Association of Wnt Inhibitors, Bone Mineral Density and Lifestyle Parameters in Women with Breast Cancer Treated with Anastrozole Therapy
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Sclerostin ELISA
2.3. Dickkopf1 ELISA
Assessment of Bone Mineral Density
2.4. Statistical Analysis
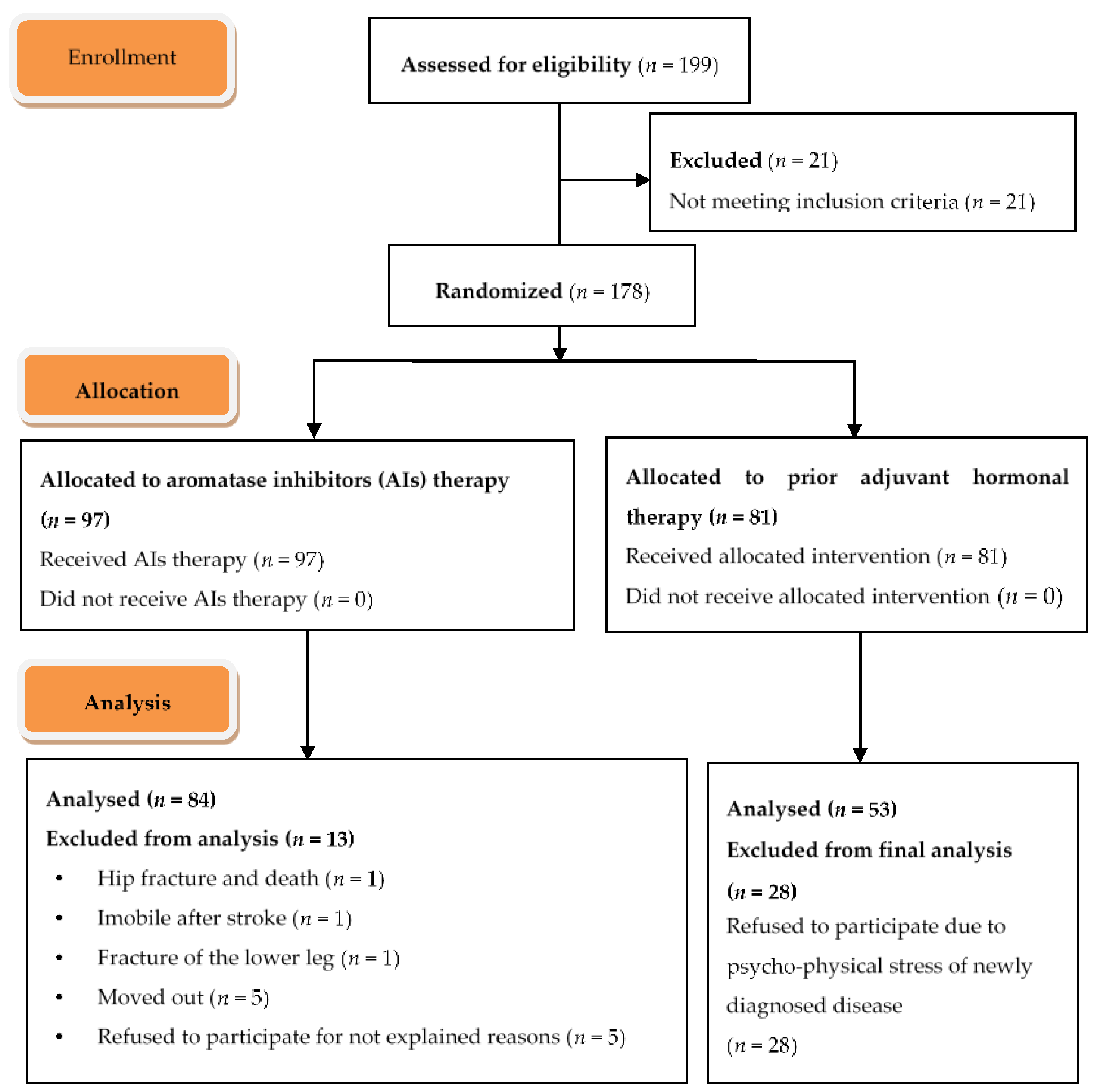
3. Results
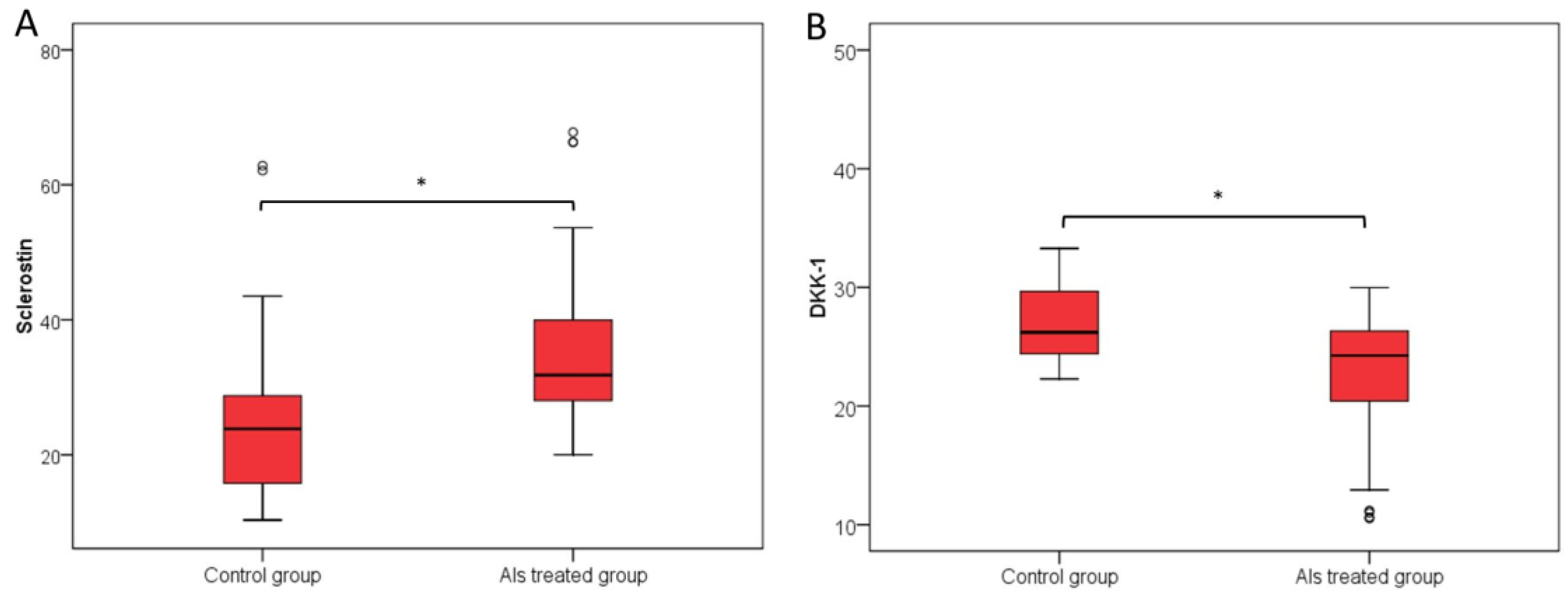
3.1. Primary Endpoint
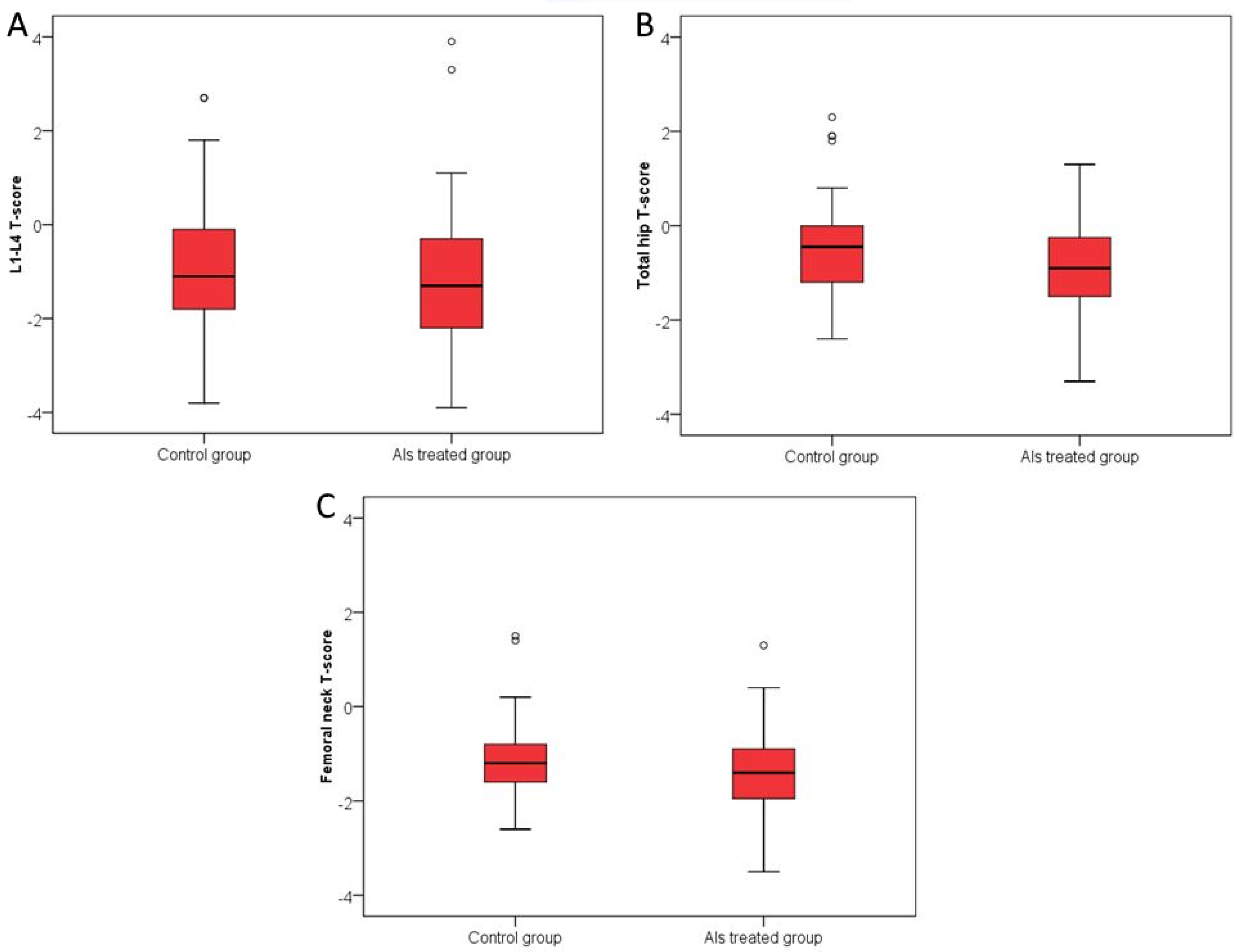
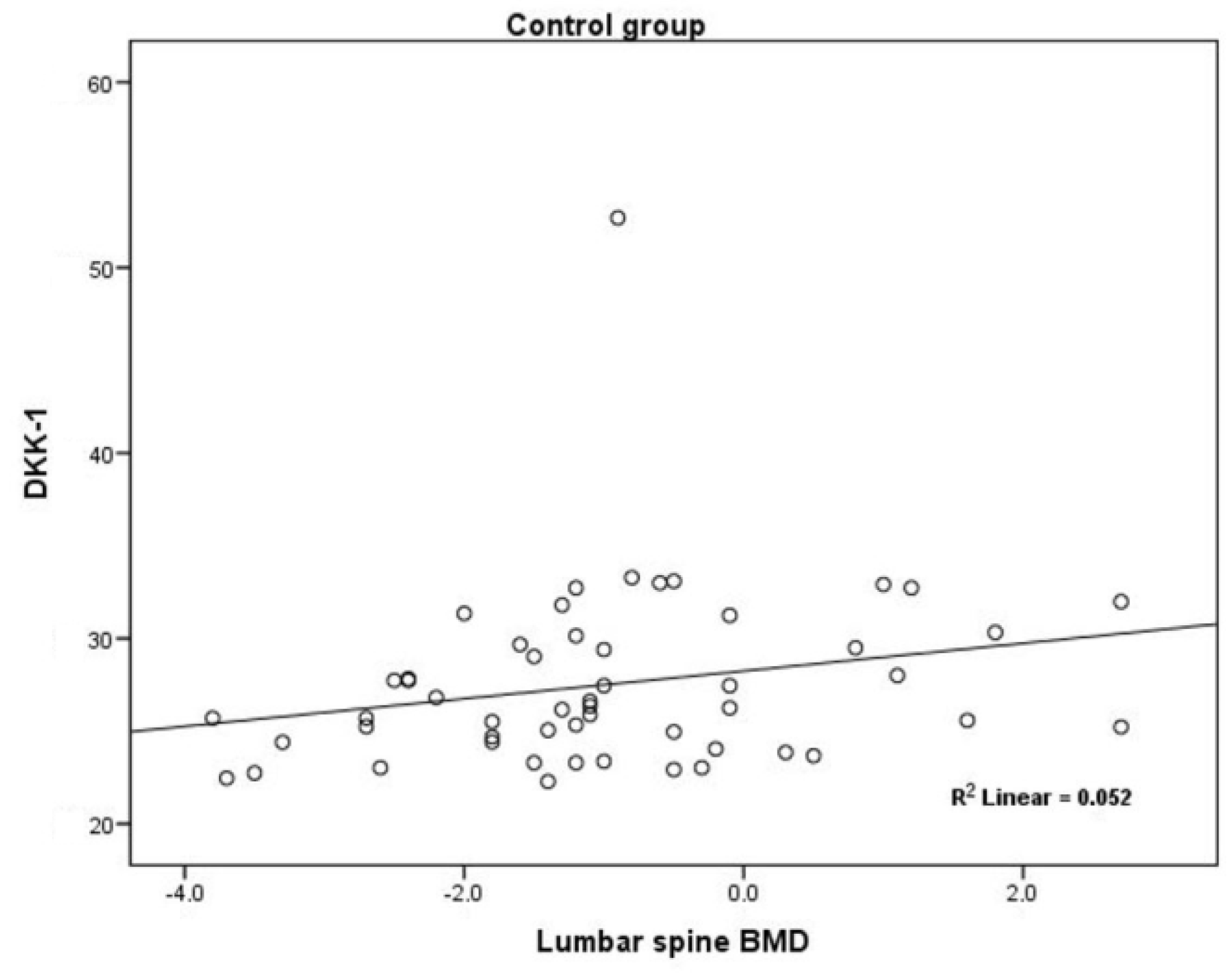
3.2. Secondary Endpoints
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dowsett, M.; Cuzick, J.; Ingle, J.; Coates, A.; Forbes, J.; Bliss, J.; Buyse, M.; Baum, M.; Buzdar, A.; Colleoni, M.; et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J. Clin. Oncol. 2010, 28, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.N. Overview of adjuvant trials of aromatase inhibitors in early breast cancer. Steroids 2011, 76, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Trudeau, M.; Shelley, W.; Messersmith, H.; Pritchard, K.I. Aromatase inhibitors in adjuvant therapy for hormone receptor positive breast cancer: A systematic review. Cancer Treat. Rev. 2008, 34, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Griggs, J.J.; Prestrud, A.A.; Temin, S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J. Oncol. Pract. 2010, 6, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S. American Society of Clinical Oncology 2014: Updates in breast and gastrointestinal cancers. Indian J. Med. Paediatr. Oncol. 2014, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Chung, Y.; Kim, S.H.; Park, S.; Bae, J.H.; Kim, G.; Lee, S.J.; Kim, J.E.; Park, B.W.; Lim, S.K.; et al. Increased sclerostin levels after further ablation of remnant estrogen by aromatase inhibitors. Endocrinol. Metab. (Seoul) 2015, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit. Rev. Oncol. Hematol. 2009, 69, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Adams, J.E.; Coleman, R.E.; Howell, A.; Hannon, R.A.; Cuzick, J.; Mackey, J.R.; Beckmann, M.W.; Clack, G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J. Clin. Oncol. 2008, 26, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Wijenayaka, A.R.; Kogawa, M.; Lim, H.P.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 2011, 6, e25900. [Google Scholar] [CrossRef] [PubMed]
- Van Bezooijen, R.L.; ten Dijke, P.; Papapoulos, S.E.; Lowik, C.W. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005, 16, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Dimopoulos, M.A.; Christoulas, D.; Migkou, M.; Iakovaki, M.; Gkotzamanidou, M.; Terpos, E. Dickkopf-1: A suitable target for the management of myeloma bone disease. Expert Opin. Ther. Targets 2009, 13, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kalder, M.; Hans, D.; Kyvernitakis, I.; Lamy, O.; Bauer, M.; Hadji, P. Effects of Exemestane and Tamoxifen treatment on bone texture analysis assessed by TBS in comparison with bone mineral density assessed by DXA in women with breast cancer. J. Clin. Densitom. 2014, 17, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, I.; Rachner, T.D.; Urbschat, A.; Hars, O.; Hofbauer, L.C.; Hadji, P. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.S.; Padhi, I.D.; Raisz, L.G.; Lorenzo, J.A. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Muschitz, C.; Kocijan, R.; Marterer, C.; Nia, A.R.; Muschitz, G.K.; Resch, H.; Pietschmann, P. Sclerostin levels and changes in bone metabolism after bariatric surgery. J. Clin. Endocrinol. Metab. 2015, 100, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Bratengeier, C.; Woloszczuk, W.; Papatheodorou, A.; Terpos, E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women--the six-month effect of risedronate and teriparatide. Osteoporos Int. 2012, 23, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Sornay-Rendu, E.; Munoz, F.; Borel, O.; Chapurlat, R.D. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: The OFELY study. Osteoporos Int. 2013, 24, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Kuhlmann, J.D.; Link, T.; Wimberger, P.; Browne, A.J.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Adjuvant tamoxifen but not aromatase inhibitor therapy decreases serum levels of the Wnt inhibitor dickkopf-1 while not affecting sclerostin in breast cancer patients. Breast Cancer Res. Treat. 2017, 164, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Voorzanger-Rousselot, N.; Goehrig, D.; Journe, F.; Doriath, V.; Body, J.J.; Clezardin, P.; Garnero, P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br. J. Cancer 2007, 97, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.S.; Murray, D.W.; Hurson, C.J.; O’Brien, J.; Doran, P.P.; O’Byrne, J.M. The role of Dkk1 in bone mass regulation: Correlating serum Dkk1 expression with bone mineral density. J. Orthop. Res. 2011, 29, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.; Viapiana, O.; Fracassi, E.; Idolazzi, L.; Dartizio, C.; Povino, M.R.; Adami, S.; Rossini, M. Sclerostin and DKK1 in postmenopausal osteoporosis treated with denosumab. J. Bone Miner. Res. 2012, 27, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Ustun, N.; Tok, F.; Kalyoncu, U.; Motor, S.; Yuksel, R.; Yagiz, A.E.; Guler, H.; Turhanoglu, A.D. Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reumatol. Port. 2014, 39, 146–151. [Google Scholar] [PubMed]
- Sankaralingam, A.; Roplekar, R.; Turner, C.; Dalton, R.N.; Hampson, G. Changes in dickkopf-1 (DKK1) and sclerostin following a loading dose of vitamin D (2) (300,000 IU). J. Osteoporos. 2014, 2014, 682763. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, O.; Peña, C.; García, J.M.; Larriba, M.J.; Ordóñez-Morán, P.; Navarro, D.; Barbáchano, A.; López de Silanes, I.; Ballestar, E.; Fraga, M.F.; et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha, 25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007, 28, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Rowe, P.S.; Lim, H.P.; Welldon, K.J.; Ormsby, R.; Wijenayaka, A.R.; Zelenchuk, L.; Evdokiou, A.; Findlay, D.M. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J. Bone Miner. Res. 2011, 26, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Rabelo Fe, S.; da Mota, L.M.; Lima, R.A.; Lima, F.A.; Barra, G.B.; de Carvalho, J.F.; Amato, A.A. The Wnt signaling pathway and rheumatoid arthritis. Autoimmun Rev. 2010, 9, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Modder, U.I.; Hoey, K.A.; Amin, S.; McCready, L.K.; Achenbach, S.J.; Riggs, B.L.; Melton, L.J.; Khosla, S. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J. Bone Miner. Res. 2011, 26, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Liakou, C.G.; Mastorakos, G.; Makris, K.; Fatouros, I.G.; Avloniti, A.; Marketos, H.; Antoniou, J.D.; Galanos, A.; Dontas, I.; Rizos, D.; et al. Changes of serum sclerostin and Dickkopf-1 levels during the menstrual cycle. A pilot study. Endocrine 2016, 54, 543–551. [Google Scholar] [CrossRef] [PubMed]
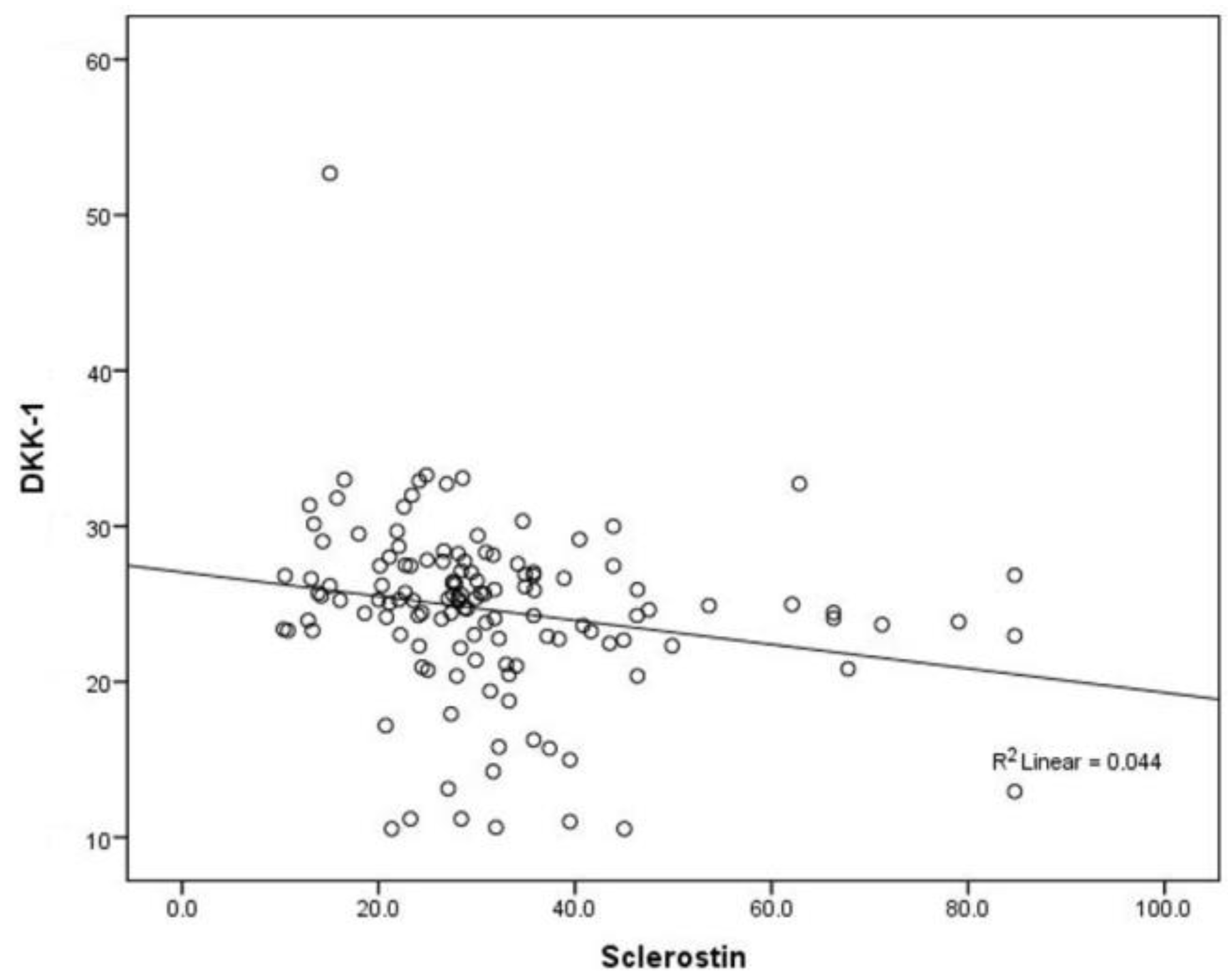
| Median (Interquartile Range) | p * | ||
| Group w/o AIs Therapy | AIs Treated Group | ||
| Age (year) | 62 (55–67) | 64 (56–70) | 0.06 |
| Hight (cm) | 163 (158–168) | 162 (158–165) | 0.33 |
| Height in youth (cm) | 165 (160–169) | 164 (160–167) | 0.69 |
| Weight (kg) | 72 (65.8–82.3) | 71 (63–80) | 0.48 |
| BMI (kg/m2) | 27 (24.5–30.9) | 27.1 (24–31.2) | 0.84 |
| The age of the menopause | 50 (45–52) | 49 (44–51) | 0.17 |
| The age of the first menstrual period | 13 (12–14) | 13 (12–14) | 0.74 |
| Number (%) Patients | p† | ||
| Group w/o AIs Therapy | AIs Treated Group | ||
| Previous hip fracture | 0 | 2 (2.4) | 0.52 |
| Previous fracture without trauma | 11 (20.8) | 11 (13.4) | 0.34 |
| Parent with hip fracture | 7 (12.7) | 6 (7.3) | 0.37 |
| Smoking | 7 (12.7) | 23 (28) | 0.04 |
| Alcohol consumption | 0 | 1 (1.2) | >0.99 |
| Vitamin D intake | 8 (14.5) | 31 (37.8) | 0.004 |
| Calcium intake | 12 (21.8) | 25 (30.5) | 0.43 |
| Regular cycles | 49 (92.5) | 73 (89) | 0.57 |
| Physical activity (work in the garden, at home) | 18 (32.7) | 9 (11) | 0.002 |
| Exercise | 45 (81.8) | 55 (67.1) | 0.03 |
| The frequency of exercise | |||
| Once a week | 7 (16) | 14 (26) | 0.16 |
| Two times a week | 8 (18) | 15 (28) | |
| Three times a week | 11 (24) | 6 (11) | |
| Four times a week | 19 (42) | 18 (34) | |
| Total | 45 (100) | 53 (100) | |
| Exercise earlier in the youth | 45 (85) | 47 (57) | 0.001 |
| Sclerostin (pmol/L) | Mean (Standard Deviation) | p * | † Pairwise Comparisons p-Value (95% CI) | |
|---|---|---|---|---|
| Group w/o AIs Therapy | AIs Treated Group | |||
| Calcium intake | ||||
| No | 26.8 (15.7) | 34.66 (13.4) | 0.03 | 0.10 (−12.2 to 1.11) |
| Yes | 23.6 (8.9) | 38.8 (16.1) | 0.02 | 0.03 (1.21 to 22.9) |
| Consumption of dairy products | ||||
| No | 24.7 (9.7) | 36.7 (15.4) | 0.03 | 0.03 (1.36 to 21.9) |
| Yes | 26.8 (16.3) | 35.4 (13.7) | 0.03 | 0.06 (−0.23 to 13.6) |
| The age of menopause prior 45 years of life | ||||
| No | 26.2 (14.6) | 34.9 (13.7) | 0.09 | 0.03 (0.68 to 14.30) |
| Yes | 26.0 (14.9) | 38.1 (15.3) | 0.21 | 0.10 (−2.17 to 21.97) |
| Presence of regular menstrual cycles | ||||
| No | 38.03 (18.9) | 31.3 (5.9) | 0.89 | 0.36 (-32.5 to 13.4) |
| Yes | 25.2 (13.8) | 36.5 (14.9) | 0.002 | 0.002 (3.57 to 15.69) |
| DKK1 (pmol/L) | Mean (Standard Deviation) | p * | † Pairwise Comparisons p-Value (95% CI) | |
|---|---|---|---|---|
| Group w/o AIs Therapy | AIs Treated Group | |||
| Calcium intake | ||||
| No | 27.5 (5.1) | 22.4 (5.4) | <0.001 | <0.001 (2.47 to 7.36) |
| Yes | 27.4 (3.6) | 22.9 (4.9) | 0.13 | 0.01 (1.13 to 8.58) |
| Consumption of dairy products | ||||
| No | 28.0 (7.2) | 23.5 (5.3) | 0.19 | 0.11 (−0.91 to 8.65) |
| Yes | 27.2 (3.3) | 22.0 (5.1) | <0.001 | <0.001 (3.13 to 7.25) |
| The age of menopause prior 45 years of life | ||||
| No | 27.4 (5.2) | 22.7 (4.8) | <0.001 | <0.001 (2.55 to 7.29) |
| Yes | 27.6 (3.0) | 22.1 (6.1) | <0.001 | 0.02 (0.64 to 7.35) |
| Presence of regular menstrual cycles | ||||
| No | 26.3 (3.8) | 24.9 (5.5) | 0.34 | 0.71 (−9.29 to 6.69) |
| Yes | 27.5 (4.9) | 22.2 (5.1) | <0.001 | <0.001 (3.30 to 7.52) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojanić, K.; Bilić Ćurčić, I.; Kuna, L.; Kizivat, T.; Smolic, R.; Raguž Lučić, N.; Kralik, K.; Šerić, V.; Ivanac, G.; Tucak-Zorić, S.; et al. Association of Wnt Inhibitors, Bone Mineral Density and Lifestyle Parameters in Women with Breast Cancer Treated with Anastrozole Therapy. J. Clin. Med. 2018, 7, 287. https://doi.org/10.3390/jcm7090287
Bojanić K, Bilić Ćurčić I, Kuna L, Kizivat T, Smolic R, Raguž Lučić N, Kralik K, Šerić V, Ivanac G, Tucak-Zorić S, et al. Association of Wnt Inhibitors, Bone Mineral Density and Lifestyle Parameters in Women with Breast Cancer Treated with Anastrozole Therapy. Journal of Clinical Medicine. 2018; 7(9):287. https://doi.org/10.3390/jcm7090287
Chicago/Turabian StyleBojanić, Kristina, Ines Bilić Ćurčić, Lucija Kuna, Tomislav Kizivat, Robert Smolic, Nikola Raguž Lučić, Kristina Kralik, Vatroslav Šerić, Gordana Ivanac, Sandra Tucak-Zorić, and et al. 2018. "Association of Wnt Inhibitors, Bone Mineral Density and Lifestyle Parameters in Women with Breast Cancer Treated with Anastrozole Therapy" Journal of Clinical Medicine 7, no. 9: 287. https://doi.org/10.3390/jcm7090287
APA StyleBojanić, K., Bilić Ćurčić, I., Kuna, L., Kizivat, T., Smolic, R., Raguž Lučić, N., Kralik, K., Šerić, V., Ivanac, G., Tucak-Zorić, S., Včev, A., & Smolić, M. (2018). Association of Wnt Inhibitors, Bone Mineral Density and Lifestyle Parameters in Women with Breast Cancer Treated with Anastrozole Therapy. Journal of Clinical Medicine, 7(9), 287. https://doi.org/10.3390/jcm7090287





