Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anesthesia, Surgical Technique and Immunosuppression
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Utsumi, M.; Umeda, Y.; Sadamori, H.; Nagasaka, T.; Takaki, A.; Matsuda, H.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Satoh, D.; et al. Risk factors for acute renal injury in living donor liver transplantation: Evaluation of the RIFLE criteria. Transpl. Int. 2013, 26, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.; Xia, Q.; Wang, S.; Qiu, Y.; Che, M.; Dai, H.; Qian, J.; Ni, Z.; Axelsson, J.; et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant. Proc. 2010, 42, 3634–3638. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Nolasco, F.; Carvalho, D.; Sampaio, S.; Baptista, A.; Pessegueiro, P.; Monteiro, E.; Mourão, L.; Barroso, E. Impact of RIFLE classification in liver transplantation. Clin. Transplant. 2010, 24, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Singhapricha, T.; Hu, K.Q.; Hong, J.C.; Steadman, R.H.; Busuttil, R.W.; Xia, V.W. Postliver transplant acute renal injury and failure by the RIFLE criteria in patients with normal pretransplant serum creatinine concentrations: A matched study. Transplantation 2011, 91, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Lebron Gallardo, M.; Herrera Gutierrez, M.E.; Seller Perez, G.; Curiel Balsera, E.; Fernandez Ortega, J.F.; Quesada Garcia, G. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transpl. 2004, 10, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Barri, Y.M.; Sanchez, E.Q.; Jennings, L.W.; Melton, L.B.; Hays, S.; Levy, M.F.; Klintmalm, G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef]
- Gameiro, J.; Agapito Fonseca, J.; Jorge, S.; Lopes, J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018, 7, 307. [Google Scholar] [CrossRef]
- Paramesh, A.S.; Roayaie, S.; Doan, Y.; Schwartz, M.E.; Emre, S.; Fishbein, T.; Florman, S.; Gondolesi, G.E.; Krieger, N.; Ames, S.; et al. Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin. Transplant. 2004, 18, 94–99. [Google Scholar] [CrossRef]
- Trinh, E.; Alam, A.; Tchervenkov, J.; Cantarovich, M. Impact of acute kidney injury following liver transplantation on long-term outcomes. Clin. Transplant. 2017, 31, e12863. [Google Scholar] [CrossRef]
- Shin, S.R.; Kim, W.H.; Kim, D.J.; Shin, I.W.; Sohn, J.T. Prediction and Prevention of Acute Kidney Injury after Cardiac Surgery. Biomed. Res. Int. 2016, 2016, 2985148. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Defining acute renal failure: Physiological principles. Intensive Care Med. 2004, 30, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.T.; Le Gall, J.R.; Dos Reis Miranda, D.; Pinsky, M.R.; Tetta, C. Physiologic endpoints (efficacy) for acute renal failure studies. Curr. Opin. Crit. Care 2002, 8, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Alge, J.L.; Arthur, J.M. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin. J. Am. Soc. Nephrol. 2015, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chenitz, K.B.; Lane-Fall, M.B. Decreased urine output and acute kidney injury in the postanesthesia care unit. Anesthesiol. Clin. 2012, 30, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Koyama, K.; Goto, Y.; Koinuma, T.; Tonai, K.; Shima, J.; Wada, M.; Nunomiya, S. Body weight definitions for evaluating a urinary diagnosis of acute kidney injury in patients with sepsis. BMC Nephrol. 2018, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, A.M.; Sladen, R.N. Acute kidney injury in cardiac surgery. Curr. Opin. Anaesthesiol. 2015, 28, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Linas, S.L.; Berns, A.S.; Henrich, W.L.; Miller, T.R.; Gabow, P.A.; Schrier, R.W. Nonoliguric acute renal failure. N. Engl. J. Med. 1977, 296, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Pathophysiology of obstructive nephropathy. Kidney Int. 1983, 23, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Shim, H.S.; Kim, W.H.; Kim, H.J.; Kim, D.J.; Lee, S.H.; Kim, C.S.; Gwak, M.S.; Kim, G.S. Clinical Risk Scoring Models for Prediction of Acute Kidney Injury after Living Donor Liver Transplantation: A Retrospective Observational Study. PLoS ONE 2015, 10, e0136230. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yoon, S.; Yang, S.M.; Kim, W.; Ryu, H.G.; Jung, C.W.; Suh, K.S.; Lee, K. Prediction of Acute Kidney Injury after Liver Transplantation: Machine Learning Approaches vs. Logistic Regression Model. J. Clin. Med. 2018, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Wu, V.C.; Huang, W.C.; Yeh, Y.C.; Wu, M.S.; Huang, C.C.; Wu, K.D.; Fang, J.T.; Wu, C.J.; CAKS Group. Norepinephrine Administration Is Associated with Higher Mortality in Dialysis Requiring Acute Kidney Injury Patients with Septic Shock. J. Clin. Med. 2018, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Kashfi, A.; Cattral, M.S.; Selzner, N.; McGilvray, I.D.; Greig, P.D.; Levy, G.A.; Renner, E.L.; Grant, D.R. Live donor liver transplantation in high MELD score recipients. Ann. Surg. 2010, 251, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Goren, O.; Matot, I. Perioperative acute kidney injury. Br. J. Anaesth. 2015, 115 (Suppl. S2), ii3–ii14. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Guerin, C.; Girard, R.; Selli, J.M.; Perdrix, J.P.; Ayzac, L. Initial versus delayed acute renal failure in the intensive care unit. A multicenter prospective epidemiological study. Rhone-Alpes Area Study Group on Acute Renal Failure. Am. J. Respir. Crit. Care Med. 2000, 161, 872–879. [Google Scholar] [CrossRef]
- Teixeira, C.; Garzotto, F.; Piccinni, P.; Brienza, N.; Iannuzzi, M.; Gramaticopolo, S.; Forfori, F.; Pelaia, P.; Rocco, M.; Ronco, C.; et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit. Care 2013, 17, R14. [Google Scholar] [CrossRef]
- Alpert, R.A.; Roizen, M.F.; Hamilton, W.K.; Stoney, R.J.; Ehrenfeld, W.K.; Poler, S.M.; Wylie, E.J. Intraoperative urinary output does not predict postoperative renal function in patients undergoing abdominal aortic revascularization. Surgery 1984, 95, 707–711. [Google Scholar] [CrossRef]
- Hori, D.; Katz, N.M.; Fine, D.M.; Ono, M.; Barodka, V.M.; Lester, L.C.; Yenokyan, G.; Hogue, C.W. Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury. Br. J. Anaesth. 2016, 117, 733–740. [Google Scholar] [CrossRef]
- Mizota, T.; Yamamoto, Y.; Hamada, M.; Matsukawa, S.; Shimizu, S.; Kai, S. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br. J. Anaesth. 2017, 119, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Kunst, G.; Ostermann, M. Intraoperative permissive oliguria - how much is too much? Br. J. Anaesth. 2017, 119, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Trzeciak, S.; Puri, N.K. Assessment of the adequacy of oxygen delivery. Curr. Opin. Crit. Care 2016, 22, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, D.; Kronish, K.; Ramsay, M.A. Anesthesia for Liver Transplantation. Anesthesiol. Clin. 2017, 35, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, M.R.; Marchi, L.D.; Plotkin, J.S. Hemodynamic monitoring during liver transplantation: A state of the art review. World, J. Hepatol. 2015, 7, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.; Sansano, T.; Palenciano, C.G.; Roqués, V.; Clavel, N.; González, P.; Robles, R.; Bueno, F.S.; Ramírez, P.; Parrilla, P. Does mixed venous oxygen saturation reflect the changes in cardiac output during liver transplantation? Transplant. Proc. 2002, 34, 277. [Google Scholar] [CrossRef]
- De Geus, H.R.; Betjes, M.G.; Bakker, J. Biomarkers for the prediction of acute kidney injury: A narrative review on current status and future challenges. Clin. Kidney, J. 2012, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Moledina, D.G.; Coca, S.G.; Thiessen-Philbrook, H.R.; Garg, A.X. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016, 89, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Brier, M.E.; Slaughter, M.S.; Klein, J.B.; McLeish, K.R. Biomarker enhanced risk prediction for development of AKI after cardiac surgery. BMC Nephrol. 2018, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Haase-Fielitz, A.; Haase, M.; Devarajan, P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann. Clin. Biochem. 2014, 51, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Baron-Stefaniak, J.; Schiefer, J.; Miller, E.J.; Berlakovich, G.A.; Baron, D.M.; Faybik, P. Comparison of macrophage migration inhibitory factor and neutrophil gelatinase-associated lipocalin-2 to predict acute kidney injury after liver transplantation: An observational pilot study. PLoS ONE 2017, 12, e0183162. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, C.; Tian, J.; Zha, Y.; Xiong, Y.; Sun, Z.; Chen, P.; Li, J.; Yang, T.; Ma, C.; et al. Urinary Angiotensinogen Level Predicts AKI in Acute Decompensated Heart Failure: A Prospective, Two-Stage Study. J. Am. Soc. Nephrol. 2015, 26, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Van Aken, H.; Martens, S.; Rossaint, J.; Singbartl, K.; Görlich, D.; Kellum, J.A.; Zarbock, A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 2014, 9, e93460. [Google Scholar] [CrossRef] [PubMed]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Zarbock, A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth Analg. 2017, 125, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Brienza, N.; Giglio, M.T.; Marucci, M.; Fiore, T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit. Care Med. 2009, 37, 2079–2090. [Google Scholar] [CrossRef]
- Sandham, J.D.; Hull, R.D.; Brant, R.F.; Knox, L.; Pineo, G.F.; Doig, C.J.; Laporta, D.P.; Viner, S.; Passerini, L.; Devitt, H.; et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N. Engl. J. Med. 2003, 348, 5–14. [Google Scholar] [CrossRef]
- El-Masry, A.; Mukhtar, A.M.; el-Sherbeny, A.M.; Fathy, M.; el-Meteini, M. Comparison of central venous oxygen saturation and mixed venous oxygen saturation during liver transplantation. Anaesthesia 2009, 64, 378–382. [Google Scholar] [CrossRef]
- Hilmi, I.A.; Damian, D.; Al-Khafaji, A.; Planinsic, R.; Boucek, C.; Sakai, T.; Chang, C.C.; Kellum, J.A. Acute kidney injury following orthotopic liver transplantation: Incidence, risk factors, and effects on patient and graft outcomes. Br. J. Anaesth. 2015, 114, 919–926. [Google Scholar] [CrossRef]

| Characteristic | No AKI Group (n = 378) | AKI Group (n = 205) | p-Values |
|---|---|---|---|
| Demographic data | |||
| Age, years | 53 (47–58) | 53 (49–58) | 0.405 |
| Female, n | 85 (22.5) | 63 (30.7) | 0.029 |
| Body-mass index, kg/m2 | 22.9 (21.0–24.8) | 23.3 (21.6–26.1) | 0.008 |
| Background medical status | |||
| Hypertension, n | 39 (10.3) | 12 (5.9) | 0.069 |
| Diabetes mellitus, n | 47 (12.4) | 22 (10.7) | 0.544 |
| Alcoholic liver cirrhosis, n | 44 (11.6) | 25 (12.2) | 0.843 |
| HBV hepatitis, n | 159 (42.1) | 71 (34.6) | 0.080 |
| HCV hepatitis, n | 25 (6.6) | 12 (5.9) | 0.719 |
| Hepatocellular carcinoma, n | 209 (55.3) | 113 (55.1) | 0.969 |
| Cholestatic disease, n | 7 (1.9) | 6 (2.9) | 0.401 |
| Preoperative hemoglobin, g/dL | 11.5 (9.7–13.2) | 10.2 (8.9–11.9) | <0.001 |
| Preoperative serum albumin level, g/dL | 3.0 (2.5–3.6) | 2.8 (2.5–3.2) | 0.002 |
| Preoperative serum creatinine, mg/dL | 0.90 (0.75–1.10) | 0.85 (0.68–1.04) | 0.014 |
| Preoperative estimated glomerular filtration rate, mL/min | 88 (70–109) | 94 (75–122) | 0.021 |
| MELD score | 13 (9–21) | 17 (11–21) | 0.001 |
| Child-Turcotte-Pugh score | 8 (6–11) | 9 (7–11) | <0.001 |
| Child class, n (A/ B/ C) | 109 (33.5)/96 (29.5)/120 (36.9) | 28 (16.2)/61 (35.3)/84 (48.6) | <0.001 |
| Preoperative LVEF. % | 65 (61–68) | 65 (62–68) | 0.254 |
| Preoperative beta-blocker, n | 29 (7.7) | 13 (6.3) | 0.645 |
| Preoperative diuretics, n | 16 (4.2) | 12 (5.9) | 0.382 |
| Donor/ graft factors | |||
| Age, years | 31 (23–41) | 30 (23–38) | 0.302 |
| Estimated GRWR | 1.17 (1.01–1.46) | 1.16 (1.00–1.41 | 0.396 |
| ABO incompatible, n | 19 (5.0) | 10 (4.9) | 0.937 |
| Operation and anesthesia details | |||
| Operation time, h | 7.0 (6.1–8.2) | 7.6 (6.5–8.6) | 0.001 |
| Cold ischemic time, min | 71 (58–83) | 78 (65–95) | <0.001 |
| Warm ischemic time, min | 31 (23–40) | 32 (26–41) | 0.142 |
| Intraoperative furosemide use, n | 71 (18.8) | 52 (25.4) | 0.063 |
| Intraoperative furosemide dose, mg | 0 (0–0) | 0 (0–5) | 0.040 |
| Intraoperative use of epinephrine, n | 148 (39.2) | 81 (39.5) | 0.933 |
| Intraoperative dose of epinephrine, mcg | 0 (0–10) | 0 (0–5) | 0.603 |
| Intraoperative mean blood glucose, mg/dL | 163 (144–178) | 164 (145–183) | 0.216 |
| Intraoperative highest blood glucose, mg/dL | 211 (194–230) | 218 (200–238) | 0.012 |
| Mean trough level of tacrolimus during posmiddleerative first week (ng/mL) | 6.2 (3.2–8.5) | 6.5 (3.4–9.1) | 0.098 |
| Bleeding and transfusion amount | |||
| pRBC transfusion, units | 4 (0–10) | 6 (3–12) | <0.001 |
| FFP transfusion, units | 4 (0–8) | 6 (2–12) | <0.001 |
| Blood loss per body weight, mL/kg | 33 (18–64) | 47 (23–91) | <0.001 |
| Input and output during surgery | |||
| Intraoperative average urine flow rate, mL/kg/h | 1.36 (0.89–2.08) | 1.15 (0.76–1.72) | 0.007 |
| Crystalloid administration, mL/kg | 53 (37–73) | 57 (40–85) | 0.007 |
| Net fluid balance during surgery, mL/kg | 31 (13–52) | 36 (18–59) | 0.072 |
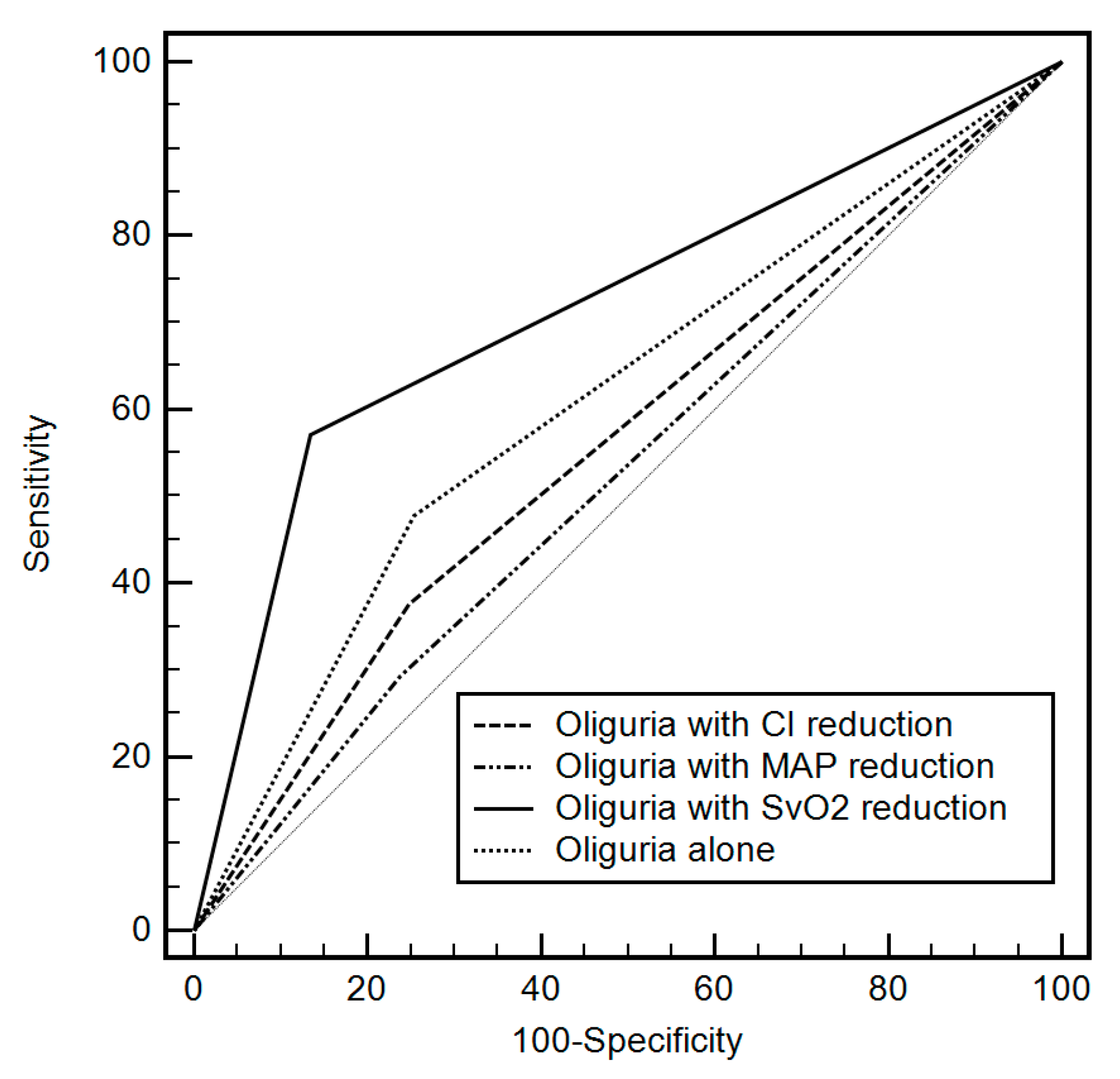
| Oliguria | Coupled Hemodynamic Variable | AUC (95% CI) | p-Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| <0.3 | None | 0.55 (0.50–0.60) | 0.031 | 16.1 | 94.7 | 62.3 | 60.5 |
| <0.5 | None | 0.60 (0.56–0.64) | <0.001 | 49.8 | 56.6 | 50.1 | 64.7 |
| <1.0 | None | 0.55 (0.50–0.60) | 0.040 | 53.7 | 48.7 | 38.3 | 69.8 |
| No | SvO2 | 0.66 (0.61–0.70) | <0.001 | 57.1 | 79.1 | 59.7 | 77.3 |
| No | Cardiac index | 0.56 (0.52–0.61) | 0.011 | 62.0 | 50.8 | 40.6 | 71.1 |
| No | Mean arterial pressure | 0.53 (0.48–0.58) | 0.219 | 52.2 | 54.0 | 38.1 | 67.5 |
| <0.3 | SvO2 | 0.61 (0.56–0.66) | <0.001 | 11.3 | 97.4 | 82.5 | 50.0 |
| <0.3 | Cardiac index | 0.55 (0.50–0.60) | 0.046 | 16.1 | 93.9 | 58.9 | 67.4 |
| <0.3 | Mean arterial pressure | 0.53 (0.48–0.58) | 0.191 | 13.2 | 91.4 | 51.9 | 66.5 |
| <0.5 | SvO2 | 0.72 (0.68–0.75) | <0.001 | 59.1 | 86.5 | 69.6 | 78.8 |
| <0.5 | Cardiac index | 0.56 (0.52–0.60) | 0.011 | 37.6 | 75.1 | 45.0 | 68.9 |
| <0.5 | Mean arterial pressure | 0.53 (0.49–0.57) | 0.276 | 29.3 | 73.2 | 40.0 | 65.5 |
| <1.0 | SvO2 | 0.58 (0.53–0.63) | 0.001 | 37.1 | 79.1 | 49.0 | 69.9 |
| <1.0 | Cardiac index | 0.52 (0.47–0.57) | 0.418 | 39.5 | 64.6 | 37.7 | 66.3 |
| <1.0 | Mean arterial pressure | 0.50 (0.45–0.55) | 0.929 | 30.2 | 69.3 | 34.8 | 64.7 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Without urine output or hemodynamic variables | |||
| Body-mass index, kg/m2 | 1.09 | 1.03–1.16 | 0.004 |
| Preoperative hemoglobin, g/dL | 0.86 | 0.77–0.95 | 0.004 |
| Preoperative albumin, g/dL | 0.88 | 0.68–1.15 | 0.158 |
| Operation time, h | 1.07 | 0.94–1.22 | 0.123 |
| Cold ischemic time, min | 1.02 | 1.01–1.03 | <0.001 |
| Red blood cell transfusion, unit | 1.10 | 1.05–1.12 | 0.010 |
| Crystalloid administration, per 10 mL/kg | 1.07 | 1.00–1.15 | 0.068 |
| Including urine output coupled with hemodynamic variables | |||
| Body-mass index, kg/m2 | 1.12 | 1.05–1.20 | 0.001 |
| Preoperative hemoglobin, g/dL | 0.89 | 0.79–0.99 | 0.041 |
| Preoperative diuretics | 1.68 | 0.58–4.89 | 0.139 |
| Operation time, h | 1.12 | 0.91–1.34 | 0.236 |
| Cold ischemic time, min | 1.02 | 1.01–1.03 | 0.003 |
| Crystalloid administration, per 10 mL/kg | 1.08 | 1.02–1.15 | 0.011 |
| SvO2 reduction with oliguria <0.5 mL/kg/h | 7.64 | 4.82–12.11 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.H.; Lee, H.-C.; Lim, L.; Ryu, H.-G.; Jung, C.-W. Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation. J. Clin. Med. 2019, 8, 29. https://doi.org/10.3390/jcm8010029
Kim WH, Lee H-C, Lim L, Ryu H-G, Jung C-W. Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation. Journal of Clinical Medicine. 2019; 8(1):29. https://doi.org/10.3390/jcm8010029
Chicago/Turabian StyleKim, Won Ho, Hyung-Chul Lee, Leerang Lim, Ho-Geol Ryu, and Chul-Woo Jung. 2019. "Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation" Journal of Clinical Medicine 8, no. 1: 29. https://doi.org/10.3390/jcm8010029
APA StyleKim, W. H., Lee, H.-C., Lim, L., Ryu, H.-G., & Jung, C.-W. (2019). Intraoperative Oliguria with Decreased SvO2 Predicts Acute Kidney Injury after Living Donor Liver Transplantation. Journal of Clinical Medicine, 8(1), 29. https://doi.org/10.3390/jcm8010029





