Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Questionnaire Survey
2.3. Measurement of GS and SMI and our Study
2.4. Statistical Considerations
3. Results
3.1. Patient Baseline Characteristics
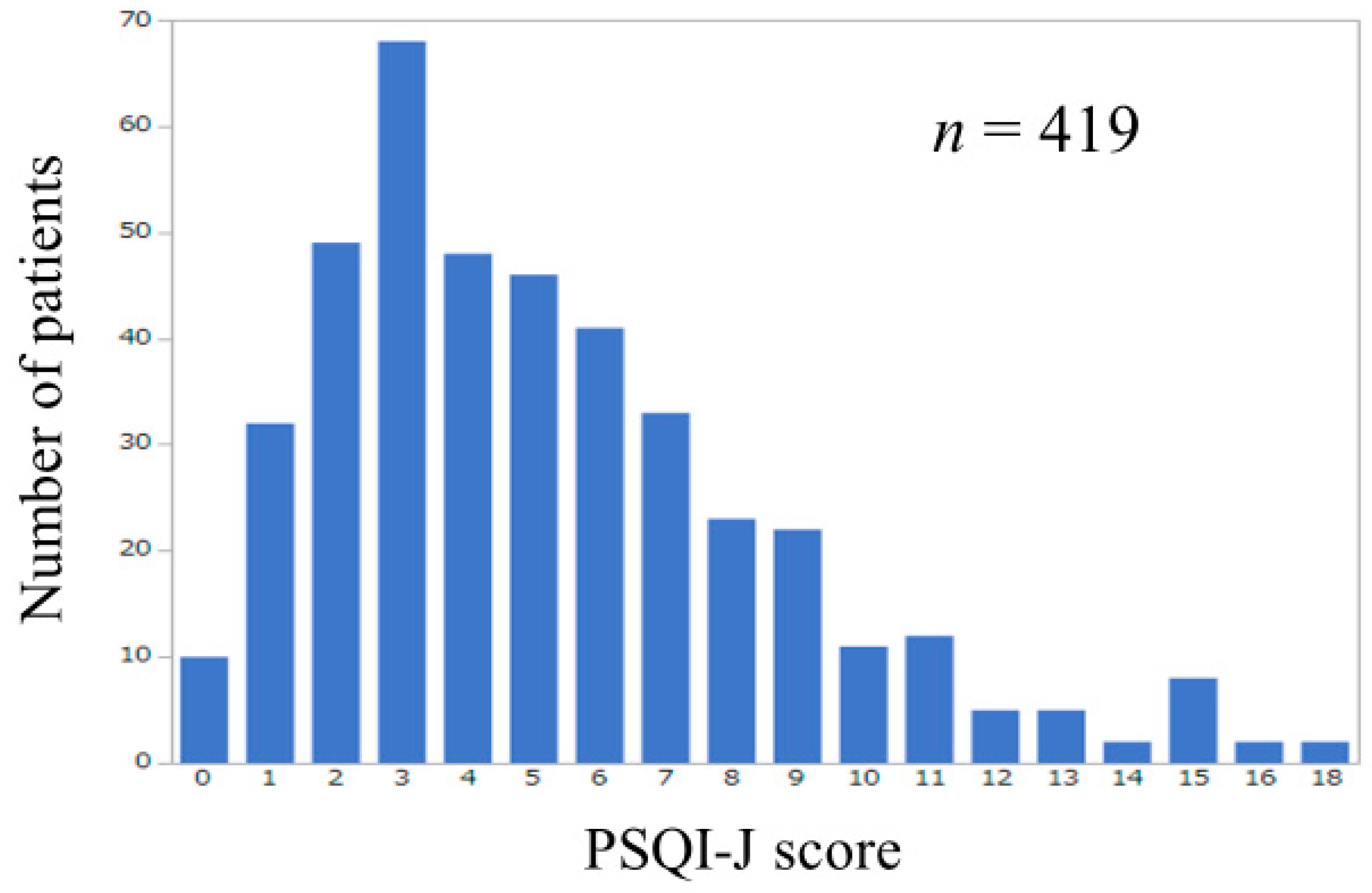
3.2. Influence of GS and SMM on PSQI-J for All Cases (n = 419)
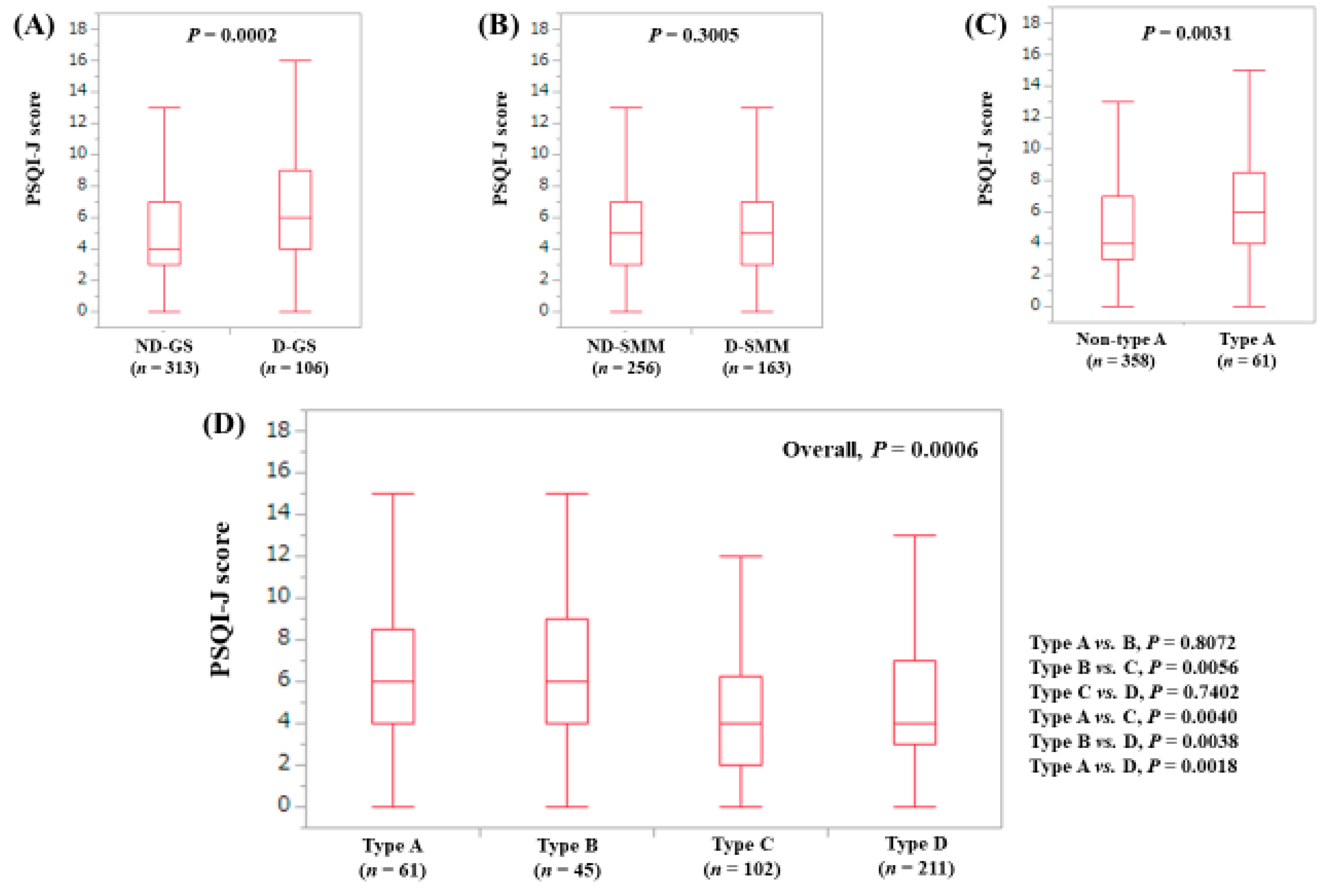
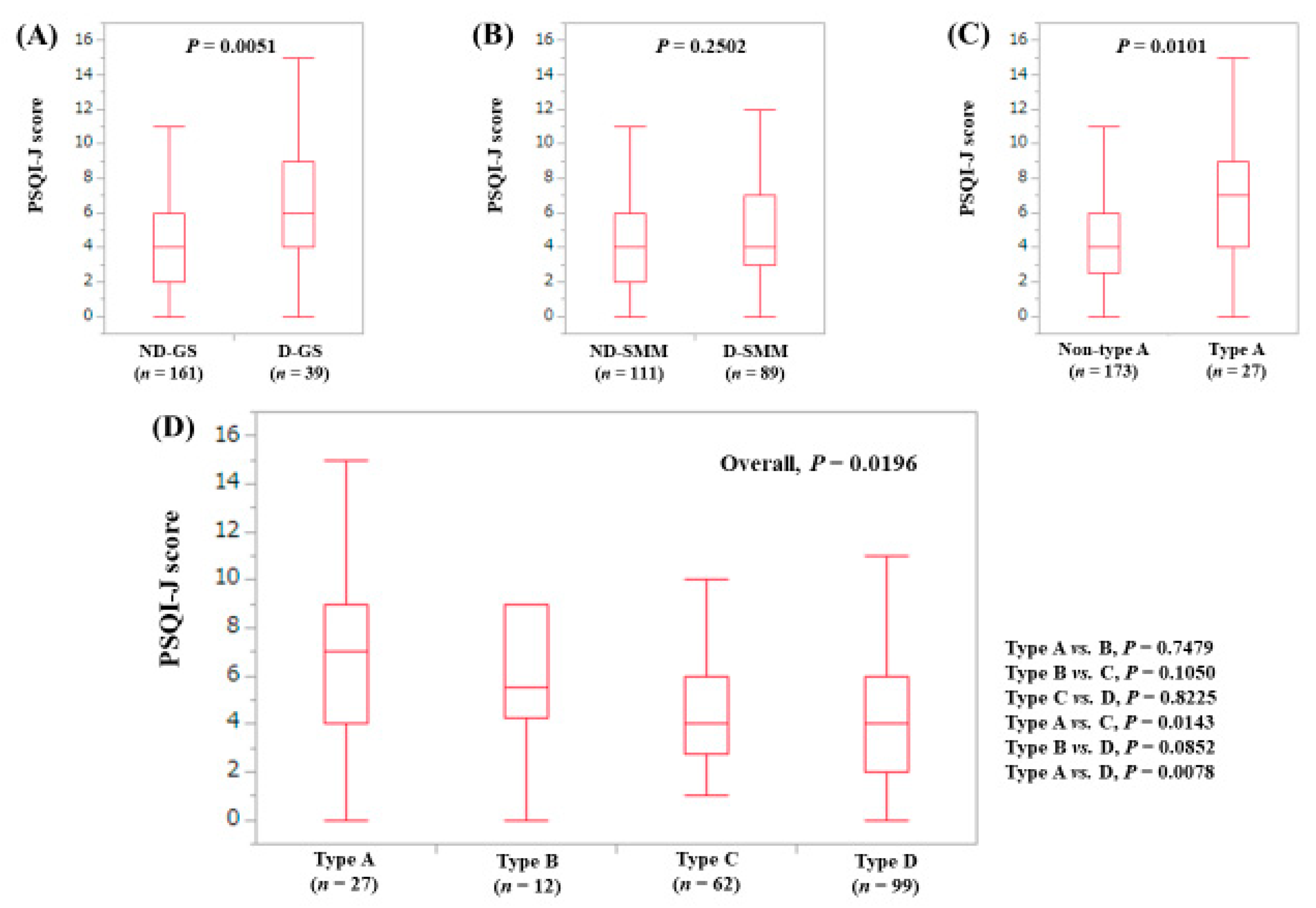
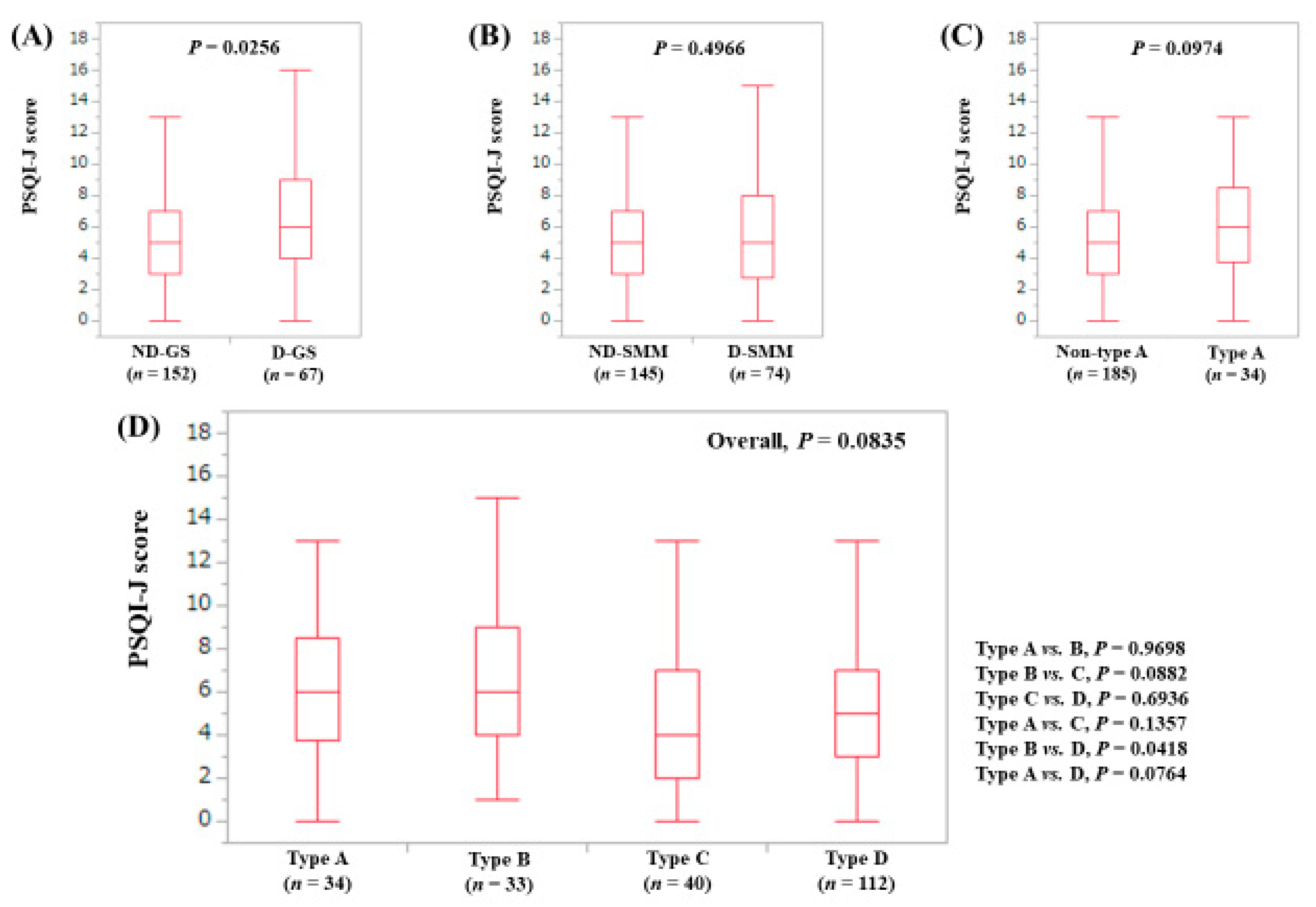
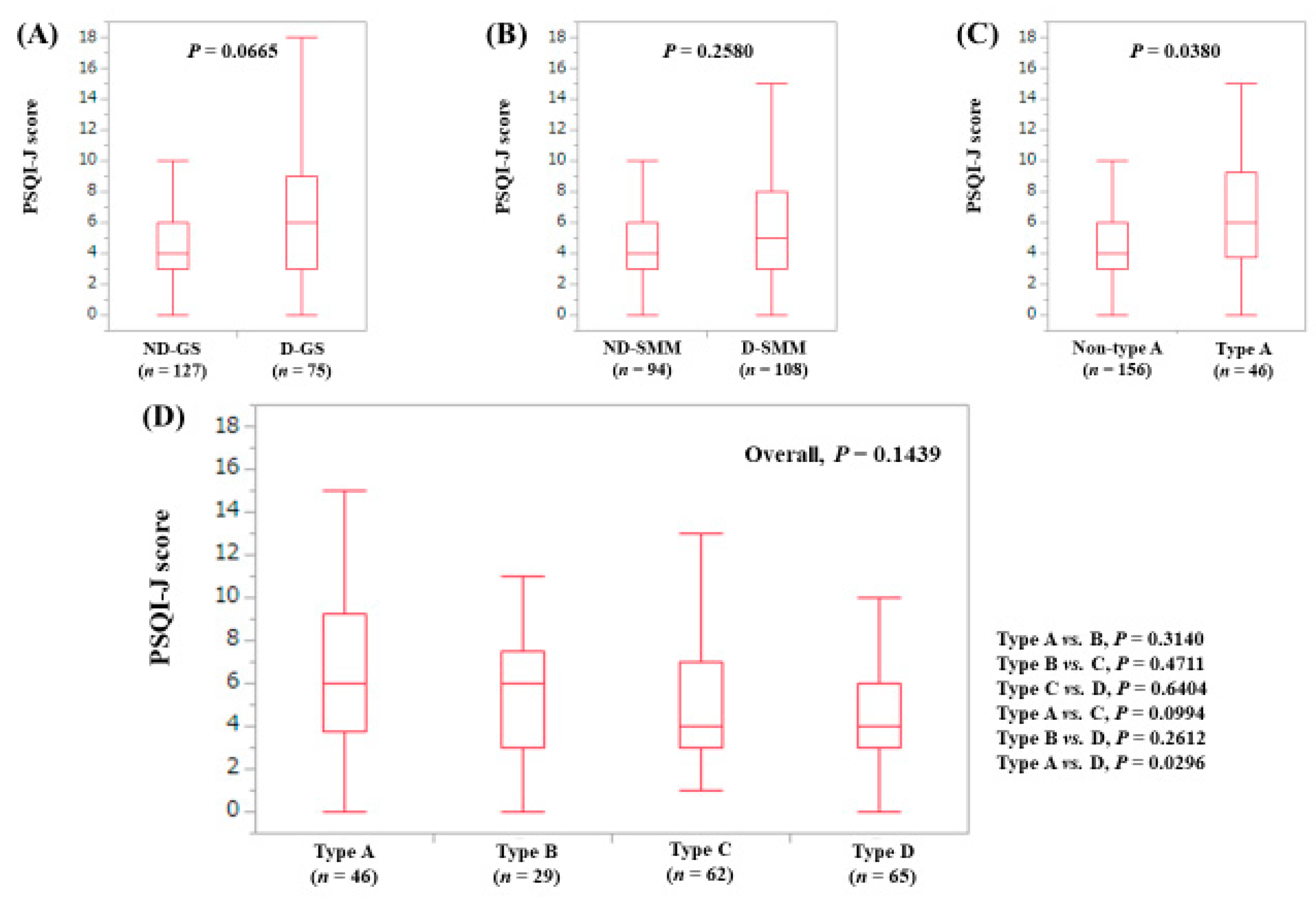
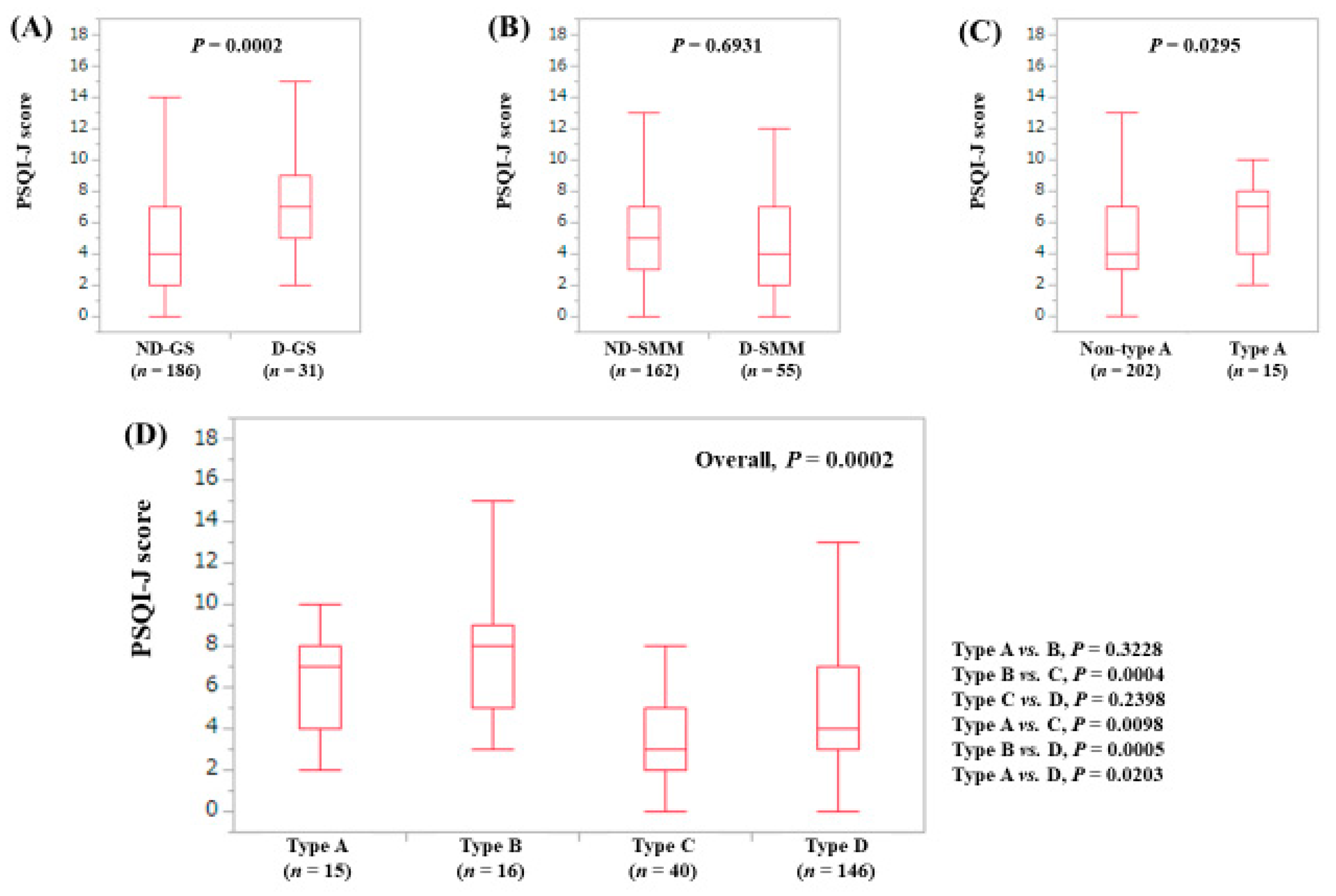
3.3. Stratified Analysis 1: Influence of GS and SMM on PSQI-J for LC Patients (n = 156)
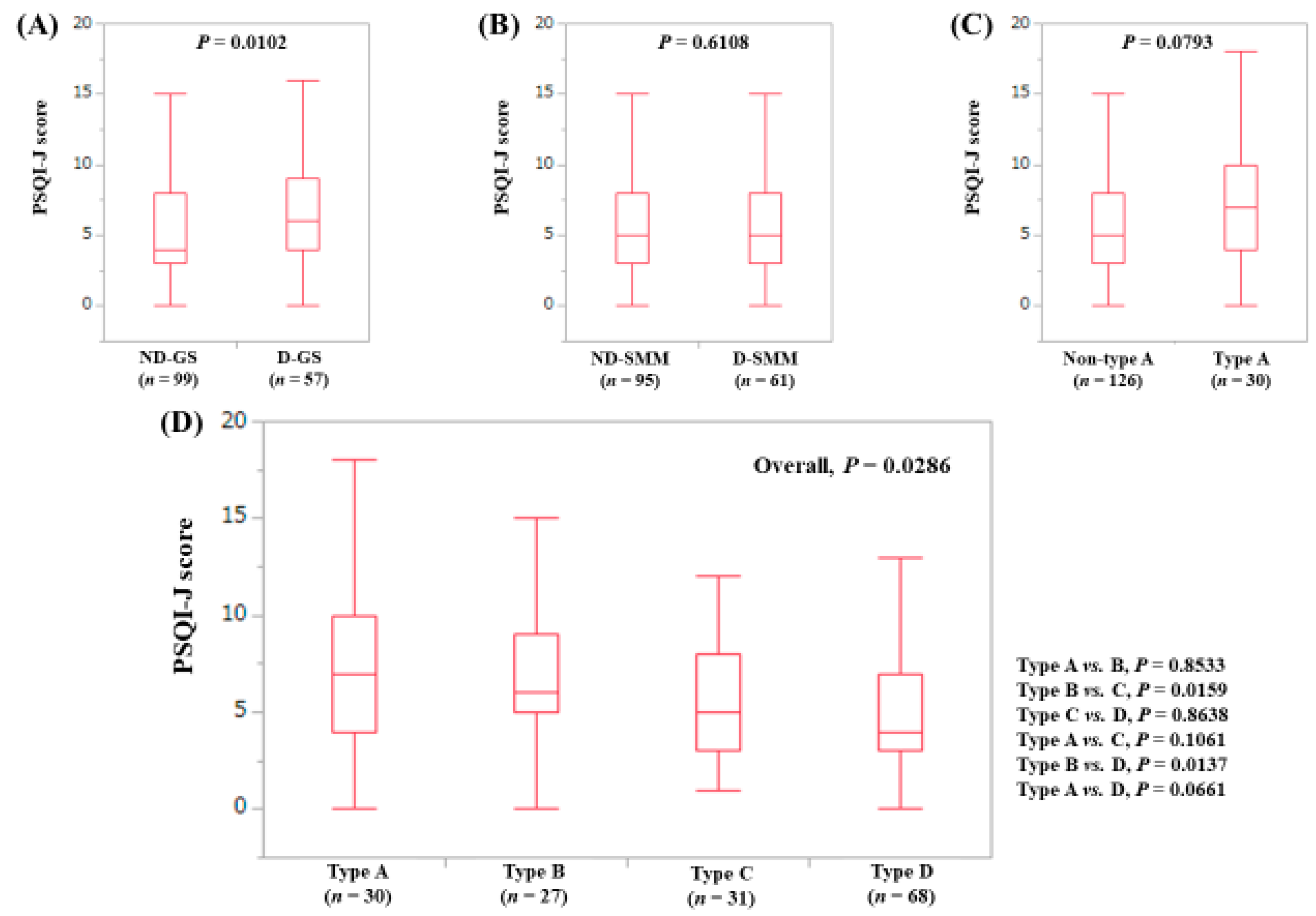
3.4. Stratified Analysis 2: Influence of GS and SMM on PSQI-J for Non-LC Patients (n = 263)
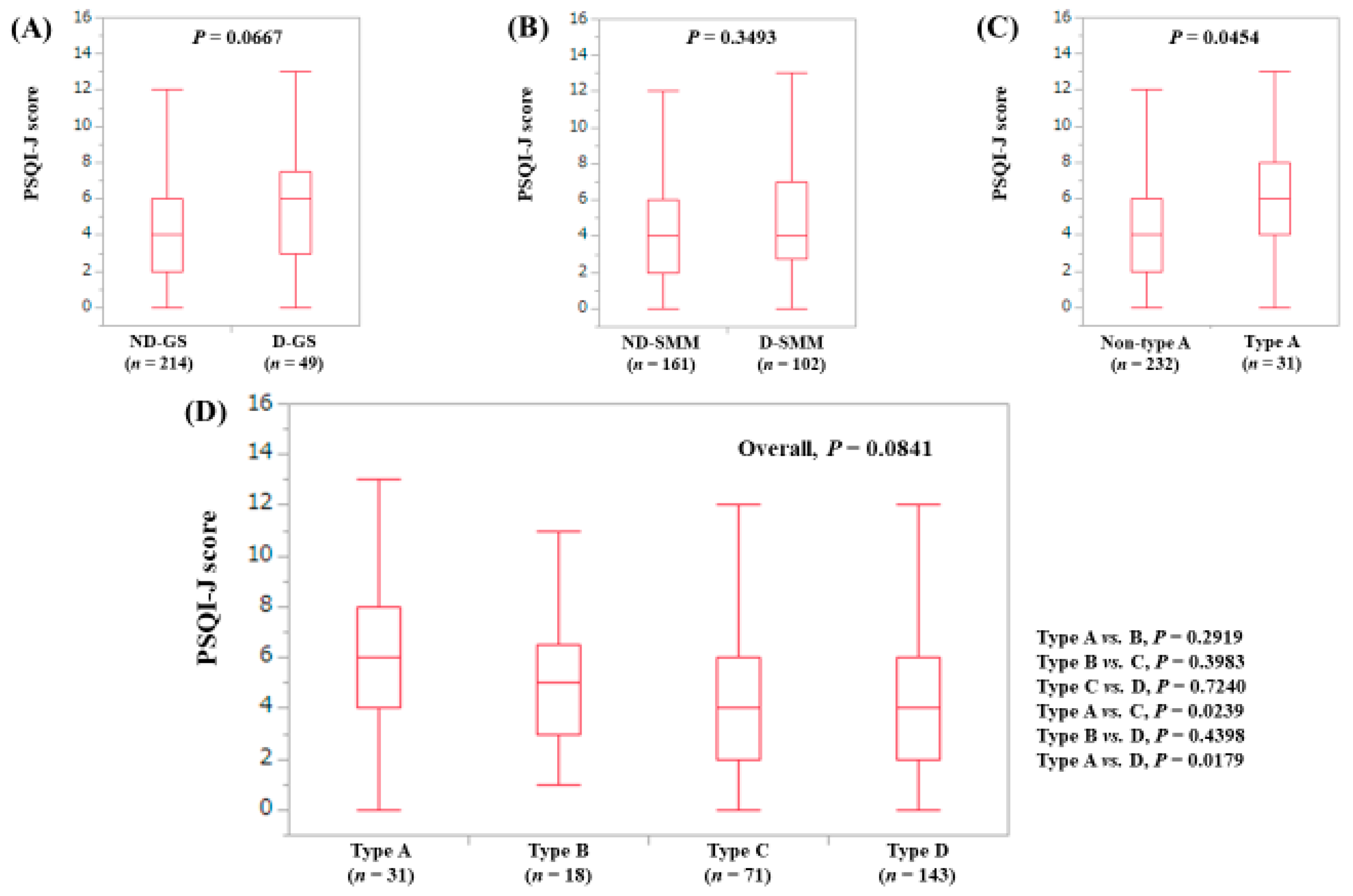
3.5. Stratified Analysis 3: Influence of GS and SMM on PSQI-J for Male Patients (n = 200)
3.6. Stratified Analysis 4: Influence of GS and SMM on PSQI-J for Female Patients (n = 219)
3.7. Stratified Analysis 5: Influence of GS and SMM on PSQI-J for Patients Aged 65 Years or More (n = 202)
3.8. Stratified Analysis 6: Influence of GS and SMM on PSQI-J for Patients Aged Less Than 65 Years (n = 217)
3.9. Univariate and Multivariate Analyses of Factors Associated with PSQI-J Score 6 or More
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, K.; Jones, D.E.; Wilton, K.; Newton, J.L. Restless leg syndrome is a treatable cause of sleep disturbance and fatigue in primary biliary cirrhosis. Liver Int. 2013, 33, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, M.; Gonen, I.; Ermis, F.; Oktay, M.; Besir, F.H.; Kutlucan, A.; Sahin, A.; Ataoglu, S. The prevalence of fibromyalgia among patients with hepatitis B virus infection. Int. J. Clin. Exp. Med. 2013, 6, 804–808. [Google Scholar] [PubMed]
- Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Drago, F.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; et al. Comparison of sleep disorders in chronic hepatitis C patients treated with interferon-based therapy and direct acting antivirals using actigraphy. Hepatol. Res. 2016, 46, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Mir, H.M.; Stepanova, M.; Afendy, H.; Cable, R.; Younossi, Z.M. Association of Sleep Disorders with Nonalcoholic Fatty Liver Disease (NAFLD): A Population-based Study. J. Clin. Exp. Hepatol. 2013, 3, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Erlangsen, A.; Runeson, B.; Bolton, J.M.; Wilcox, H.C.; Forman, J.L.; Krogh, J.; Shear, M.K.; Nordentoft, M.; Conwell, Y. Association Between Spousal Suicide and Mental, Physical, and Social Health Outcomes: A Longitudinal and Nationwide Register-Based Study. JAMA Psychiatry 2017, 74, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Cheng, H.J.; Wu, J.S.; Yang, Y.C.; Chou, C.Y.; Chang, C.J.; Lu, F.H. The association of sleep duration and sleep quality with non-alcoholic fatty liver disease in a Taiwanese population. Obes. Res. Clin. Pract. 2018, 12, 500–505. [Google Scholar] [CrossRef]
- Labenz, C.; Baron, J.S.; Toenges, G.; Schattenberg, J.M.; Nagel, M.; Sprinzl, M.F.; Nguyen-Tat, M.; Zimmermann, T.; Huber, Y.; Marquardt, J.U.; et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment. Pharmacol. Ther. 2018, 48, 313–321. [Google Scholar] [CrossRef]
- Bernsmeier, C.; Weisskopf, D.M.; Pflueger, M.O.; Mosimann, J.; Campana, B.; Terracciano, L.; Beglinger, C.; Heim, M.H.; Cajochen, C. Sleep Disruption and Daytime Sleepiness Correlating with Disease Severity and Insulin Resistance in Non-Alcoholic Fatty Liver Disease: A Comparison with Healthy Controls. PLoS ONE 2015, 10, e0143293. [Google Scholar] [CrossRef]
- Iwasa, M.; Karino, Y.; Kawaguchi, T.; Nakanishi, H.; Miyaaki, H.; Shiraki, M.; Nakajima, T.; Sawada, Y.; Yoshiji, H.; Okita, K.; et al. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: A nationwide study. Liver Int. 2018. [Google Scholar] [CrossRef]
- Ghabril, M.; Jackson, M.; Gotur, R.; Weber, R.; Orman, E.; Vuppalanchi, R.; Chalasani, N. Most Individuals with Advanced Cirrhosis Have Sleep Disturbances, Which Are Associated with Poor Quality of Life. Clin. Gastroenterol. Hepatol. 2017, 15, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, J.; Cabrera, J.; Lataif, L.; Penev, P.; Zee, P.; Blei, A.T. High prevalence of sleep disturbance in cirrhosis. Hepatology 1998, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Middleton, B.; Mani, A.R.; Skene, D.J.; Morgan, M.Y. Sleep and circadian abnormalities in patients with cirrhosis: Features of delayed sleep phase syndrome? Metab. Brain Dis. 2009, 24, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Mariotto, S.; Ferrari, S.; Calabrese, M.; Zanusso, G.; Gajofatto, A.; Sansonno, D.; Dammacco, F. Hepatitis C virus-associated neurocognitive and neuropsychiatric disorders: Advances in 2015. World J. Gastroenterol. 2015, 21, 11974–11983. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Middleton, B.; Skene, D.J.; Morgan, M.Y. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009, 29, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Mostacci, B.; Ferlisi, M.; Baldi Antognini, A.; Sama, C.; Morelli, C.; Mondini, S.; Cirignotta, F. Sleep disturbance and daytime sleepiness in patients with cirrhosis: A case control study. Neurol. Sci. 2008, 29, 237–240. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J. Clinical relevance of sarcopenia in patients with cirrhosis. World J. Gastroenterol. 2014, 20, 8061–8071. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Review article: Sarcopenia in cirrhosis—Aetiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Otten, L. Financial impact of sarcopenia or low muscle mass—A short review. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Shirai, H.; Kaido, T.; Hamaguchi, Y.; Kobayashi, A.; Okumura, S.; Yao, S.; Yagi, S.; Kamo, N.; Taura, K.; Okajima, H.; et al. Preoperative Low Muscle Mass and Low Muscle Quality Negatively Impact on Pulmonary Function in Patients Undergoing Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2018, 7, 76–89. [Google Scholar] [CrossRef]
- De Bandt, J.P.; Jegatheesan, P.; Tennoune-El-Hafaia, N. Muscle Loss in Chronic Liver Diseases: The Example of Nonalcoholic Liver Disease. Nutrients 2018, 10, 1195. [Google Scholar] [CrossRef]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, H.D.; Tapper, E.B. Interventions to improve physical function and prevent adverse events in cirrhosis. Gastroenterol. Rep. (Oxf.) 2018, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Tsuchiya, K.; Tamaki, N.; Hirayama, I.; Tanaka, T.; Sato, M.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Kuzuya, T.; et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010, 52, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Abad, V.C.; Guilleminault, C. Insomnia in Elderly Patients: Recommendations for Pharmacological Management. Drugs Aging 2018, 35, 791–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Duffy, J.F. Circadian Rhythm Sleep-Wake Disorders in Older Adults. Sleep Med. Clin. 2018, 13, 39–50. [Google Scholar] [CrossRef]
- Gulia, K.K.; Kumar, V.M. Sleep disorders in the elderly: A growing challenge. Psychogeriatrics 2018, 18, 155–165. [Google Scholar] [CrossRef]
- Durazzo, M.; Belci, P.; Collo, A.; Prandi, V.; Pistone, E.; Martorana, M.; Gambino, R.; Bo, S. Gender specific medicine in liver diseases: A point of view. World J. Gastroenterol. 2014, 20, 2127–2135. [Google Scholar] [CrossRef]
- Saner, N.J.; Bishop, D.J.; Bartlett, J.D. Is exercise a viable therapeutic intervention to mitigate mitochondrial dysfunction and insulin resistance induced by sleep loss? Sleep Med. Rev. 2018, 37, 60–68. [Google Scholar] [CrossRef]
- Hussain, M.M.; Pan, X. Circadian regulators of intestinal lipid absorption. J. Lipid Res. 2015, 56, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, B.C.; Puri, V.; Sachdeva, S.; Srivastava, S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab Brain Dis. 2017, 32, 595–605. [Google Scholar] [CrossRef] [PubMed]
| Male | Female | |||
|---|---|---|---|---|
| GS < 26 kg | SMI < 7.0 kg/m2 | GS < 18 kg | SMI < 5.7 kg/m2 | |
| Type A | Yes | Yes | Yes | Yes |
| Type B | Yes | No | Yes | No |
| Type C | No | Yes | No | Yes |
| Type D | No | No | No | No |
| Variables | All Cases (n = 419) | Type A (n = 61) | Type B (n = 45) | Type C (n = 102) | Type D (n = 211) | Overall (Type A, B, C and D) P Value |
|---|---|---|---|---|---|---|
| Age (years) | 64 (25–90) | 71 (25–90) | 69 (33–83) | 67 (29–84) | 58 (26–90) | <0.0001 |
| Gender, male/female | 200/219 | 27/34 | 12/33 | 62/40 | 99/112 | 0.0016 |
| HBV/HCV/HBV and HCV/NBNC | 61/259/8/91 | 5/42/2/12 | 7/28/0/10 | 15/67/3/17 | 34/122/3/52 | 0.5540 |
| Presence of LC, yes/no | 156/263 | 30/31 | 27/18 | 31/71 | 68/143 | 0.0004 |
| Body mass index (kg/m2) | 22.6 (14.8–41.4) | 21.3 (17.2–26.9) | 25.5 (19.8–32.8) | 20.6 (16.0–28.3) | 24.1 (14.8–41.4) | <0.0001 |
| Total bilirubin (mg/dL) | 0.8 (0.3–5.6) | 0.7 (0.4–2.7) | 0.85 (0.3–4.0) | 0.9 (0.3–2.4) | 0.8 (0.3–5.6) | 0.1415 |
| Serum albumin (g/dL) | 4.2 (1.8–5.2) | 4.1 (2.4–4.7) | 4.1 (2.5–5.2) | 4.3 (2.3–5.0) | 4.2 (1.8–4.9) | <0.0001 |
| Prothrombin time (%) | 88.7 (23.0–122.9) | 85.3 (46.5–122.5) | 80.4 (44.6–110.1) | 90.0 (23.0–120.2) | 89.8 (28.3–122.9) | 0.0002 |
| Platelet count (×104/mm3) | 16.8 (1.4–42.2) | 15.3 (2.8–39.7) | 13.1 (3.6–30.0) | 15.3 (4.1–33.0) | 17.8 (1.4–42.2) | 0.0012 |
| Total cholesterol (mg/dL) | 177.5 (80–420) | 175 (80–420) | 158 (88–244) | 182 (99–290) | 181 (90–292) | 0.0080 |
| AST (IU/L) | 28 (10–222) | 29 (10–191) | 34 (15–133) | 27 (11–143) | 27 (12–222) | 0.2732 |
| ALT (IU/L) | 24 (5–297) | 27 (5–263) | 28 (5–213) | 23 (5–188) | 24 (8–297) | 0.8079 |
| ALP (IU/L) | 239 (91–5065) | 253.5 (121–5065) | 249 (95–579) | 231 (112–650) | 234 (91–1206) | 0.1702 |
| GGT (IU/L) | 28 (7–542) | 26 (8–462) | 37 (11–311) | 26.5 (10–386) | 27 (7–542) | 0.8156 |
| eGFR (mL/min/1.73 m2) | 83 (5–162) | 80 (87–141) | 83.5 (31–146) | 83 (5–136) | 83.5 (34–162) | 0.0497 |
| HbA1c (NGSP) | 5.7 (3.7–10.4) | 5.6 (4.7–8.8) | 5.6 (4.1–8.2) | 5.7 (3.8–10.1) | 5.6 (3.7–10.4) | 0.9597 |
| Serum sodium (mmol/L) | 140 (124–148) | 140 (124–144) | 140 (130–146) | 140 (131–148) | 140 (126–144) | 0.2000 |
| PSQI-J score | 5 (0–18) | 6 (0–18) | 6 (0–15) | 4 (0–16) | 4 (0–18) | 0.0006 |
| Variables | PSQI-J Score 6 or More (n = 166) | PSQI-J Score Less than 6 (n = 253) | P Value |
|---|---|---|---|
| Age (years) | 65 (26–90) | 62 (25–90) | 0.2326 |
| Gender, male/female | 70/96 | 130/123 | 0.0721 |
| HBV/HCV/HBV and HCV/NBNC | 21/106/3/36 | 40/153/5/55 | 0.8294 |
| Body mass index (kg/m2) | 22.9 (16.0–41.4) | 22.5 (14.8–36.5) | 0.1589 |
| Presence of LC, yes/no | 74/92 | 82/171 | 0.0132 |
| Type, A/B/C/D | 36/26/32/72 | 25/19/70/139 | <0.0001 |
| Total bilirubin (mg/dL) | 0.8 (0.3–5.6) | 0.8 (0.3–4.0) | 0.6316 |
| Serum albumin (g/dL) | 4.1 (1.8–5.0) | 4.2 (2.3–5.2) | 0.0041 |
| Prothrombin time (%) | 87.5 (38.4–117.6) | 90.0 (23.0–122.9) | 0.1593 |
| Platelet count (×104/mm3) | 16.5 (2.8–42.2) | 16.9 (1.4–37.6) | 0.5222 |
| AST (IU/L) | 29.5 (12–191) | 28 (10–222) | 0.6180 |
| ALT (IU/L) | 25 (5–297) | 24 (5–213) | 0.5807 |
| ALP (IU/L) | 235 (105–5065) | 244 (91–1206) | 0.9472 |
| GGT (IU/L) | 28.5 (8–462) | 27 (7–542) | 0.5677 |
| Total cholesterol (mg/dL) | 175.5 (80–420) | 179.5 (88–290) | 0.3166 |
| eGFR (mL/min/1.73m2) | 83 (83–162) | 83 (5–147) | 0.9792 |
| Serum sodium (mmol/L) | 140 (124–148) | 140 (131–146) | 0.6521 |
| HbA1c (NGSP) | 5.7 (3.7–10.1) | 5.7 (3.8–10.4) | 0.7734 |
| Multivariate Analysis | |||
|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | |
| Presence of LC | |||
| Yes | 0.796 | 0.483–1.310 | 0.3704 |
| No | 1.000 | Reference | |
| Gender | |||
| Male | 1.369 | 0.900–2.082 | 0.1412 |
| Female | 1.000 | Reference | |
| Serum albumin (per 1.0 g/dL) | 1.320 | 0.831–2.095 | 0.2390 |
| Type | |||
| A (D-GS and D-SMM) | 0.393 | 0.217–0.713 | 0.0021 |
| B (D-GS and ND-SMM) | 0.453 | 0.230–0.892 | 0.0220 |
| C (ND-GS and D-SMM) | 1.020 | 0.609–1.710 | 0.9386 |
| D (ND-GS and ND-SMM) | 1.000 | Reference | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases. J. Clin. Med. 2019, 8, 16. https://doi.org/10.3390/jcm8010016
Nishikawa H, Enomoto H, Yoh K, Iwata Y, Sakai Y, Kishino K, Ikeda N, Takashima T, Aizawa N, Takata R, et al. Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases. Journal of Clinical Medicine. 2019; 8(1):16. https://doi.org/10.3390/jcm8010016
Chicago/Turabian StyleNishikawa, Hiroki, Hirayuki Enomoto, Kazunori Yoh, Yoshinori Iwata, Yoshiyuki Sakai, Kyohei Kishino, Naoto Ikeda, Tomoyuki Takashima, Nobuhiro Aizawa, Ryo Takata, and et al. 2019. "Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases" Journal of Clinical Medicine 8, no. 1: 16. https://doi.org/10.3390/jcm8010016
APA StyleNishikawa, H., Enomoto, H., Yoh, K., Iwata, Y., Sakai, Y., Kishino, K., Ikeda, N., Takashima, T., Aizawa, N., Takata, R., Hasegawa, K., Ishii, N., Yuri, Y., Nishimura, T., Iijima, H., & Nishiguchi, S. (2019). Effect of Sarcopenia on Sleep Disturbance in Patients with Chronic Liver Diseases. Journal of Clinical Medicine, 8(1), 16. https://doi.org/10.3390/jcm8010016




