Effects of Low Energy Availability on Reproductive Functions and Their Underlying Neuroendocrine Mechanisms
Abstract
1. Introduction
2. Effects of Nutrition on Reproductive Functions
3. The Effects of a Negative Energy Balance on GnRH/LH Secretion
4. Hormonal and Neuropeptide Pathways That Connect Metabolic Status and GnRH Neurons
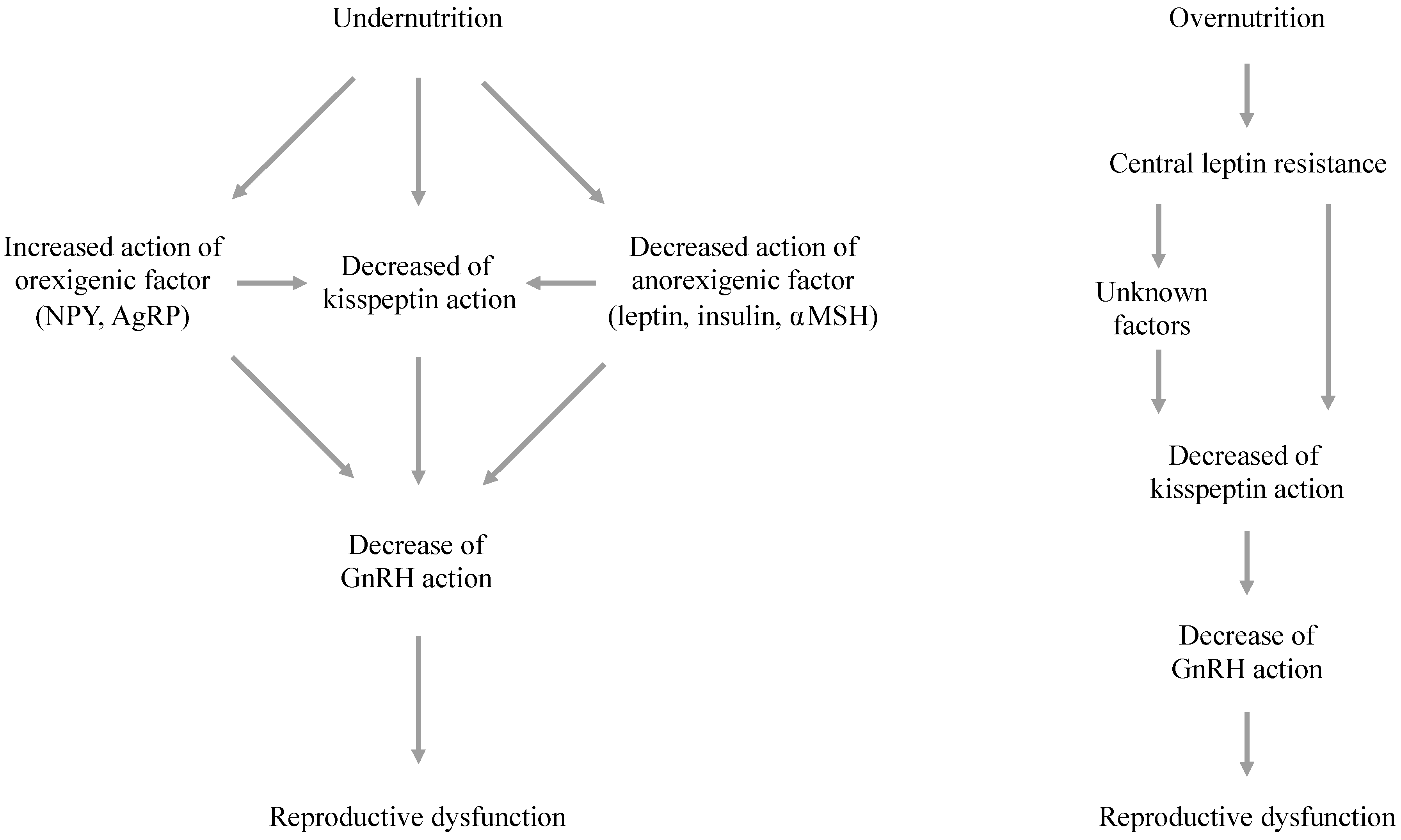
5. The Effects of Energy Availability on Hypothalamic Kisspeptin Signaling
6. Mechanisms Responsible for Metabolic Effects on the Kisspeptin System
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gordon, C.M. Clinical practice. Functional hypothalamic amenorrhea. N. Engl. J. Med. 2010, 363, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional hypothalamic amenorrhea: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Calvo, M.T.; Argente, J. Nutritional and pubertal disorders. Endocr. Dev. 2016, 29, 153–173. [Google Scholar] [PubMed]
- Schneider, J.E. Energy balance and reproduction. Physiol. Behav. 2004, 81, 289–317. [Google Scholar] [CrossRef] [PubMed]
- El Ghoch, M.; Gatti, D.; Calugi, S.; Viapiana, O.; Bazzani, P.V.; Dalle Grave, R. The association between weight gain/restoration and bone mineral density in adolescents with anorexia nervosa: A systematic review. Nutrients 2016, 8, 769. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Klibanski, A. Anorexia nervosa and bone metabolism. Bone 2014, 66, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, J.J.; Mitchell, J.E. Bone mineral density and anorexia nervosa in women. Am. J. Psychiatry 1991, 148, 768–774. [Google Scholar] [PubMed]
- Sachs, K.V.; Mlis, B.H.; Mehler, P.S.; Krantz, M.J. Cardiovascular complications of anorexia nervosa: A systematic review. Int. J. Eat. Disord. 2016, 49, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Kerr-Gaffney, J.; Harrison, A.; Tchanturia, K. Social anxiety in the eating disorders: A systematic review and meta-analysis. Psychol. Med. 2018, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nazar, B.P.; Bernardes, C.; Peachey, G.; Sergeant, J.; Mattos, P.; Treasure, J. The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Int. J. Eat. Disord. 2016, 49, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.J.; Balen, A.H. The adverse effects of obesity on conception and implantation. Reproduction 2010, 140, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Arrais, R.F.; Dib, S.A. The hypothalamus-pituitary-ovary axis and type 1 diabetes mellitus: A mini review. Hum. Reprod. 2006, 21, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Codner, E.; Merino, P.M.; Tena-Sempere, M. Female reproduction and type 1 diabetes: From mechanisms to clinical findings. Hum. Reprod. Update 2012, 18, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Reame, N.E.; Sauder, S.E.; Case, G.D.; Kelch, R.P.; Marshall, J.C. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: Evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J. Clin. Endocrinol. Metab. 1985, 61, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Heath, E.M. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J. Clin. Endocrinol. Metab. 1994, 78, 910–915. [Google Scholar] [PubMed]
- Loucks, A.B.; Verdun, M.; Heath, E.M. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J. Appl. Physiol. 1998, 84, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Aydin, S.; Celik, K.; Yilmaz, M. Peptides: Basic determinants of reproductive functions. Peptides 2015, 72, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Atika, B.; Ullah, F.; Shahab, M.; Behr, R. Metabolic impact on the hypothalamic kisspeptin-Kiss1r signaling pathway. Front. Endocrinol. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Shahab, M.; Behr, R. The involvement of gonadotropin inhibitory hormone and kisspeptin in the metabolic regulation of reproduction. J. Endocrinol. 2015, 225, R49–R66. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.P. The effects of exercise on pubertal progression and reproductive function in girls. J. Clin. Endocrinol. Metab. 1980, 51, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Winterer, J.; Cutler, G.B.; Loriaux, D.L. Caloric balance, brain to body ratio, and the timing of menarche. Med. Hypotheses 1984, 15, 87–91. [Google Scholar] [CrossRef]
- I’anson, H.; Manning, J.M.; Herbosa, C.G.; Pelt, J.; Friedman, C.R.; Wood, R.I.; Bucholtz, C.; Foster, D.L. Central inhibition of gonadotoropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep. Endocrinology 2000, 141, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Santoro, N.; Hall, J.; Filicori, M.; Wierman, M.; Crowley, W.F., Jr. Clinical review 15: Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J. Clin. Endocrinol. Metab. 1990, 71, 1081A–1081G. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Elzahr, D. Pulsatile gonadotropin-releasing hormone therapy for ovulatory disorders. Clin. Obstet. Gynecol. 1993, 36, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Stem, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar]
- Dietrich, M.O.; Horvath, T. Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 2013, 36, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Schwartz, M.W. Insulin and leptin revisited: Adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003, 24, 1–10. [Google Scholar] [CrossRef]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.A.; Cheung, D.S.; Weigle, D.S.; Hongping, R.; Kagigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.J.; Clifton, D.K.; Steiner, R.A. Leptin’s actions on the reproductive axis: Perspectives and mechanisms. Biol. Reprod. 1999, 60, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Quennell, J.H.; Mulligan, A.C.; Tups, A.; Liu, X.; Phipps, S.J.; Kemp, C.J.; Herbison, A.E.; Grattan, D.R.; Anderson, G.M. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009, 150, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Bruning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Muller-Wieland, D.; Kahn, C.R. Role of brain insulin receptor in control of body weight and reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- DiVall, S.A.; Williams, T.R.; Carver, S.E.; Koch, L.; Bruning, J.C.; Kahn, C.R.; Wondisford, F.; Radovick, S.; Wolfe, A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Investig. 2010, 120, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, C.; Borgquist, A.; Nestor, C.C.; Smith, A.W.; Bosch, M.A.; Ku, S.; Wagner, E.J.; Ronnekleiv, O.K.; Kelly, M.J. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014, 19, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Leranth, C.; MacLusky, N.J.; Shanabrough, M.; Naftolin, F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988, 449, 1–176. [Google Scholar] [CrossRef]
- Yeo, G.S.H.; Farooqi, I.S.; Aminian, S.; Halsall, D.J.; Stanhope, R.G.; O’Rahilly, S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998, 20, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.D.; Sheffer-Babila, S.; De Luca, C.; Jo, Y.H.; Liu, S.M.; Xia, Q.; Spergel, D.J.; Dun, S.L.; Dun, N.J.; Chua, S.C., Jr. Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 2012, 153, 2408–2419. [Google Scholar] [CrossRef] [PubMed]
- Celis, M.E. Release of LH in response to alpha-MSH administration. Acta Physiol. Pharmacol. Latinoam. 1985, 35, 281. [Google Scholar] [PubMed]
- Sandrock, M.; Schulz, A.; Merkwitz, C.; Schoneberg, T.; Spanel-Borowski, K.; Ricken, A. Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient mice. Reprod. Biol. Endocrinol. 2009, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, P.; Smith, M.S. Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology 1999, 140, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Catzeflis, C.; Pierroz, D.D.; Rohnerjeanrenaud, F.; Rivier, J.E.; Sizonenko, P.C.; Aubert, M.L. Neuropeptide-Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology 1993, 132, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, J.K.; Lumpkin, M.D.; Depaolo, L.V. Neuropeptide-Y suppresses pulsatile secretion of luteinizing-hormone in ovariectomized rats-possible site of action. Endocrinology 1989, 125, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.R.; Pu, S.; Kalra, P.S.; Kalra, S.P. Evidence that stimulation of two modalities of pituitary luteinizing hormone release in ovarian steroid-primed ovariectomized rats may involve neuropeptide Y Y1 and Y4 receptors. Endocrinology 1999, 140, 5171–5177. [Google Scholar] [CrossRef] [PubMed]
- Khorram, O.; Pau, K.Y.; Spies, H.G. Release of hypothalamic neuropeptide Y and effects of exogenous NPY on the release of hypothalamic GnRH and pituitary gonadotropins in intact and ovariectomized does in vitro. Peptides 1988, 9, 411–417. [Google Scholar] [CrossRef]
- Erickson, J.C.; Hollopeter, G.; Palmiter, R.D. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 1996, 274, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.W.; Basinski, M.; Bristow, P.K.; Bue-Valleskey, J.M.; Burgett, S.G.; Craft, L.; Hale, J.; Hoffmann, J.; Hsiung, H.M.; Kriauciunas, A.; et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 1995, 377, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, P.S.; Wilding, J.P. Hypothalamic neuropeptide Y and its neuroendocrine regulation by leptin. Front. Horm. Res. 2000, 26, 71–86. [Google Scholar] [PubMed]
- Brady, L.S.; Smith, M.A.; Gold, P.W.; Herkenham, M. Altered expression of hypothalamic neuropeptides mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 1990, 52, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.W.; Levine, J.E. Abnormal response of the neuropeptide Y-deficient mouse reproductive axis to food deprivation but not lactation. Endocrinology 2003, 144, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Vulliemoz, N.R.; Xiao, E.; Xia-Zhang, L.; Wardlaw, S.L.; Ferin, M. Central infusion of agouti-related peptides suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology 2005, 146, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Whiddon, B.B.; Palmiter, R.D. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc. Natl. Acad. Sci. USA 2012, 109, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Rosemary, B.S.; Thresher, R.R.; Acieno, J.S., Jr.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, Y.; Schwinof, K.W.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- De Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypothalamic hypogonadism due to loss of function of the KiSS-1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A role for kisspeptin in the regulation of gonadotrophin secretion in the mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin directly stimulates gonadotrophin-releasing hormone release via G protein-coupled receptor54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.M.; Castellano, J.M.; Fernandez-Fernandez, R.; Tovar, S.; Roa, J.; Mayen, A.; Nogueiras, R.; Vazquez, M.J.; Barreiro, M.L.; Magni, P.; et al. Characterization of the potent luteinizing hormone releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 2005, 146, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Mastronardi, C.; Seminara, S.B.; Crowley, W.F.; Ojeda, S.R.; Plant, T.M. Increased hypothalmaic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.I.; Adachi, S.; Inoue, K.; Ohkura, S.; Tsukamura, H. Metastin/kisspeptin and control of estrous cycle in rats. Rev. Endocr. Metab. Disord. 2007, 8, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, S.; Uenoyama, Y.; Yamada, S.; Homma, T.; Takase, K.; Inoue, K.; Maeda, K.I.; Tsukamura, H. Physiological role of metastin/kisspeptin in regulating gonadotrophin-releasing hormone (GnRH) secretion in female rats. Peptides 2009, 30, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Clifton, D.K.; Steiner, R.A. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 2006, 131, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.L.; Lehman, M.N. Kisspeptin neurons from mice to men: Similarities and differences. Endocrinology 2012, 153, 5105–5118. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Popa, S.M.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A. Kiss1 neurons in the forebrain as central processor for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006, 26, 6687–6694. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Tsukamura, H.; Adachi, S.; Matsui, H.; Uenoyama, Y.; Iwata, K.; Yamada, S.; Inoue, K.; Ohtaki, T.; Matsumoto, H.; et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cycle in female rats. Endocrinology 2005, 146, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Bentsen, A.H.; Mikkelsen, J.D.; Tena-Sempere, M. Kisspeptins: Bridging energy homeostasis and reproduction. Brain. Res. 2010, 1364, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Tena-Sempere, M. Metabolic control of female puberty: Potential therapeutic targets. Expert Opin. Ther. Targets 2016, 20, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Iwasa, T.; Kinouchi, R.; Yoshida, S.; Murakami, M.; Gereltsetseg, G.; Yamamoto, S.; Kuwahara, A.; Yasui, T.; Irahara, M. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr. J. 2011, 58, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Murakami, M.; Kinouchi, R.; Gereltsetseg, G.; Fujisawa, S.; Kuwahara, A.; Yasui, T.; Irahara, M. Sensitivities of mRNA expression levels of Kiss1 and its receptor, Kiss1r, to nutritional status are changed during the developmental period in female rats. J. Endocrinol. 2010, 207, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Navarro, V.M.; Fernandez-Fernandez, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.J.; Vigo, E.; Casanueva, F.F.; Aguilar, E.; et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Kalamatianos, T.; Grimshaw, S.E.; Poorun, R.; Hahn, J.D.; Coen, C.W. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): Effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J. Neuroendocrinol. 2008, 20, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.; Vigo, E.; Castellano, J.M.; Navarro, V.M.; Fernandez-Fernandez, R.; Casanueva, F.F.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology 2006, 147, 2864–2878. [Google Scholar] [CrossRef] [PubMed]
- Backholer, K.; Smith, J.T.; Rao, A.; Pereira, A.; Iqbal, J.; Ogawa, S.; Li, Q.; Clarke, I.J. Communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010, 151, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Polkowska, J.; Cieslak, M.; Wankowska, M.; Wojcik-Gladysz, A. The effect of short fasting on the hypothalamic neuronal system of kisspeptin in peripubertal female lambs. Anim. Reprod. Sci. 2015, 159, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Navarro, V.M.; Fernandez-Fernandez, R.; Roa, J.; Vigo, E.; Pineda, R.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes 2006, 55, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Quennell, J.H.; Howell, C.S.; Roa, J.; Augustine, R.A.; Grattan, D.R.; Anderson, G.M. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology 2011, 152, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic control of puberty: Roles of leptin and kisspeptins. Horm. Behav. 2013, 64, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Ullah, F.; Chan, Y.M.; Seminara, S.B.; Shahab, M. Decrease in hypothalamic Kiss1 and Kiss1r expression: A potential mechanism for fasting-induced suppression of the HPG axis in the adult male rhesus monkey (Macaca mulatta). Horm. Behav. 2011, 43, 81–85. [Google Scholar]
- Luque, R.M.; Kineman, R.D.; Tena-Sempere, M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology 2007, 148, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Cravo, R.M.; Frazao, R.; Laurent, G.; Scott, M.M.; Lachey, J.; Castro, I.A.; Margatho, L.O.; Lee, S.; Richardson, J.A.; et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. InvestIG. 2011, 121, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Chen, H.; Weingarth, D.; Trumbauer, M.E.; Novi, D.E.; Guan, X.; Yu, H.; Shen, Z.; Feng, Y.; Frazier, E.; et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 2002, 22, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Alex Thomas, M.; Xue, B. Mechanisms for AgRP neuron-mediated regulation of appetite between behaviors in rodents. Physiol. Behav. 2018, 190, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Warne, J.P.; Wu, A.W. Metabolic transceivers: In tune with the central melanocortin system. Trends. Endocrinol. Metab. 2013, 24, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.L.; Qiu, J.; Nestor, C.C.; Zhang, C.; Smith, A.W.; Whiddon, B.B.; Ronnekleiv, O.K.; Kelly, M.J.; Palmiter, R.D. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl. Acad. Sci. USA 2017, 114, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.H.; Colledge, W.H. The role of Kiss1 neurons as integrators of endocrine, metabolic, and environmental factors in the hypothalamic-pituitary-gonadal axis. Front. Endocrinol. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasa, T.; Matsuzaki, T.; Yano, K.; Mayila, Y.; Yanagihara, R.; Yamamoto, Y.; Kuwahara, A.; Irahara, M. Effects of Low Energy Availability on Reproductive Functions and Their Underlying Neuroendocrine Mechanisms. J. Clin. Med. 2018, 7, 166. https://doi.org/10.3390/jcm7070166
Iwasa T, Matsuzaki T, Yano K, Mayila Y, Yanagihara R, Yamamoto Y, Kuwahara A, Irahara M. Effects of Low Energy Availability on Reproductive Functions and Their Underlying Neuroendocrine Mechanisms. Journal of Clinical Medicine. 2018; 7(7):166. https://doi.org/10.3390/jcm7070166
Chicago/Turabian StyleIwasa, Takeshi, Toshiya Matsuzaki, Kiyohito Yano, Yiliyasi Mayila, Rie Yanagihara, Yuri Yamamoto, Akira Kuwahara, and Minoru Irahara. 2018. "Effects of Low Energy Availability on Reproductive Functions and Their Underlying Neuroendocrine Mechanisms" Journal of Clinical Medicine 7, no. 7: 166. https://doi.org/10.3390/jcm7070166
APA StyleIwasa, T., Matsuzaki, T., Yano, K., Mayila, Y., Yanagihara, R., Yamamoto, Y., Kuwahara, A., & Irahara, M. (2018). Effects of Low Energy Availability on Reproductive Functions and Their Underlying Neuroendocrine Mechanisms. Journal of Clinical Medicine, 7(7), 166. https://doi.org/10.3390/jcm7070166



