Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
- Self-reported being pregnant [43]
- Had exercised within 5 h of laboratory testing [15]
- Consumed any caffeine within 3 h of laboratory testing [44]
- Self-reported a concussion or head trauma within the past 30 days [45]
- Self-reported taking marijuana or other illegal drugs within the past 30 days [46]
- Were considered a daily alcohol user (>30 drinks/month for women; >60 drinks/month for men) [47]
2.3. Exercise Protocol
2.4. Control Protocol
2.5. Memory Assessments and Procedures
2.6. Additional Assessments
2.7. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The effects of exercise on memory function among young to middle-aged adults: Systematic review and recommendations for future research. Am. J. Health Promot. 2018, 32, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Pearson, J. The sensory components of high-capacity iconic memory and visual working memory. Front. Psychol. 2012, 3, 355. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Irwin, D.E. Voluntary eyeblinks disrupt iconic memory. Percept. Psychophys. 2006, 68, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Ogmen, H.; Herzog, M.H. A new conceptualization of human visual sensory-memory. Front. Psychol. 2016, 7, 830. [Google Scholar] [CrossRef] [PubMed]
- Arning, L.; Stock, A.K.; Kloster, E.; Epplen, J.T.; Beste, C. NPY2-receptor variation modulates iconic memory processes. Eur. Neuropsychopharmacol. 2014, 24, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Gegenfurtner, K.R.; Sperling, G. Information transfer in iconic memory experiments. J. Exp. Psychol. Hum. Percept. Perform. 1993, 19, 845–866. [Google Scholar] [CrossRef] [PubMed]
- Sperling, G. Why we need iconic memory. Behav. Brain Sci. 1983, 6, 37–39. [Google Scholar] [CrossRef]
- Sperling, G. The information available in brief visual presentations. Psychol. Monogr. 1960, 74, 1–29. [Google Scholar] [CrossRef]
- Aru, J.; Bachmann, T. Expectation creates something out of nothing: The role of attention in iconic memory reconsidered. Conscious Cogn. 2017, 53, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.; Erol, M.; Clarke, J. When expectation confounds iconic memory: A reply to Bachmann and Aru (2016). Conscious Cogn. 2017, 49, 363–364. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, T.; Aru, J. When expectation confounds iconic memory. Conscious Cogn. 2016, 45, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Persuh, M.; Genzer, B.; Melara, R.D. Iconic memory requires attention. Front. Hum. Neurosci. 2012, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.; Erol, M.; Clarke, J.; Bert, J. No iconic memory without attention. Conscious Cogn. 2016, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Labban, J.D.; Etnier, J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Kane, C.J. Exercise and cognitive function: A randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin. Proc. 2015, 90, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Crush, E.A.; Loprinzi, P.D. Dose-response effects of exercise duration and recovery on cognitive functioning. Percept. Mot. Skills 2017, 124, 1164–1193. [Google Scholar] [CrossRef] [PubMed]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-dependent effects of cardiovascular exercise on memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Turner, A.; Hale, B.J.; Sproule, J. Beyond the catecholamines hypothesis for an acute exercise-cognition interaction: A neurochemical perspective. In Exercise-Cognition Interaction: Neuroscience Perspectives; McMorris, T., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 65–103. [Google Scholar]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Tessaro, V.H.; Teixeira, L.A.; Murakava, K.; Roschel, H.; Gualano, B.; Takito, M.Y. Influence of acute high-intensity aerobic interval exercise bout on selective attention and short-term memory tasks. Percept. Mot. Skills 2014, 118, 63–72. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Davranche, K.; Jones, G.; Hall, B.; Corbett, J.; Minter, C. Acute incremental exercise, performance of a central executive task, and sympathoadrenal system and hypothalamic-pituitary-adrenal axis activity. Int. J. Psychophysiol. 2009, 73, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Geva, R.; Zivan, M.; Warsha, A.; Olchik, D. Alerting, orienting or executive attention networks: Differential patters of pupil dilations. Front. Behav. Neurosci. 2013, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A. Attentional state: From automatic detection to willful focused concentration. In Attention and Meaning. The Attentional Basis of Meaning; Marchetti, G., Benedetti, G., Alharbi, A., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 133–150. [Google Scholar]
- Kinomura, S.; Larsson, J.; Gulyas, B.; Roland, P.E. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996, 271, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Gehring, W.J.; Kozak, R. More attention must be paid: The neurobiology of attentional effort. Brain Res. Rev. 2006, 51, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, G.A.; Kaufman, M.P. Caudal ventrolateral medullary cells responsive to muscular contraction. J. Appl. Physiol. 1987, 62, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Audiffren, M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 2011, 35, 1305–1325. [Google Scholar] [CrossRef] [PubMed]
- Rajab, A.S.; Crane, D.E.; Middleton, L.E.; Robertson, A.D.; Hampson, M.; MacIntosh, B.J. A single session of exercise increases connectivity in sensorimotor-related brain networks: A resting-state fMRI study in young healthy adults. Front. Hum. Neurosci. 2014, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Myers, K.G.; Guo, Y.; Ocampo, M.A.; Pang, R.D.; Jakowec, M.W.; Holschneider, D.P. Functional reorganization of motor and limbic circuits after exercise training in a rat model of bilateral parkinsonism. PLoS ONE 2013, 8, e80058. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Kerszberg, M.; Changeux, J.P. A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. USA 1998, 95, 14529–14534. [Google Scholar] [CrossRef] [PubMed]
- Daffner, K.R.; Scinto, L.F.; Weitzman, A.M.; Faust, R.; Rentz, D.M.; Budson, A.E.; Holcomb, P.J. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J. Cogn. Neurosci. 2003, 15, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Enders, H.; Cortese, F.; Maurer, C.; Baltich, J.; Protzner, A.B.; Nigg, B.M. Changes in cortical activity measured with EEG during a high-intensity cycling exercise. J. Neurophysiol. 2016, 115, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, T.; Komatsu, K.; Sakatani, K. Acute effects of physical exercise on prefrontal cortex activity in older adults: A functional near-infrared spectroscopy study. Adv. Exp. Med. Biol. 2013, 765, 293–298. [Google Scholar] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal effects of acute walking exercise on learning and memory function. Am. J. Health Promot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jubelt, L.E.; Barr, R.S.; Goff, D.C.; Logvinenko, T.; Weiss, A.P.; Evins, A.E. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology 2008, 199, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Klaming, R.; Annese, J.; Veltman, D.J.; Comijs, H.C. Episodic memory function is affected by lifestyle factors: A 14-year follow-up study in an elderly population. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2017, 24, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.D.; Rendell, P.G. A review of the impact of pregnancy on memory function. J. Clin. Exp. Neuropsychol. 2007, 29, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M.; Buckley, T.P.; Baena, E.; Ryan, L. Caffeine enhances memory performance in young adults during their non-optimal time of day. Front. Psychol. 2016, 7, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wammes, J.D.; Good, T.J.; Fernandes, M.A. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017, 111, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C.; Freeman, T.P.; Xia, J.X.; Shaban, N.D.C.; Curran, H.V. Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: A placebo-controlled trial. Psychol. Med. 2017, 47, 2708–2719. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, A.P.; Fama, R.; Sullivan, E.V. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcohol. Clin. Exp. Res. 2017, 41, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Prichard, E.C.; Christman, S.D. Inconsistent-handed advantage in episodic memory extends to paragraph-level materials. Memory 2017, 25, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Ball, T.J.; Joy, E.A.; Gren, L.H.; Shaw, J.M. Concurrent validity of a self-reported physical activity “vital sign” questionnaire with adult primary care patients. Prev. Chronic Dis. 2016, 13, E16. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Ponce, P. Memorcise and Alzheimer’s disease. Phys. Sportsmed. 2018, 46, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Sng, E.; Frith, E. ‘Memorcise’: Implications for patient compliance and medication adherence. Phys. Sportsmed. 2018, 46, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E. Memorcise in the context of Parkinson’s disease. J. Cogn. Enhanc. 2018. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Herod, S.M.; Cardinal, B.J.; Noakes, T.D. Physical activity and the brain: A review of this dynamic, bi-directional relationship. Brain Res. 2013, 1539, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.; Loprinzi, P.D. A bi-directional model of exercise and episodic memory function. Med. Hypotheses 2018, 117, 3–6. [Google Scholar] [CrossRef]
- Lash, T.L. The harm done to reproducibility by the culture of null hypothesis significance testing. Am. J. Epidemiol. 2017, 186, 627–635. [Google Scholar] [CrossRef] [PubMed]
- McShane, B.B.; Gal, D.; Gelman, A.; Robert, C.; Tackett, J.L. Abandon Statistical Significance. Available online: https://arxiv.org/abs/1709.07588 (accessed on 24 May 2018).
- Hutchinson, J.C.; Tenenbaum, G. Attention focus during physical effort: The mediating role of task intensity. Psychol. Sport Exerc. 2007, 8, 233–245. [Google Scholar] [CrossRef]
| Variable | Exercise (n = 20) | Control (n = 20) |
|---|---|---|
| Age, mean years | 21.0 (1.0) | 20.8 (0.9) |
| % Female | 75.0 | 55.0 |
| % white | 85.0 | 65.0 |
| Waist circumference, mean cm | 87.6 (13.6) | 84.2 (9.6) |
| MVPA, mean min/week | 198.0 (155.2) | 224.1 (193.4) |
| Heart Rate, mean | ||
| Resting | 73.8 (12.5) | 68.2 (12.3) |
| Midpoint | 124.9 (22.7) | - |
| Endpoint | 126.8 (23.6) | - |
| Post | 84.2 (15.9) | 68.9 (11.8) |
| Speed, mean mph | 3.6 (0.1) | - |
| Variable | Exercise (n = 20) | Control (n = 20) | ||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 1 | Trial 2 | |
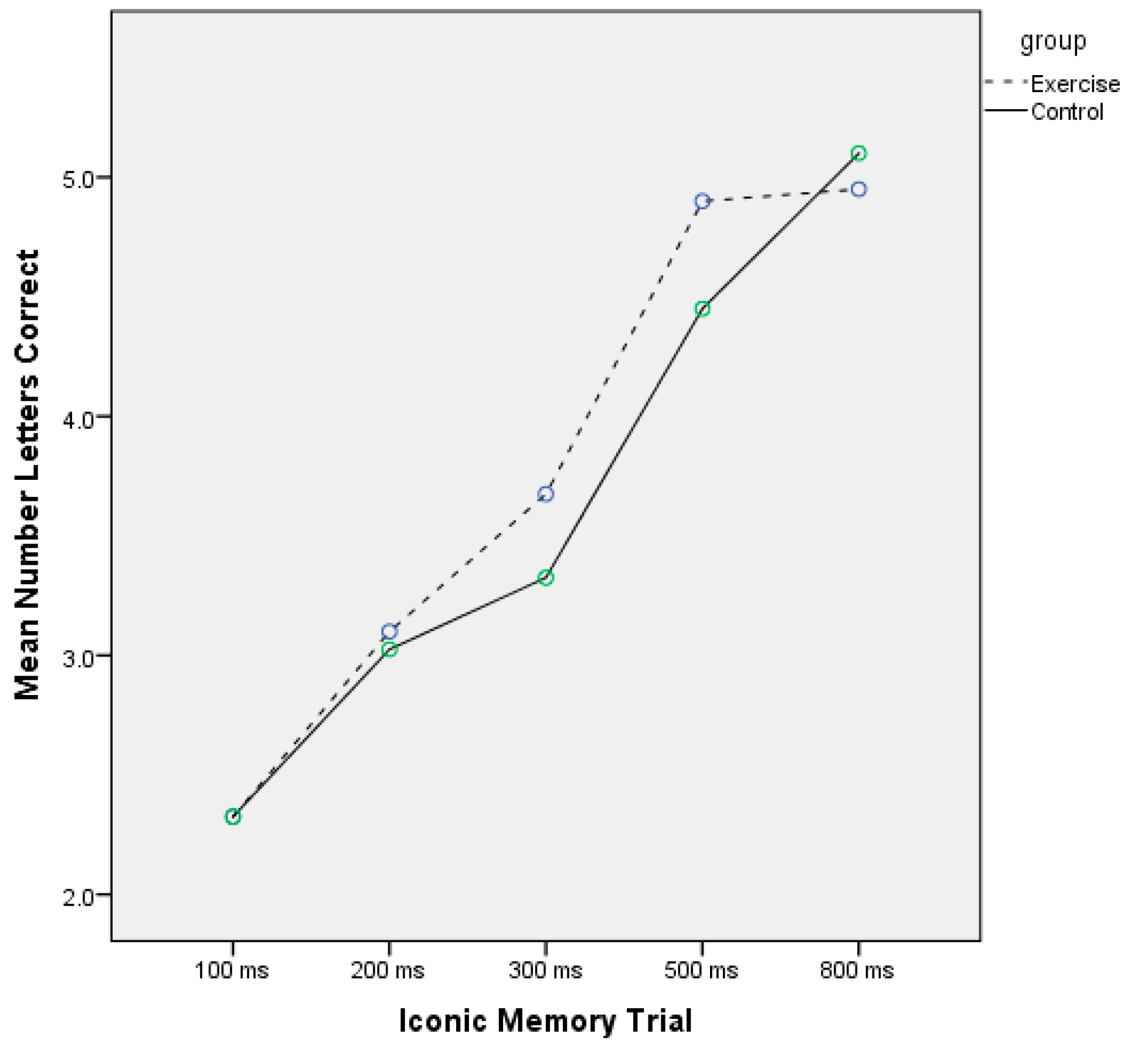
| Iconic memory, mean | ||||
| 100 ms | ||||
| Number correct | 1.95 (1.3) | 2.70 (0.9) | 2.10 (1.0) | 2.55 (0.8) |
| Number of mislocation errors | 0.45 (0.6) | 0.45 (0.6) | 0.25 (0.5) | 0.60 (1.0) |
| Number of intrusion errors | 0.65 (1.3) | 0.35 (0.8) | 0.20 (0.5) | 0.10 (0.3) |
| 200 ms | ||||
| Number correct | 3.05 (1.0) | 3.15 (1.0) | 3.10 (0.7) | 2.95 (0.8) |
| Number of mislocation errors | 0.85 (0.9) | 0.45 (0.7) | 0.45 (0.6) | 0.45 (0.7) |
| Number of intrusion errors | 0.25 (0.7) | 0.35 (0.7) | 0.10 (0.3) | 0.25 (0.6) |
| 300 ms | ||||
| Number correct | 3.80 (1.1) | 3.55 (1.0) | 3.25 (0.9) | 3.40 (0.9) |
| Number of mislocation errors | 0.40 (0.9) | 0.70 (0.9) | 0.50 (0.8) | 0.60 (1.0) |
| Number of intrusion errors | 0.35 (0.9) | 0.40 (0.8) | 0.30 (0.5) | 0.25 (0.44) |
| 500 ms | ||||
| Number correct | 4.90 (1.4) | 4.90 (1.2) | 4.40 (1.2) | 4.50 (1.4) |
| Number of mislocation errors | 0.40 (0.8) | 0.85 (1.1) | 0.30 (0.7) | 0.20 (0.5) |
| Number of intrusion errors | 0.45 (0.9) | 0.25 (0.5) | 0.30 (0.7) | 0.10 (0.3) |
| 800 ms | ||||
| Number correct | 4.10 (1.3) | 5.80 (1.3) | 4.35 (1.4) | 5.85 (1.0) |
| Number of mislocation errors | 1.05 (1.6) | 0.55 (1.0) | 0.65 (1.0) | 0.10 (0.4) |
| Number of intrusion errors | 0.30 (0.5) | 0.50 (0.8) | 0.40 (0.8) | 0.30 (0.8) |
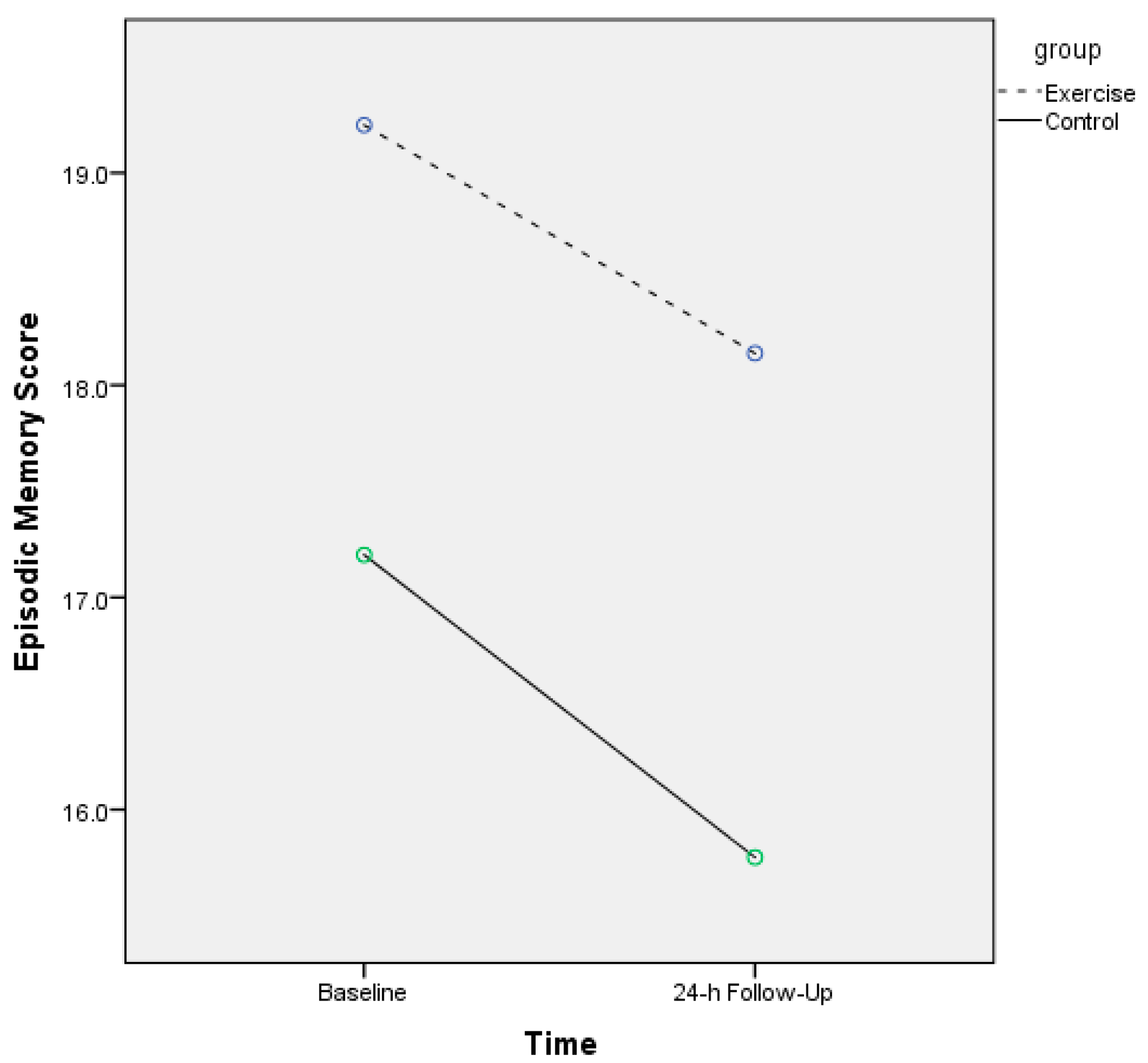
| Episodic memory, mean | ||||
| Short-term | 19.22 (6.7) | 17.20 (7.9) | ||
| Long-term | 18.15 (6.2) | 15.77 (7.3) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanes, D.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory. J. Clin. Med. 2018, 7, 146. https://doi.org/10.3390/jcm7060146
Yanes D, Loprinzi PD. Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory. Journal of Clinical Medicine. 2018; 7(6):146. https://doi.org/10.3390/jcm7060146
Chicago/Turabian StyleYanes, Danielle, and Paul D. Loprinzi. 2018. "Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory" Journal of Clinical Medicine 7, no. 6: 146. https://doi.org/10.3390/jcm7060146
APA StyleYanes, D., & Loprinzi, P. D. (2018). Experimental Effects of Acute Exercise on Iconic Memory, Short-Term Episodic, and Long-Term Episodic Memory. Journal of Clinical Medicine, 7(6), 146. https://doi.org/10.3390/jcm7060146





