, D.K.; Chittams, J.; Sammarco, T.; Compher, C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. JPEN J. Parenter. Enter. Nutr. 2016, 40, 45–51. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef]
- Mogensen, K.M.; Robinson, M.K.; Casey, J.D.; Gunasekera, N.S.; Moromizato, T.; Rawn, J.D.; Christopher, K.B. Nutritional Status and Mortality in the Critically Ill. Crit. Care Med. 2015, 43, 2605–2615. [Google Scholar] [CrossRef]
- Zager, S.; Mendu, M.L.; Chang, D.; Bazick, H.S.; Braun, A.B.; Gibbons, F.K.; Christopher, K.B. Neighborhood poverty rate and mortality in patients receiving critical care in the academic medical center setting. Chest 2011, 139, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Mogensen, K.M.; Casey, J.D.; McKane, C.K.; Moromizato, T.; Rawn, J.D.; Christopher, K.B. The relationship among obesity, nutritional status, and mortality in the critically ill*. Crit. Care Med. 2015, 43, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Swails, W.S.; Samour, P.Q.; Babineau, T.J.; Bistrian, B.R. A proposed revision of current ICD-9-CM malnutrition code definitions. J. Am. Diet. Assoc. 1996, 96, 370–373. [Google Scholar] [CrossRef]
- Blackburn, G.L.; Bistrian, B.R.; Maini, B.S.; Schlamm, H.T.; Smith, M.F. Nutritional and metabolic assessment of the hospitalized patient. JPEN J. Parenter. Enter. Nutr. 1977, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Obesity and body weight standards. Annu. Rev. Public Health 1986, 7, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- McMahon, G.M.; Mendu, M.L.; Gibbons, F.K.; Christopher, K.B. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012, 38, 1834–1842. [Google Scholar] [CrossRef]
- Thickett, D.R.; Moromizato, T.; Litonjua, A.A.; Amrein, K.; Quraishi, S.A.; Lee-Sarwar, K.A.; Mogensen, K.M.; Purtle, S.W.; Gibbons, F.K.; Camargo, C.A., Jr.; et al. Association between prehospital vitamin D status and incident acute respiratory failure in critically ill patients: A retrospective cohort study. BMJ Open Respir. Res. 2015, 2, e000074. [Google Scholar] [CrossRef]
- Braun, A.B.; Litonjua, A.A.; Moromizato, T.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill*. Crit. Care Med. 2012, 40, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.; Gehlbach, S.; Lemeshow, S.; Teres, D. Resource utilization among intensive care patients. Managed care vs traditional insurance. Arch. Intern. Med. 1992, 152, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Litonjua, A.A.; Moromizato, T.; Quraishi, S.A.; Gibbons, F.K.; Pieber, T.R.; Camargo, C.A., Jr.; Giovannucci, E.; Christopher, K.B. Increases in pre-hospitalization serum 25(OH)D concentrations are associated with improved 30-day mortality after hospital admission: A cohort study. Clin. Nutr. 2016, 35, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, K.M.; Horkan, C.M.; Purtle, S.W.; Moromizato, T.; Rawn, J.D.; Robinson, M.K.; Christopher, K.B. Malnutrition, Critical Illness Survivors, and Postdischarge Outcomes: A Cohort Study. JPEN J. Parenter. Enter. Nutr. 2017, 42, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hedeker, D. A mixed-effects multinomial logistic regression model. Stat. Med. 2003, 22, 1433–1446. [Google Scholar] [CrossRef]
- Hartzel, J.; Agresti, A.; Caffo, B. Multinomial logit random effects models. Stat. Model. 2001, 1, 81–102. [Google Scholar] [CrossRef]
- Nicholson, B.D.; Shinkins, B.; Pathiraja, I.; Roberts, N.W.; James, T.J.; Mallett, S.; Perera, R.; Primrose, J.N.; Mant, D. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst. Rev. 2015, CD011134. [Google Scholar] [CrossRef]
- Desai, S.V.; Law, T.J.; Needham, D.M. Long-term complications of critical care. Crit. Care Med. 2011, 39, 371–379. [Google Scholar] [CrossRef]
- Horkan, C.M.; Purtle, S.W.; Mendu, M.L.; Moromizato, T.; Gibbons, F.K.; Christopher, K.B. The association of acute kidney injury in the critically ill and postdischarge outcomes: A cohort study. Crit. Care Med. 2015, 43, 354–364. [Google Scholar] [CrossRef]
- Puleo, F.; Arvanitakis, M.; Van Gossum, A.; Preiser, J.C. Gut failure in the ICU. Semin. Respir. Crit. Care Med. 2011, 32, 626–638. [Google Scholar] [CrossRef] [PubMed]
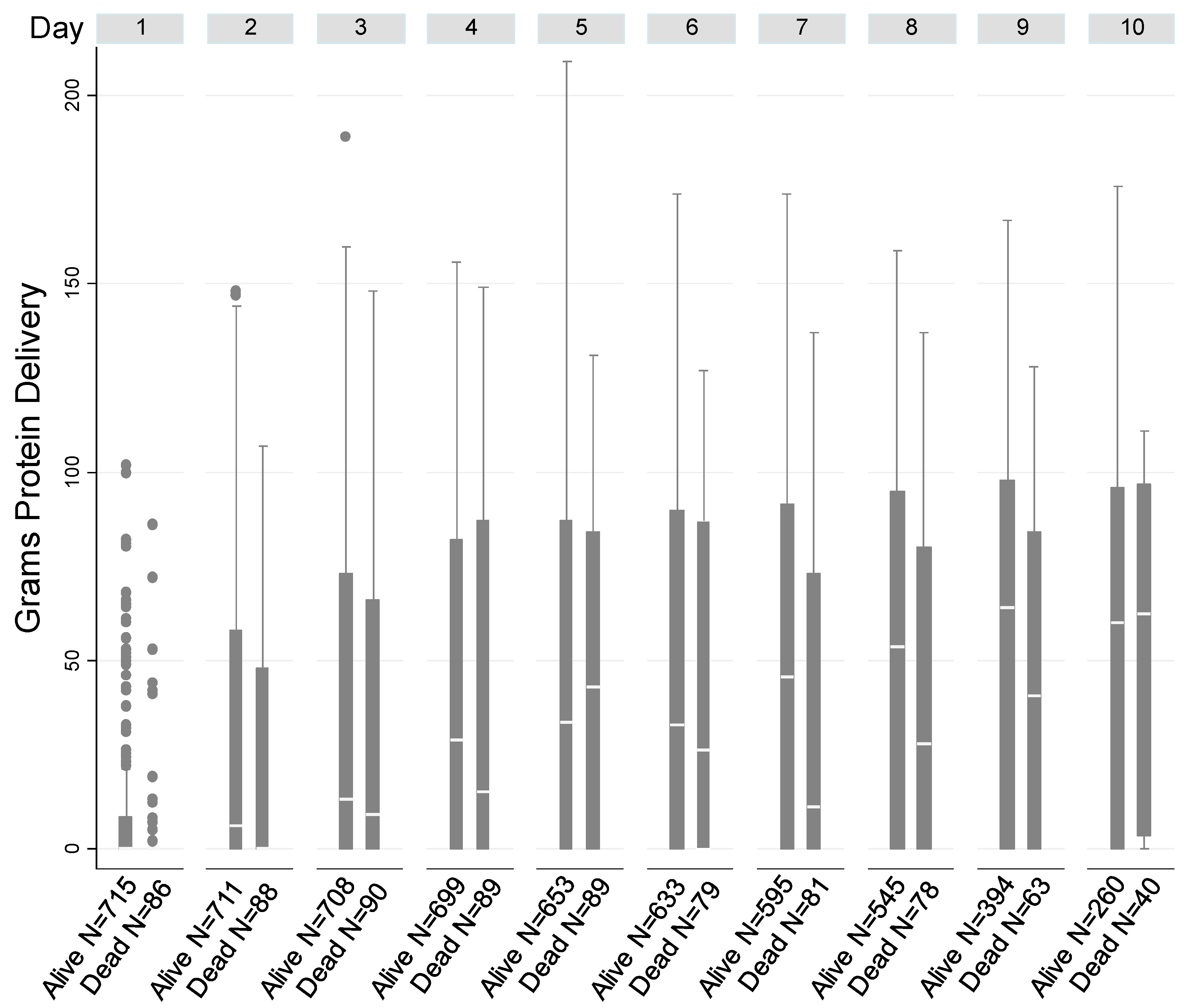
| Characteristics | Alive at 90-Days Post-Discharge | Expired by 90-Days Post-Discharge | Total | p-Value | Unadjusted OR (95%CI) for 90-Day Post-Discharge Mortality |
|---|---|---|---|---|---|
| 690 | 111 | 801 | |||
| Age-Mean ± SD | 61.1 ± 16.7 | 69.8 ± 14 | 62.3 ± 16.6 | <0.001 * | 1.04 (1.02, 1.05) |
| Male Gender-No. (%) | 384 (56) | 58 (52) | 442 (55) | 0.50 | 0.87 (0.58, 1.30) |
| Non-White Race-No. (%) | 154 (22) | 15 (14) | 169 (21) | 0.035 | 0.54 (0.31, 0.96) |
| Deyo-Charlson Index-No. (%) | 0.003 | ||||
| 0 | 182 (26.38) | 15 (13.39) | 197 (24.56) | 1.00 (Referent) | |
| 1–2 | 440 (63.77) | 75 (66.96) | 515 (64.21) | 2.07 (1.16, 3.70) | |
| ≥3 | 68 (9.86) | 22 (19.64) | 90 (11.22) | 3.93 (1.92, 8.01) | |
| Acute Organ Failures-No. (%) | 0.030 | ||||
| 0 | 86 (12) | 16 (14) | 102 (13) | 1.00 (Referent) | |
| 1 | 217 (31) | 29 (26) | 246 (31) | 0.72 (0.37, 1.39) | |
| 2 | 192 (28) | 26 (23) | 218 (27) | 0.73 (0.37, 1.43) | |
| 3 | 136 (20) | 20 (18) | 156 (19) | 0.79 (0.39, 1.61) | |
| ≥4 | 59 (9) | 20 (18) | 79 (10) | 1.82 (0.87, 3.80) | |
| Sepsis-No. (%) | 138 (20) | 28 (25) | 166 (21) | 0.21 | 1.35 (0.85, 2.15) |
| Intubation-No. (%) | 400 (58) | 60 (54) | 460 (57) | 0.44 | 0.85 (0.57, 1.28) |
| Acute Organ Failure Score-Mean ± SD | 10.2 ± 4.4 | 11.4 ± 4.6 | 10.4 ± 4.5 | 0.0082 * | 1.06 (1.02, 1.11) |
| Vasopressors/Inotropes-No. (%) | 264 (38) | 51 (46) | 315 (39) | 0.12 | 1.37 (0.92, 2.05) |
| Metastatic Malignancy-No. (%) | 296 (43) | 58 (52) | 354 (44) | 0.066 | 1.46 (0.97, 2.18) |
| Acute Kidney Injury-No. (%)† | 55 (10) | 6 (7) | 61 (10) | 0.381 | 0.68 (0.28, 1.63) |
| Chronic Kidney Disease-No. (%)†† | 169 (31) | 42 (49) | 211 (33) | 0.001 | 1.23 (1.06, 1.41) |
| Malnutrition-No. (%) | 0.035 | ||||
| At Risk for Malnutrition | 291 (43) | 33 (30) | 324 (41) | 1.00 (Referent) | |
| Non-Specific Malnutrition | 343 (51) | 65 (60) | 408 (52) | 1.67 (1.07, 2.61) | |
| Protein-Energy Malnutrition | 45 (7) | 11 (10) | 56 (7) | 2.16 (1.02, 4.57) |
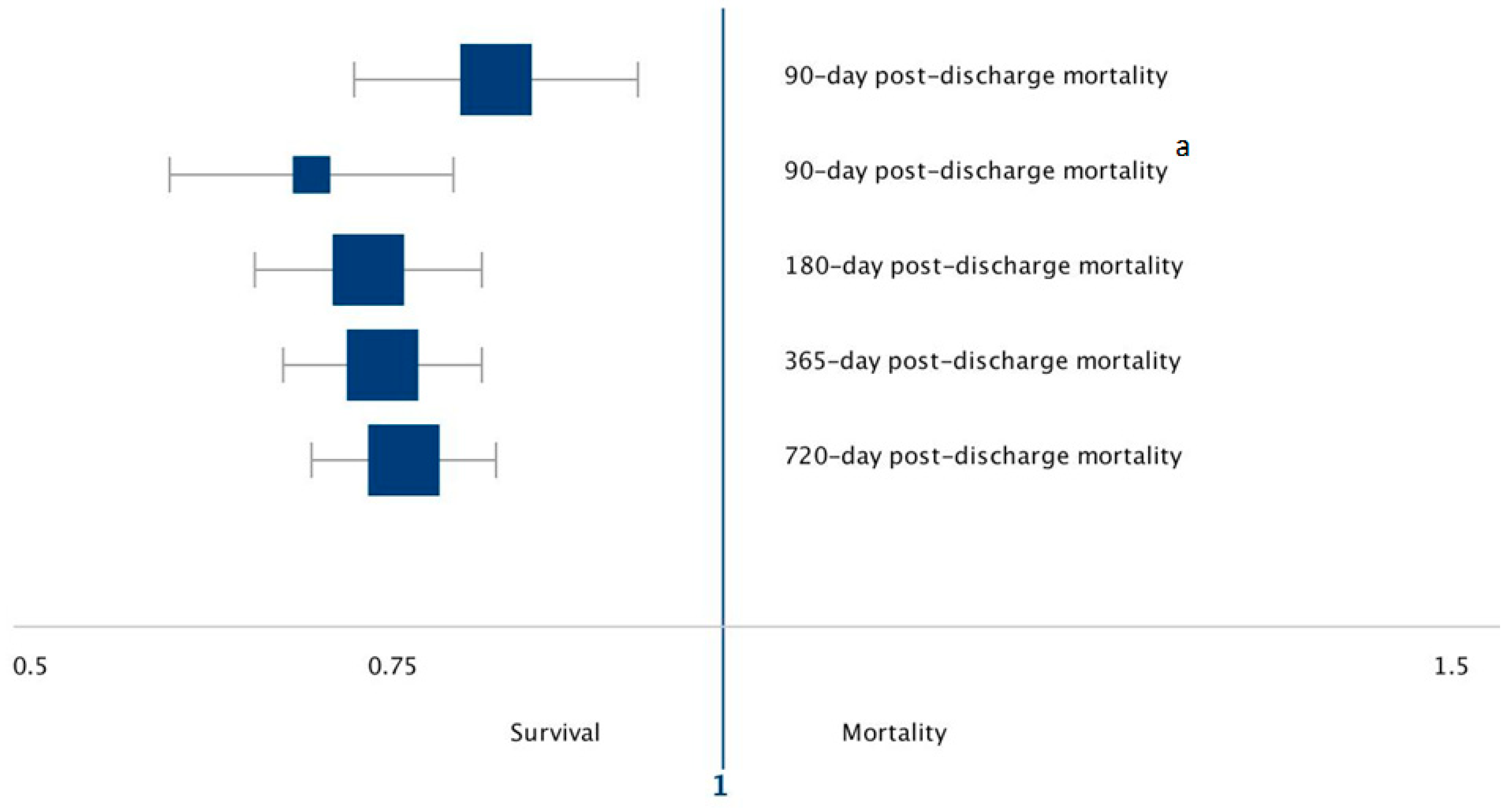
| Outcome | OR | 95% CI | p-Value | Association for Each 1 g/kg/Day Increase in Protein Delivery |
|---|---|---|---|---|
| 90-day post-discharge Mortality | ||||
| Full Cohort (n = 801) | 0.83 | 0.74–0.94 | 0.002 | 17% decrease odds of death |
| Malnutrition (n = 473) | 0.70 | 0.61–0.81 | <0.001 | 30% decrease odds of death |
| 180-day post-discharge Mortality | ||||
| Full Cohort (n = 801) | 0.74 | 0.67–0.83 | <0.001 | 26% decrease odds of death |
| 365-day post-discharge Mortality | ||||
| Full Cohort (n = 801) | 0.76 | 0.69–0.83 | <0.001 | 24% decrease odds of death |
| 720-day post-discharge Mortality | ||||
| Full Cohort (n = 801) | 0.77 | 0.71–0.84 | <0.001 | 23% decrease odds of death |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weijs, P.J.M.; Mogensen, K.M.; Rawn, J.D.; Christopher, K.B. Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study. J. Clin. Med. 2019, 8, 43. https://doi.org/10.3390/jcm8010043
Weijs PJM, Mogensen KM, Rawn JD, Christopher KB. Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study. Journal of Clinical Medicine. 2019; 8(1):43. https://doi.org/10.3390/jcm8010043
Chicago/Turabian StyleWeijs, Peter J.M., Kris M. Mogensen, James D. Rawn, and Kenneth B. Christopher. 2019. "Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study" Journal of Clinical Medicine 8, no. 1: 43. https://doi.org/10.3390/jcm8010043
APA StyleWeijs, P. J. M., Mogensen, K. M., Rawn, J. D., & Christopher, K. B. (2019). Protein Intake, Nutritional Status and Outcomes in ICU Survivors: A Single Center Cohort Study. Journal of Clinical Medicine, 8(1), 43. https://doi.org/10.3390/jcm8010043






