Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention
Abstract
:1. Introduction
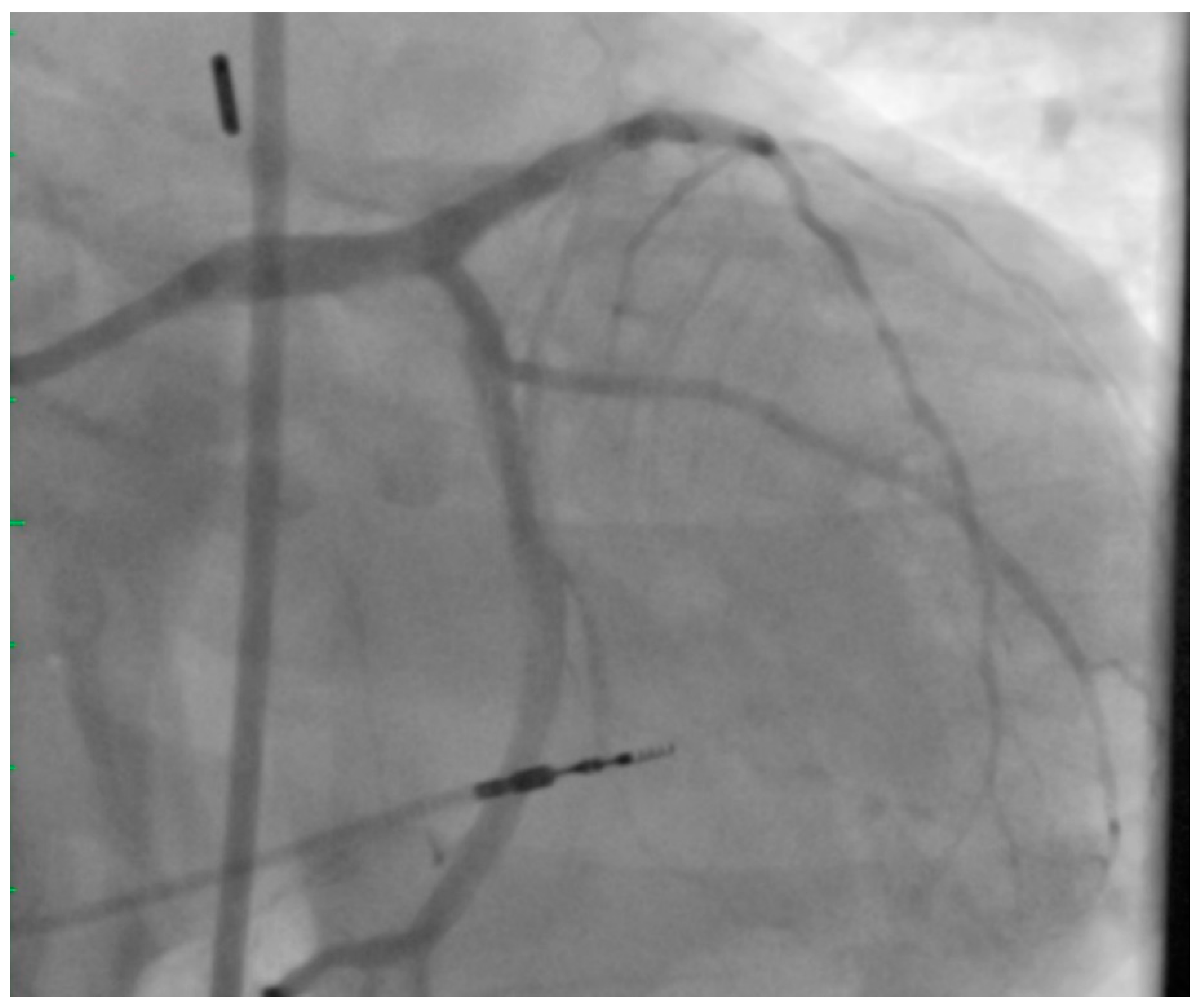
2. Left Main PCI
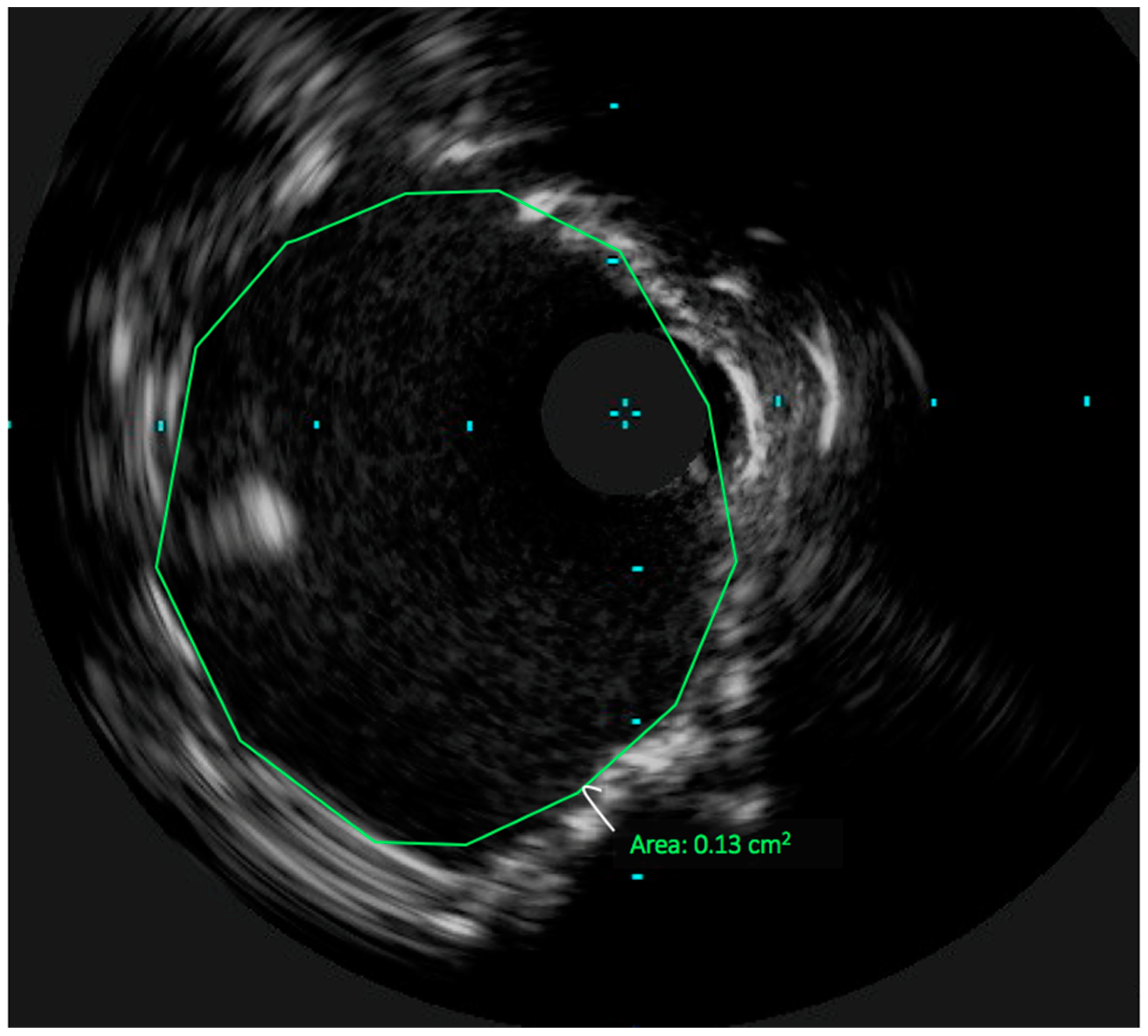
3. Technical Considerations: The Role of Intravascular Ultrasound in UPLM PCI
4. Achieving Adequate Left Main Minimal Luminal Area
5. Atherectomy in UPLM PCI
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ragosta, M.; Dee, S.; Sarembock, I.J.; Lipson, L.C.; Gimple, L.W.; Powers, E.R. Prevalence of unfavorable angiographic characteristics for percutaneous intervention in patients with unprotected left main coronary artery disease. Catheter. Cardiovasc. Interv. 2006, 68, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Grüntzig, A.R.; Senning, A.; Siegenthaler, W.E. Nonoperative DIlation of Coronary-Artery Stenosis. N. Engl. J. Med. 1979, 301, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Genereux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M., III; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Chinnakondepalli, K.; Magnuson, E.A.; Kandzari, D.E.; Puskas, J.D.; Ben-Yehuda, O.; van Es, G.A.; Taggart, D.P.; Morice, M.C.; Lembo, N.J.; et al. Quality of Life after Everolimus-Eluting Stents or Bypass Surgery for Treatment of Left Main Disease. J. Am. Coll. Cardiol. 2017, 70, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Rab, T.; Sheiban, I.; Louvard, Y.; Sawaya, F.J.; Zhang, J.J.; Chen, S.L. Current Interventions for the Left Main Bifurcation. JACC Cardiovasc. Interv. 2017, 10, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Sheiban, I.; Moretti, C.; D’Ascenzo, F.; Chieffo, A.; Taha, S.; Connor, S.O.; Chandran, S.; de la Torre Hernández, J.M.; Chen, S.; Varbella, F.; et al. Long-Term (>/=10 Years) Safety of Percutaneous Treatment of Unprotected Left Main Stenosis With Drug-Eluting Stents. Am. J. Cardiol. 2016, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, R.; Sotomi, Y.; Lee, C.W.; Ahn, J.M.; Farooq, V.; Tateishi, H.; Tenekecioglu, E.; Zeng, Y.; Suwannasom, P.; Collet, C.; et al. Outcomes after Percutaneous Coronary Intervention or Bypass Surgery in Patients With Unprotected Left Main Disease. J. Am. Coll. Cardiol. 2016, 68, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.M.; Roh, J.H.; Kim, Y.H.; Park, D.W.; Yun, S.C.; Lee, P.H.; Chang, M.; Park, H.W.; Lee, S.W.; Lee, C.W.; et al. Randomized Trial of Stents Versus Bypass Surgery for Left Main Coronary Artery Disease: 5-Year Outcomes of the PRECOMBAT Study. J. Am. Coll. Cardiol. 2015, 65, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ahn, J.M.; Kim, Y.H.; Park, D.W.; Yun, S.C.; Yoon, S.H.; Park, H.W.; Chang, M.; Lee, J.Y.; Kang, S.J.; et al. Temporal trends in revascularization strategy and outcomes in left main coronary artery stenosis: Data from the ASAN Medical Center-Left MAIN Revascularization registry. Circ. Cardiovasc. Interv. 2015, 8, e001846. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.; Valenti, R.; Parodi, G.; Vergara, R.; Buonamici, P.; Cerisano, G.; Carrabba, N.; Antoniucci, D. Angiographic and Clinical Outcomes After Everolimus-Eluting Stenting for Unprotected Left Main Disease and High Anatomic Coronary Complexity. JACC Cardiovasc. Interv. 2016, 9, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Tamburino, C.; Capranzano, P.; Capodanno, D.; Tagliareni, F.; Biondi-Zoccai, G.; Sanfilippo, A.; Caggegi, A.; Barrano, G.; Monaco, S.; Tomasello, S.D.; et al. Plaque distribution patterns in distal left main coronary artery to predict outcomes after stent implantation. JACC Cardiovasc. Interv. 2010, 3, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Girasis, C.; Lassen, J.F. Differences between the left main and other bifurcations. EuroIntervention 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Ahn, J.M.; Song, H.; Kim, W.J.; Lee, J.Y.; Park, D.W.; Yun, S.C.; Lee, S.W.; Kim, Y.H.; Lee, C.W.; et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ. Cardiovasc. Interv. 2011, 4, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Parikh, P.; Patel, A.; Chag, M.; Chandarana, A.; Parikh, R.; Parikh, K. Orbital atherectomy system in treating calcified coronary lesions: 3-Year follow-up in first human use study (ORBIT I. trial). Cardiovasc. Revasc. Med. 2014, 15, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Kovach, J.A.; Mintz, G.S.; Pichard, A.D.; Kent, K.M.; Popma, J.J.; Satler, L.F.; Leon, M.B. Sequential intravascular ultrasound of the mechanisms of rotational atherectomy and adjunct balloon angioplasty. J. Am. Coll. Cardiol. 1993, 22, 1024–1032. [Google Scholar] [CrossRef]
- Brogan, W.C., III; Popma, J.J.; Mintz, G.S.; Pichard, A.D.; Satler, L.F.; Kent, K.M.; Leon, M.B. Rotational coronary atherectomy after unsuccessful coronary balloon angioplasty. Am. J. Cardiol. 1993, 71, 794–798. [Google Scholar] [CrossRef]
- Rathore, S.; Matsuo, H.; Terashima, M.; Kinoshita, Y.; Kimura, M.; Tsuchikane, E.; Nasu, K.; Ehara, M.; Asakura, Y.; Katoh, O.; et al. Rotational atherectomy for fibro-calcific coronary artery disease in drug eluting stent era: Procedural outcomes and angiographic follow-up results. Catheter. Cardiovasc. Interv. 2010, 75, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Zhang, J.J.; Han, Y.; Kan, J.; Chen, L.; Qiu, C.; Jiang, T.; Tao, L.; Zeng, H.; Li, L.; et al. Double Kissing Crush Versus Provisional Stenting for Left Main Distal Bifurcation Lesions: DKCRUSH-V Randomized Trial. J. Am. Coll. Cardiol. 2017, 70, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.A.; Deumite, N.; Chaitman, B.R.; Davis, K.B.; Killip, T.; Rogers, W.J. Asymptomatic left main coronary artery disease in the Coronary Artery Surgery Study (CASS) registry. Circulation 1989, 79, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, A.R.; Cuculi, F.; Banning, A.P. Incidence, predictors and management of left main coronary artery stent restenosis: A comprehensive review in the era of drug-eluting stents. EuroIntervention 2013, 8, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [PubMed]
- Morice, M.C.; Serruys, P.W.; Kappetein, A.P.; Feldman, T.E.; Stahle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Choi, J.W.; Ruzyllo, W.; et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation 2014, 129, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Mäkikallio, T.; Holm, N.R.; Lindsay, M.; Spence, M.S.; Erglis, A.; Menown, I.B.; Trovik, T.; Eskola, M.; Romppanen, H.; Kellerth, T.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): A prospective, randomised, open-label, non-inferiority trial. Lancet 2016, 388, 2743–2752. [Google Scholar] [CrossRef]
- De Rosa, S.; Sabatino, J.; Indolfi, C. Long-term outcomes of coronary artery bypass grafting versus stent-PCI for unprotected left main disease: A meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 240. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R., Jr.; Morel, M.A.; Van Dyck, N.; et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013, 381, 629–638. [Google Scholar] [CrossRef]
- De la Torre Hernandez, J.M.; Baz Alonso, J.A.; Gomez Hospital, J.A.; Alfonso Manterola, F.; Garcia Camarero, T.; Gimeno de Carlos, F.; Roura Ferrer, G.; Recalde, A.S.; Martínez-Luengas, I.L.; Gomez Lara, J.; et al. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: Pooled analysis at the patient-level of 4 registries. JACC Cardiovasc. Interv. 2014, 7, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, Y.H.; Park, D.W.; Lee, S.W.; Kim, W.J.; Suh, J.; Yun, S.C.; Lee, C.W.; Hong, M.K.; Lee, J.H.; et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ. Cardiovasc. Interv. 2009, 2, 167–177. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Hernandez, J.M.; Hernandez Hernandez, F.; Alfonso, F.; Rumoroso, J.R.; Lopez-Palop, R.; Sadaba, M.; Carrillo, P.; Rondan, J.; Lozano, I.; Ruiz Nodar, J.M.; et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J. Am. Coll. Cardiol. 2011, 58, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc. Imaging 2015, 8, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, Q.; Liu, D.; Zhang, S.; Zhang, Y.; Li, Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med. J. 2015, 36, 549–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogacka, R.; Latib, A.; Colombo, A. IVUS-Guided Stent Implantation to Improve Outcome: A Promise Waiting to be Fulfilled. Curr. Cardiol. Rev. 2009, 5, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Oshima, A.; Hayase, M.; Metz, J.A.; Bailey, S.R.; Cleman, M.W.; Deutsch, E.; Diver, D.J.; Moses, J.W.; Oesterle, S.N.; et al. Final Results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) Study. Circulation 2000, 102, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Kim, B.K.; Shin, D.H.; Nam, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kang, T.S.; Kang, W.C.; Her, A.Y.; et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA 2015, 314, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Buszman, P.E.; Kiesz, S.R.; Bochenek, A.; Peszek-Przybyla, E.; Szkrobka, I.; Debinski, M.; Bialkowska, B.; Dudek, D.; Gruszka, A.; Zurakowski, A.; et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J. Am. Coll. Cardiol. 2008, 51, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Dangas, G.D.; Solinas, E.; Aoki, J.; Parise, H.; Kimura, M.; Franklin-Bond, T.; Dasgupta, N.K.; Kirtane, A.J.; Moussa, I.; et al. Effectiveness of drug-eluting stent implantation for patients with unprotected left main coronary artery stenosis. Am. J. Cardiol. 2008, 101, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Mintz, G.S. Clinical utility of intravascular imaging and physiology in coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.G.; Roubin, G.S.; King, S.B.; Douglas, J.S., Jr.; Weintraub, W.S.; Thomas, R.G.; Cox, W.R. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation 1988, 77, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, R.L.; Selzer, F.; Johnston, J.; Laskey, W.K.; Klugherz, B.D.; Block, P.; Cohen, H.; Detre, K.; Williams, D.O. Relation of percutaneous coronary intervention of complex lesions to clinical outcomes (from the NHLBI Dynamic Registry). Am. J. Cardiol. 2002, 90, 216–221. [Google Scholar] [CrossRef]
- Attizzani, G.F.; Patricio, L.; Bezerra, H.G. Optical coherence tomography assessment of calcified plaque modification after rotational atherectomy. Catheter. Cardiovasc. Interv. 2013, 81, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Behrens, A.N.; Martinsen, B.J. Atherectomy Devices for the Treatment of Calcified Coronary Lesions. Interv. Cardiol. Clin. 2016, 5, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Mintz, G.S.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; Popma, J.J.; Hong, M.K.; Laird, J.R.; Leon, M.B. Comparative early and nine-month results of rotational atherectomy, stents, and the combination of both for calcified lesions in large coronary arteries. Am. J. Cardiol. 1998, 81, 552–557. [Google Scholar] [CrossRef]
- Chiang, M.H.; Lee, W.L.; Tsao, C.R.; Chang, W.C.; Su, C.S.; Liu, T.J.; Liang, K.W.; Ting, C.T. The use and clinical outcomes of rotablation in challenging cases in the drug-eluting stent era. J. Chin. Med. Assoc. 2013, 76, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; Barbero, U.; D’Ascenzo, F.; Latib, A.; Pennacchi, M.; Rossi, M.L.; Ugo, F.; Meliga, E.; Kawamoto, H.; Moretti, C.; et al. Rotational atherectomy in very long lesions: Results for the ROTATE registry. Catheter. Cardiovasc. Interv. 2016, 88, E164–E172. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Meliga, E.; Latib, A.; Park, S.J.; Onuma, Y.; Capranzano, P.; Valgimigli, M.; Jegere, S.; Makkar, R.R.; Palacios, I.F.; et al. Drug-eluting stent for left main coronary artery disease. The DELTA registry: A multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc. Interv. 2012, 5, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lara, J.; Pinar, E.; Valdesuso, R.; Lacunza, J.; Gimeno, J.R.; Hurtado, J.A.; Valdés-Chávarri, M. Percutaneous coronary intervention with rotational atherectomy for severely calcified unprotected left main: Immediate and two-years follow-up results. Catheter. Cardiovasc. Interv. 2012, 80, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, H.; Takagi, K.; Tahara, S.; Fujino, Y.; Warisawa, T.; Kawamoto, H.; Watanabe, Y.; Mitomo, S.; Karube, K.; Matsumoto, T.; et al. Impact of Rotational Atherectomy on Heavily Calcified, Unprotected Left Main Disease. Circ. J. 2014, 78, 1867–1872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-J.; Hong, M.-K.; Lee, C.W.; Kim, J.-J.; Song, J.-K.; Kang, D.-H.; Park, S.-W.; Mintz, G.S. Elective stenting of unprotected left main coronary artery stenosis. J. Am. Coll. Cardiol. 2001, 38, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Ariyaratne, T.V.; Ademi, Z.; Yap, C.H.; Billah, B.; Rosenfeldt, F.; Yan, B.P.; Reid, C.M. Prolonged effectiveness of coronary artery bypass surgery versus drug-eluting stents in diabetics with multi-vessel disease: An updated systematic review and meta-analysis. Int. J. Cardiol. 2014, 176, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.; Brinton, M.; Ali, Z.; Mario, C.D.; Maehara, A.; Illindala, U.; Whitbourn, R.; Gotberg, M.; Van Miegham, N.; Hill, J.; et al. DISRUPT CAD: A Multicenter, Prospective, Single-Arm Study of Percutaneous Lithoplastyprior to Stent Implantation in Heavily Calcified Coronary Lesions. Available online: https://www.tctmd.com/slide/disrupt-cad-multicenterprospective-single-arm-study-percutaneouslithoplasty-prior-stent (accessed on 15 April 2018).
- Ali, Z.A.; Brinton, T.J.; Hill, J.M.; Maehara, A.; Matsumura, M.; Galougahi, K.; Illindala, U.; Götberg, M.; Whitbourn, R.; Van Mieghem, N.; et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions: First Description. JACC Cardiovasc. Imaging 2017, 10, 897–906. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, C.A.; Pfau, S.E. Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention. J. Clin. Med. 2018, 7, 180. https://doi.org/10.3390/jcm7070180
Shah CA, Pfau SE. Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2018; 7(7):180. https://doi.org/10.3390/jcm7070180
Chicago/Turabian StyleShah, Chirag A., and Steven E. Pfau. 2018. "Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention" Journal of Clinical Medicine 7, no. 7: 180. https://doi.org/10.3390/jcm7070180
APA StyleShah, C. A., & Pfau, S. E. (2018). Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention. Journal of Clinical Medicine, 7(7), 180. https://doi.org/10.3390/jcm7070180



