Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell culture
2.2. Human Study Populations
2.3. Flow Cytometry
2.4. Quantification of Soluble Metabolic and Inflammatory Markers
2.5. Leukocyte-Endothelial Cell Interactions under Flow Conditions
2.6. Immunofluorescence Studies
2.7. Statistical Analysis
3. Results
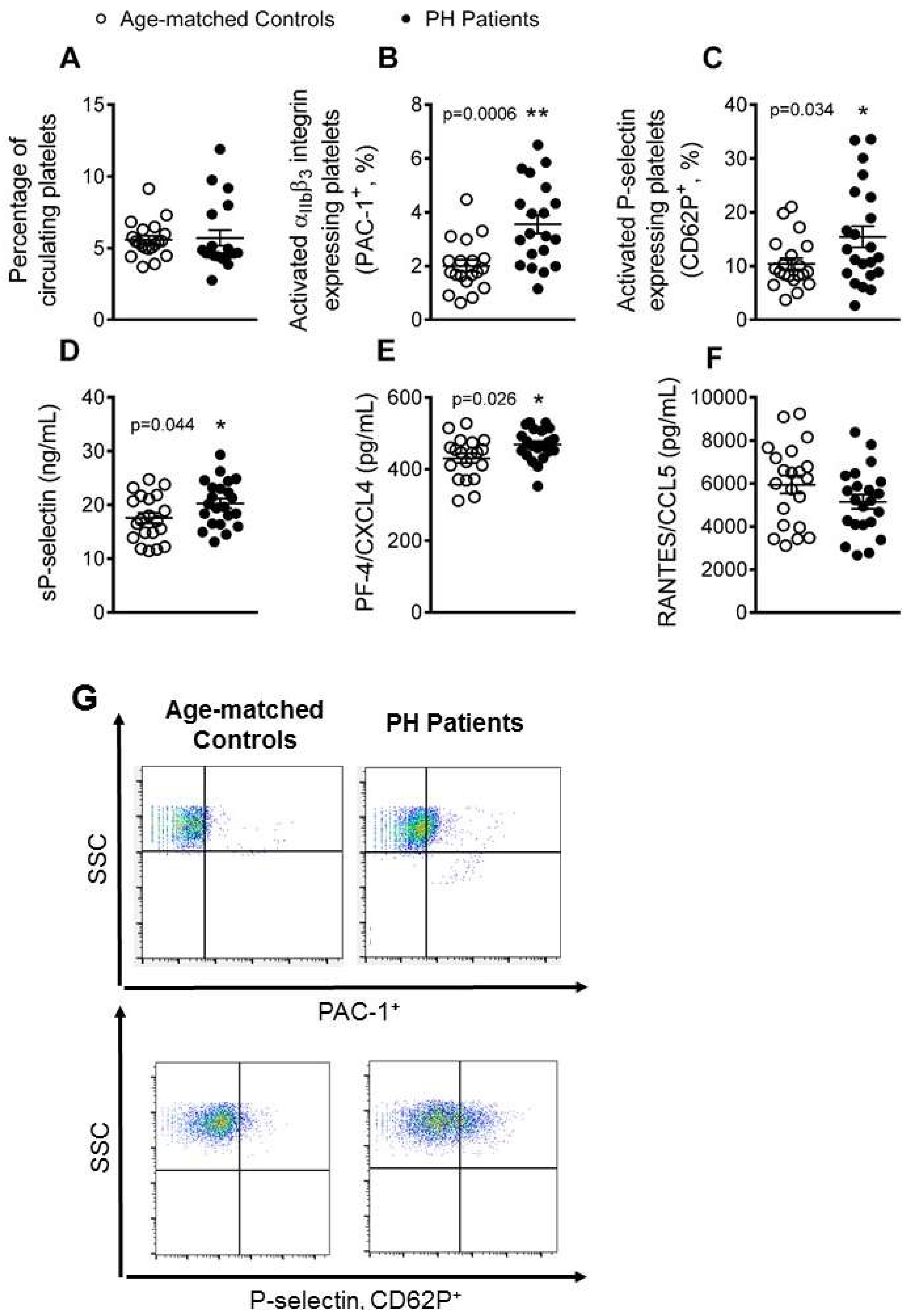
3.1. Platelet Activation Is Enhanced in Patients with PH
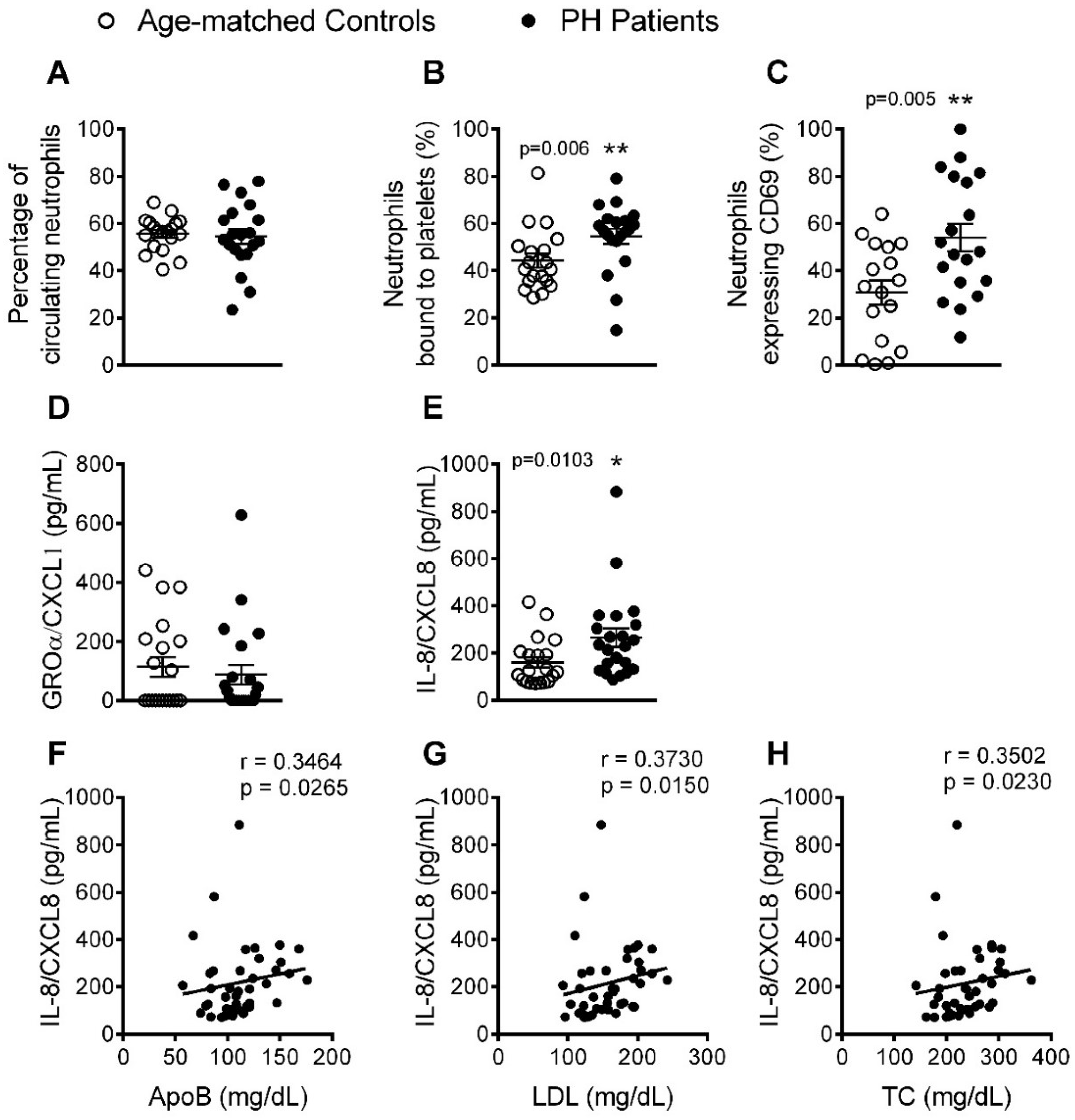
3.2. The Percentage of Platelet-Neutrophil Aggregates, Activated Neutrophils, and Circulating Levels of IL-8, Are Elevated in Patients with PH
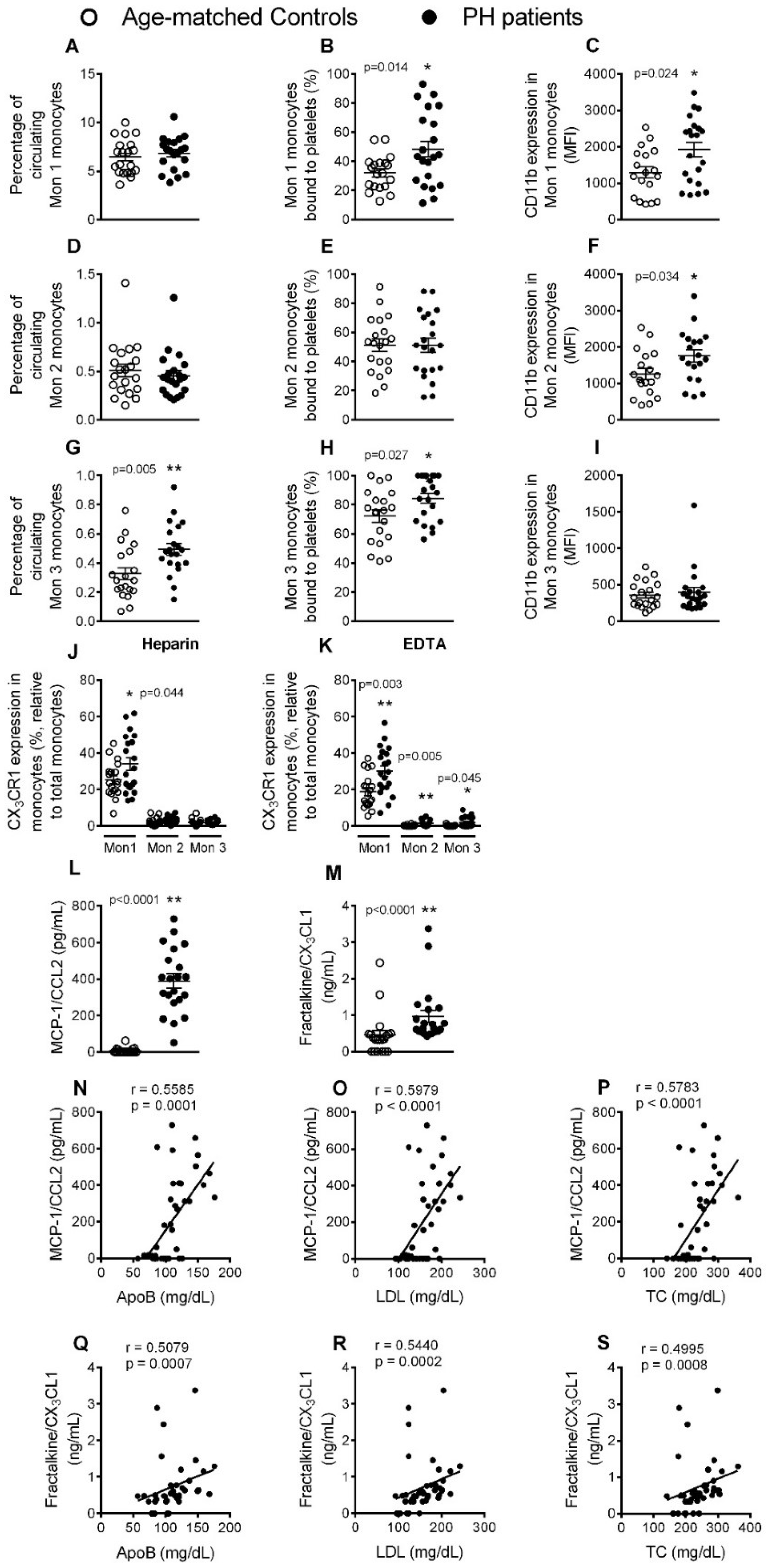
3.3. Circulating Mon 3 Monocytes, Platelet-Mon 1 and 3 Aggregates, Activated Mon 1 and 2 Monocytes, and Plasma Levels of CCL2 and CX3CL1, Are All Elevated in Patients with PH
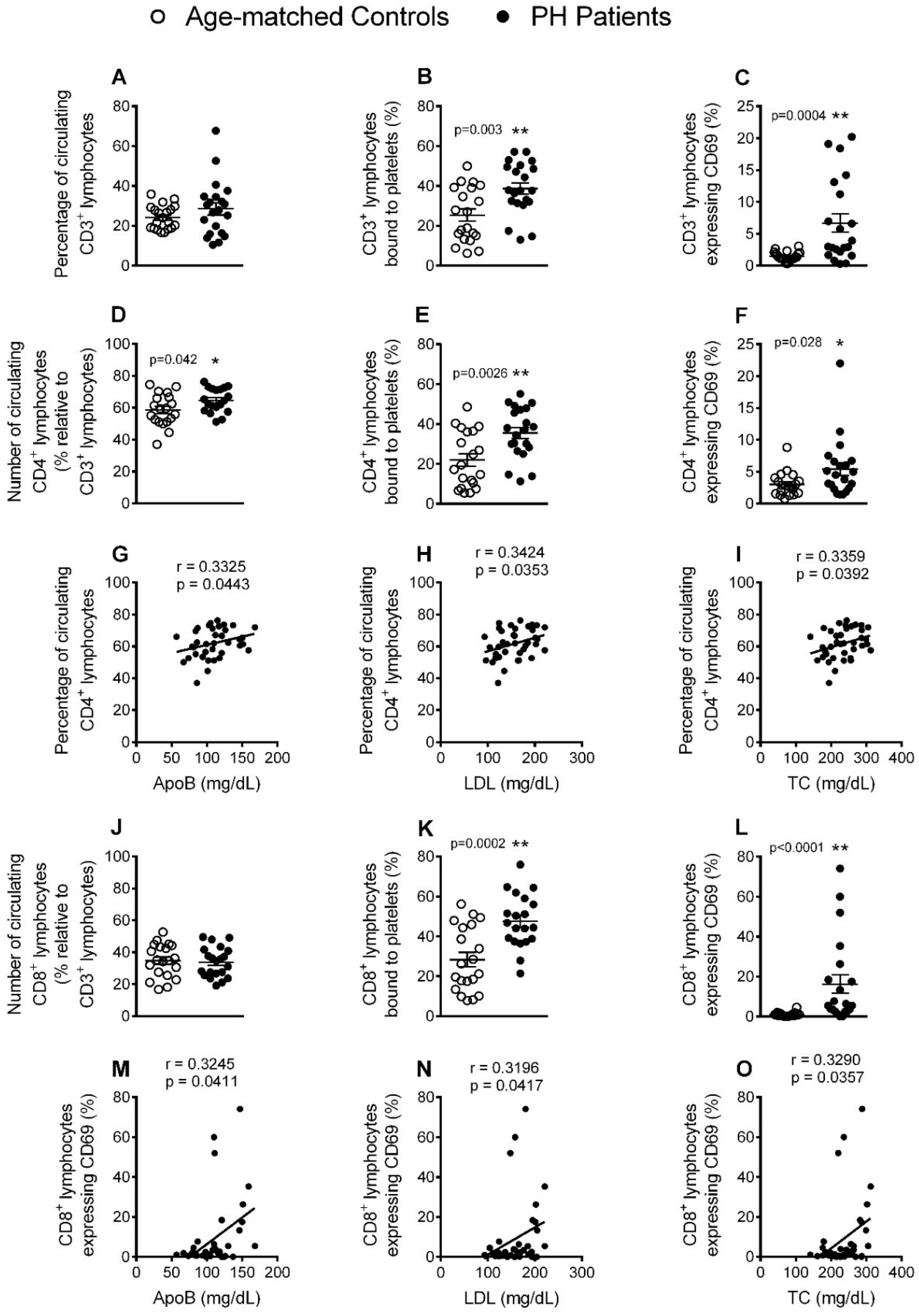
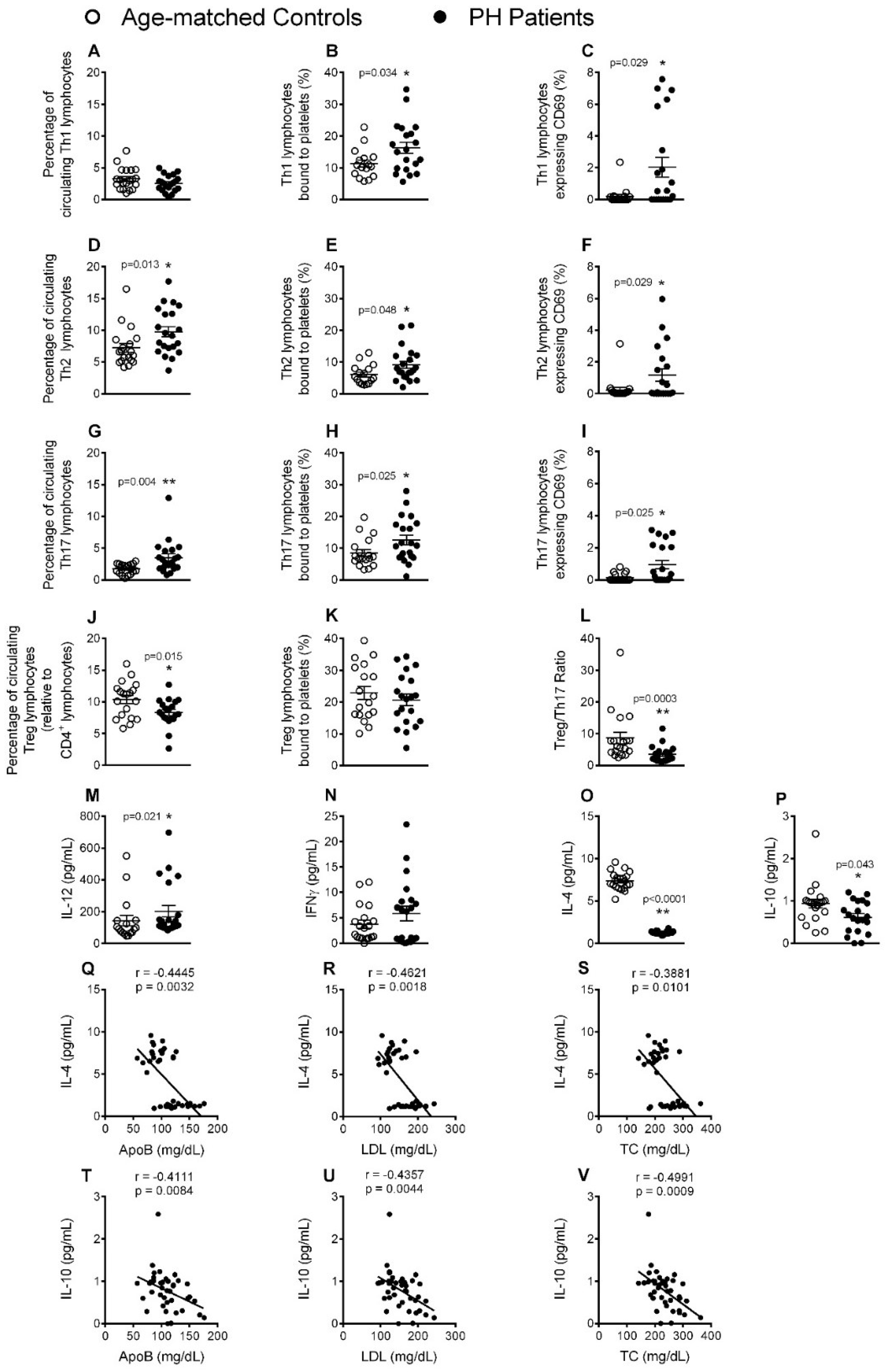
3.4. Circulating CD4+ Lymphocytes, Platelet-Lymphocyte (CD4+ and CD8+) Aggregates and Lymphocyte (CD4+ and CD8+) Activation Are Significantly increased in Patients with PH
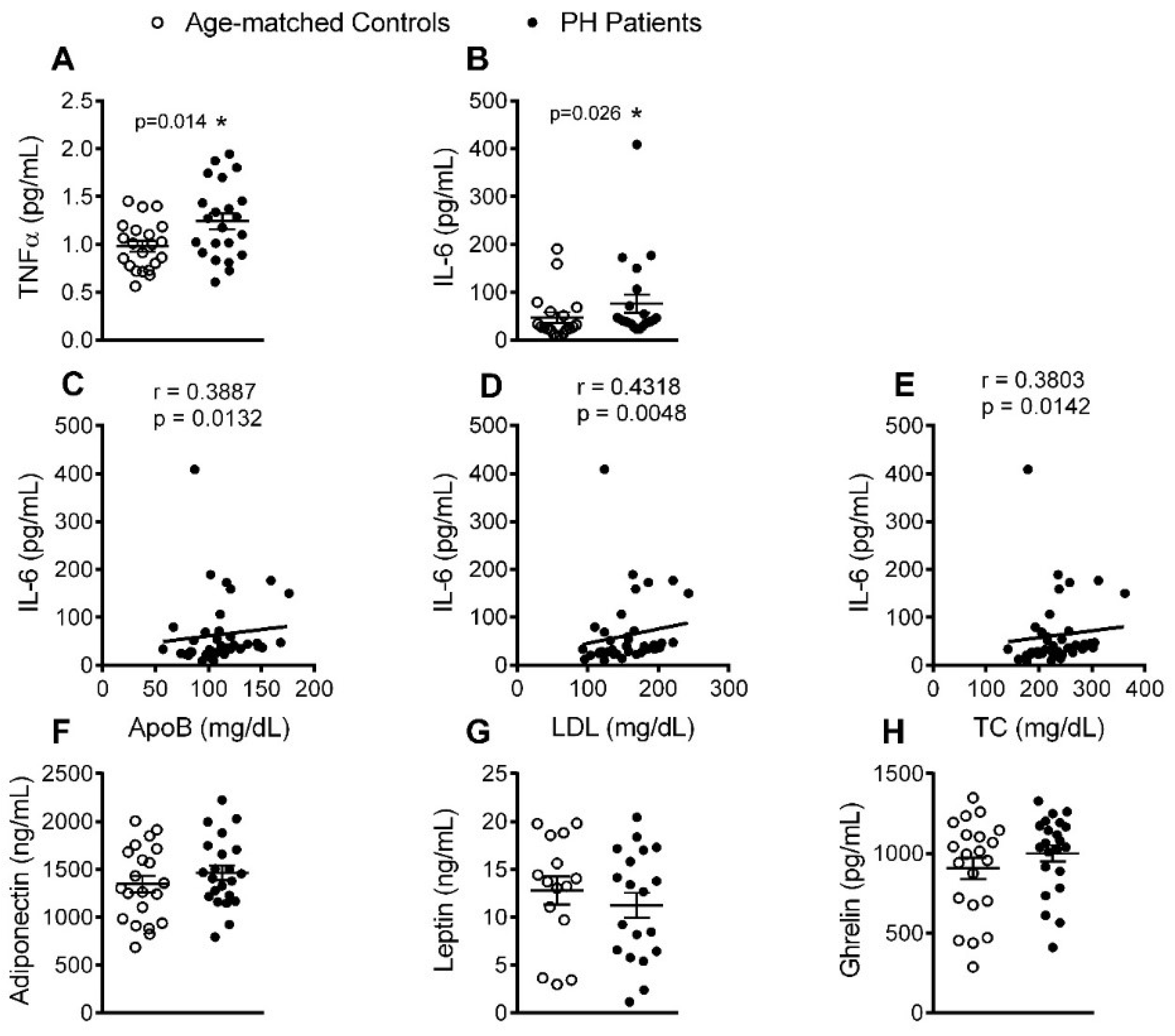
3.5. Circulating Levels of Pro-Inflammatory Cytokines but not Adipokines Are Increased in PH Patients
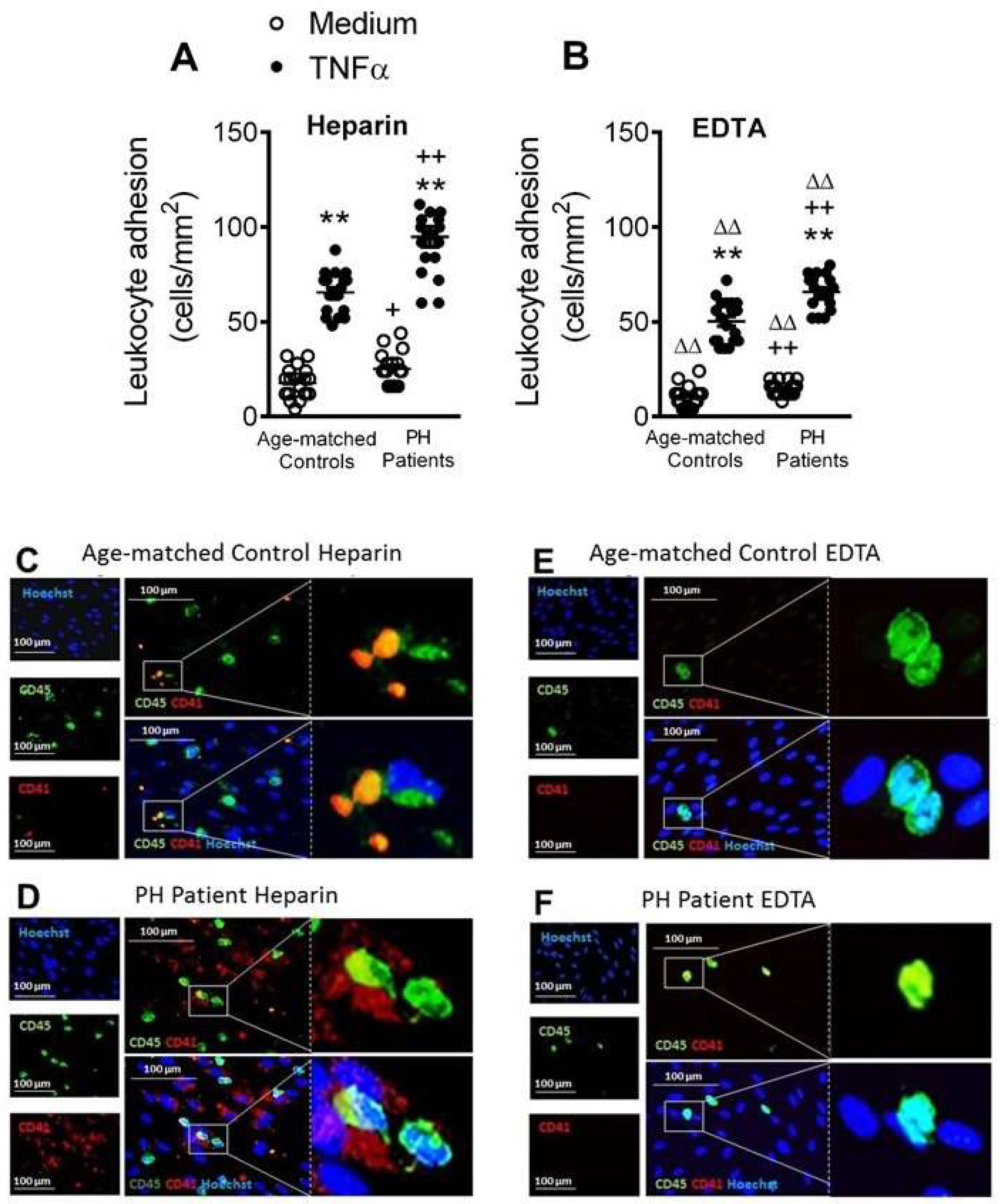
3.6. Circulating Platelet-Leukocytes and Leukocytes from PH Patients Have Increased Adhesiveness to TNFα-Stimulated HUAEC
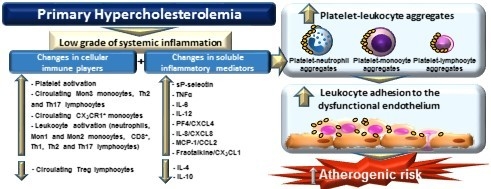
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 2017, 174, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Langslet, G.; Emery, M.; Wasserman, S.M. Evolocumab (AMG 145) for primary hypercholesterolemia. Expert Rev. Cardiovasc. Ther. 2015, 13, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Barale, C.; Frascaroli, C.; Senkeev, R.; Cavalot, F.; Russo, I. Simvastatin Effects on Inflammation and Platelet Activation Markers in Hypercholesterolemia. Biomed Res. Int. 2018, 2018, 6508709. [Google Scholar] [CrossRef] [PubMed]
- Real, J.T.; Martinez-Hervas, S.; Garcia-Garcia, A.B.; Civera, M.; Pallardo, F.V.; Ascaso, J.F.; Vina, J.R.; Chaves, F.J.; Carmena, R. Circulating mononuclear cells nuclear factor-kappa B activity, plasma xanthine oxidase, and low grade inflammatory markers in adult patients with familial hypercholesterolaemia. Eur. J. Clin. Investig. 2010, 40, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Narverud, I.; Lindvig, H.W.; Halvorsen, B.; Langslet, G.; Nenseter, M.S.; Ulven, S.M.; Ose, L.; Aukrust, P.; Retterstol, K. Subjects with familial hypercholesterolemia are characterized by an inflammatory phenotype despite long-term intensive cholesterol lowering treatment. Atherosclerosis 2014, 233, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Ye, Z.X.; Chiou, K.R.; Lin, S.J.; Charng, M.J. Vascular stiffness in familial hypercholesterolaemia is associated with C-reactive protein and cholesterol burden. Eur. J. Clin. Investig. 2007, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, T.; Tuoni, M.; Ferdeghini, M.; Ciardi, A.; Marraccini, P.; Prontera, C.; Sassi, G.; Taddei, M.; Bionda, A. Plasma cholesterol regulates soluble cell adhesion molecule expression in familial hypercholesterolemia. Circulation 1997, 96, 1381–1385. [Google Scholar] [CrossRef]
- Cortes, R.; Ivorra, C.; Martinez-Hervas, S.; Pedro, T.; Gonzalez-Albert, V.; Artero, A.; Adam, V.; Garcia-Garcia, A.B.; Ascaso, J.F.; Real, J.T.; et al. Postprandial Changes in Chemokines Related to Early Atherosclerotic Processes in Familial Hypercholesterolemic Subjects: A Preliminary Study. Arch. Med. Res. 2016, 47, 33–39. [Google Scholar] [CrossRef]
- Hansen, M.; Kuhlman, A.C.B.; Sahl, R.E.; Kelly, B.; Morville, T.; Dohlmann, T.L.; Chrois, K.M.; Larsen, S.; Helge, J.W.; Dela, F. Inflammatory biomarkers in patients in Simvastatin treatment: No effect of co-enzyme Q10 supplementation. Cytokine 2019, 113, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Holven, K.B.; Damas, J.K.; Yndestad, A.; Waehre, T.; Ueland, T.; Halvorsen, B.; Heggelund, L.; Sandberg, W.J.; Semb, A.G.; Froland, S.S.; et al. Chemokines in children with heterozygous familiar hypercholesterolemia: Selective upregulation of RANTES. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial function: A critical determinant in atherosclerosis? Circulation 2004, 109, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.C.; Sallam, R.; Doyle, M.F.; Tracy, R.P.; Huber, S.A. T helper cell polarization in healthy people: Implications for cardiovascular disease. J. Cardiovasc. Transl. Res. 2013, 6, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.; Dosquet, C.; del-Pino, M.; Denarie, N.; Megnien, J.L.; Drouet, L.; Bal dit Sollier, C.; Levenson, J.; Simon, A. Relationship of circulating biomarkers of inflammation and hemostasis with preclinical atherosclerotic burden in nonsmoking hypercholesterolemic men. Am. J. Hypertens. 2006, 19, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Von Hundelshausen, P.; Schmitt, M.M. Platelets and their chemokines in atherosclerosis-clinical applications. Front. Physiol. 2014, 5, 294. [Google Scholar] [CrossRef]
- Mazor, R.; Shurtz-Swirski, R.; Farah, R.; Kristal, B.; Shapiro, G.; Dorlechter, F.; Cohen-Mazor, M.; Meilin, E.; Tamara, S.; Sela, S. Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients. Atherosclerosis 2008, 197, 937–943. [Google Scholar] [CrossRef]
- Weber, C.; Shantsila, E.; Hristov, M.; Caligiuri, G.; Guzik, T.; Heine, G.H.; Hoefer, I.E.; Monaco, C.; Peter, K.; Rainger, E.; et al. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups “Atherosclerosis & Vascular Biology” and “Thrombosis”. Thromb Haemost 2016, 116, 626–637. [Google Scholar] [CrossRef]
- Fadini, G.P.; Simoni, F.; Cappellari, R.; Vitturi, N.; Galasso, S.; Vigili de Kreutzenberg, S.; Previato, L.; Avogaro, A. Pro-inflammatory monocyte-macrophage polarization imbalance in human hypercholesterolemia and atherosclerosis. Atherosclerosis 2014, 237, 805–808. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Dyslipidaemia: PCSK9 inhibitors and foamy monocytes in familial hypercholesterolaemia. Nat. Rev. Cardiol. 2017, 14, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Urra, X.; Villamor, N.; Amaro, S.; Gomez-Choco, M.; Obach, V.; Oleaga, L.; Planas, A.M.; Chamorro, A. Monocyte subtypes predict clinical course and prognosis in human stroke. J. Cereb. Blood Flow Metab. 2009, 29, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Ketelhuth, D.F.; Hansson, G.K. Adaptive Response of T and B Cells in Atherosclerosis. Circ. Res. 2016, 118, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Zheng, X.X.; Zhou, T.; Tong, X.H.; Luo, C.Y.; Wang, X.A. HMGB1Modulates the Treg/Th17 Ratio in Atherosclerotic Patients. J. Atherosc. Thromb. 2016, 23, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Quandt, D.; Rothe, K.; Scholz, R.; Baerwald, C.W.; Wagner, U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS ONE. 2014, 9, e93293. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T. The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo. Front. Immunol. 2018, 9, 716. [Google Scholar] [CrossRef]
- Rius, C.; Company, C.; Piqueras, L.; Cerda-Nicolas, J.M.; Gonzalez, C.; Servera, E.; Ludwig, A.; Morcillo, E.J.; Sanz, M.J. Critical role of fractalkine (CX3CL1) in cigarette smoke-induced mononuclear cell adhesion to the arterial endothelium. Thorax 2013, 68, 177–186. [Google Scholar] [CrossRef]
- Rius, C.; Piqueras, L.; Gonzalez-Navarro, H.; Albertos, F.; Company, C.; Lopez-Gines, C.; Ludwig, A.; Blanes, J.I.; Morcillo, E.J.; Sanz, M.J. Arterial and venous endothelia display differential functional fractalkine (CX3CL1) expression by angiotensin-II. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 96–104. [Google Scholar] [CrossRef]
- Marques, P.; Collado, A.; Escudero, P.; Rius, C.; Gonzalez, C.; Servera, E.; Piqueras, L.; Sanz, M.J. Cigarette Smoke Increases Endothelial CXCL16-Leukocyte CXCR6 Adhesion In Vitro and In Vivo. Potential Consequences in Chronic Obstructive Pulmonary Disease. Front. Immunol. 2017, 8, 1766. [Google Scholar] [CrossRef]
- Furio, E.; Garcia-Fuster, M.J.; Redon, J.; Marques, P.; Ortega, R.; Sanz, M.J.; Piqueras, L. CX3CR1/CX3CL1 Axis Mediates Platelet-Leukocyte Adhesion to Arterial Endothelium in Younger Patients with a History of Idiopathic Deep Vein Thrombosis. Thromb Haemost 2018, 118, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Valeri, C.R.; Furman, M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001, 104, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
| Control Volunteers (n = 21) | PH Subjects (n = 22) | p Value | |
|---|---|---|---|
| Age (years) | 48.8 ± 2.7 | 49 ± 3.1 | 0.95 |
| Gender M/F (%) | 5/16 (23.8/76.2) | 4/18 (18.2/81.8) | 0.72 |
| BMI (kg/m2) | 25.4 ± 0.7 | 25.7 ± 0.9 | 0.83 |
| Waist circumference (cm) | 85.3 ± 1.9 | 85.7 ± 2.2 | 0.90 |
| SBP (mmHg) | 115.9 ± 2.0 | 124.7 ± 3.6 * | <0.05 |
| DBP (mmHg) | 71.6 ± 1.8 | 78.5 ± 2.6 * | <0.05 |
| Glucose (mg/dL) | 86.7 ± 1.5 | 88.1 ± 1.9 | 0.57 |
| TC levels (mg/dL) | 206.1 ± 6.8 | 264.6 ± 8.9 ** | <0.01 |
| LDL levels (mg/dL) | 130.6 ± 5.4 | 182.8 ± 6.2 ** | <0.01 |
| TG (mg/dL) | 80.9 ± 7.3 | 109.7 ± 8.5 ** | <0.01 |
| HDL levels (mg/dL) | 65.9 ± 2.5 | 63.4 ± 2.9 | 0.51 |
| ApoB (mg/dL) | 92.5 ± 4.1 | 127.4 ± 5.0 ** | <0.01 |
| GOT (U/L) | 21.7 ± 0.9 | 22.8 ± 1.1 | 0.42 |
| GPT (U/L) | 18.3 ± 1.8 | 18.5 ± 1.1 | 0.90 |
| Creatinine (mg/dL) | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.48 |
| IgG (mg/dL) | 966.7 ± 41.1 | 968.5 ± 34.4 | 0.97 |
| Igm (mg/dL) | 100.4 ± 7.6 | 125.8 ± 14.1 | 0.14 |
| IgE total (IU/L) | 42.6 ± 12.0 | 50.4 ± 16.9 | 0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado, A.; Marques, P.; Domingo, E.; Perello, E.; González-Navarro, H.; Martinez-Hervás, S.; Real, J.T.; Piqueras, L.; Ascaso, J.F.; Sanz, M.-J. Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation. J. Clin. Med. 2019, 8, 18. https://doi.org/10.3390/jcm8010018
Collado A, Marques P, Domingo E, Perello E, González-Navarro H, Martinez-Hervás S, Real JT, Piqueras L, Ascaso JF, Sanz M-J. Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation. Journal of Clinical Medicine. 2019; 8(1):18. https://doi.org/10.3390/jcm8010018
Chicago/Turabian StyleCollado, Aida, Patrice Marques, Elena Domingo, Eva Perello, Herminia González-Navarro, Sergio Martinez-Hervás, José T. Real, Laura Piqueras, Juan F. Ascaso, and Maria-Jesus Sanz. 2019. "Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation" Journal of Clinical Medicine 8, no. 1: 18. https://doi.org/10.3390/jcm8010018
APA StyleCollado, A., Marques, P., Domingo, E., Perello, E., González-Navarro, H., Martinez-Hervás, S., Real, J. T., Piqueras, L., Ascaso, J. F., & Sanz, M.-J. (2019). Novel Immune Features of the Systemic Inflammation Associated with Primary Hypercholesterolemia: Changes in Cytokine/Chemokine Profile, Increased Platelet and Leukocyte Activation. Journal of Clinical Medicine, 8(1), 18. https://doi.org/10.3390/jcm8010018