Biobanking Organoids or Ground-State Stem Cells?
Abstract
1. Introduction
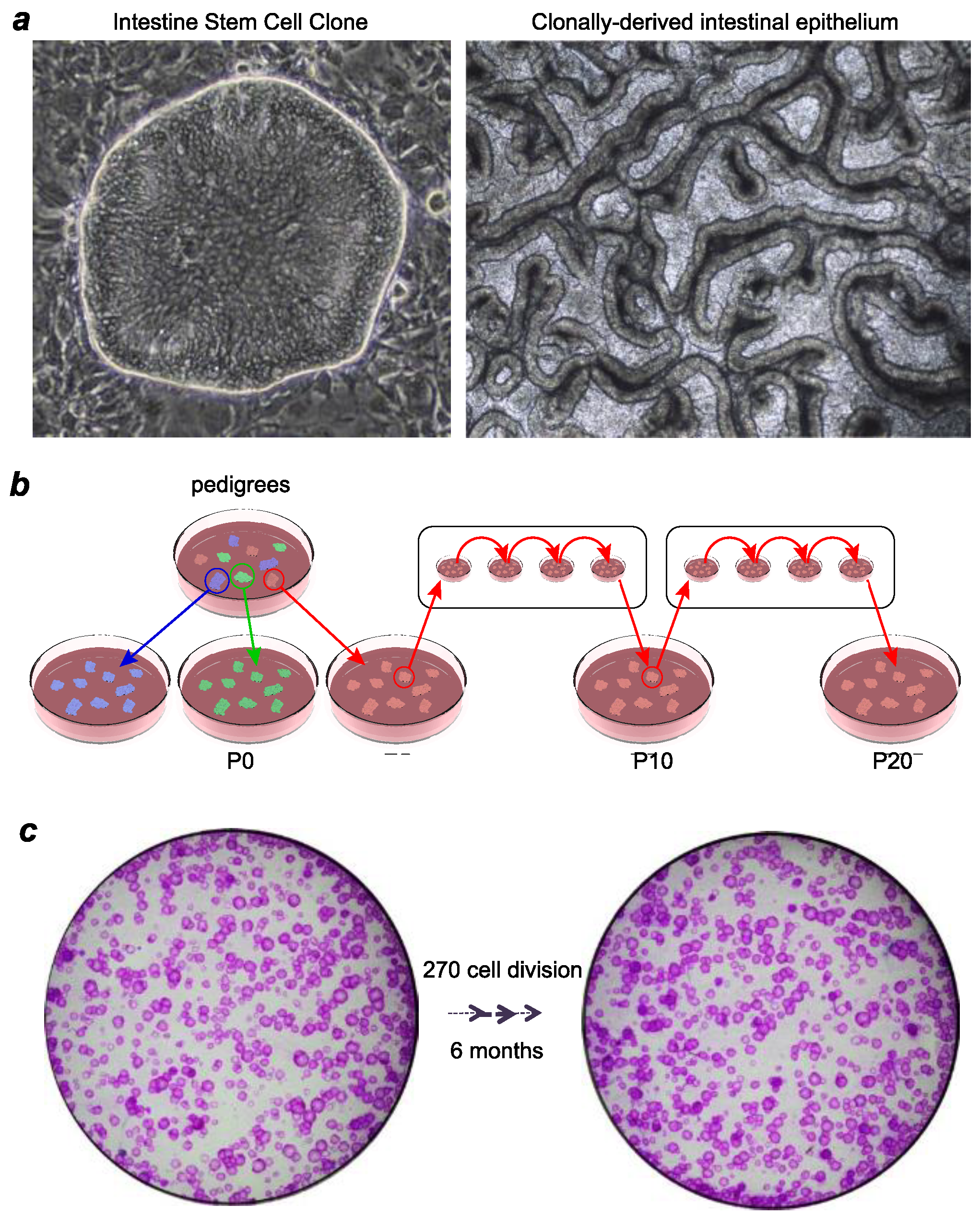
2. Technologies for Adult Epithelial Stem Cell Culturing
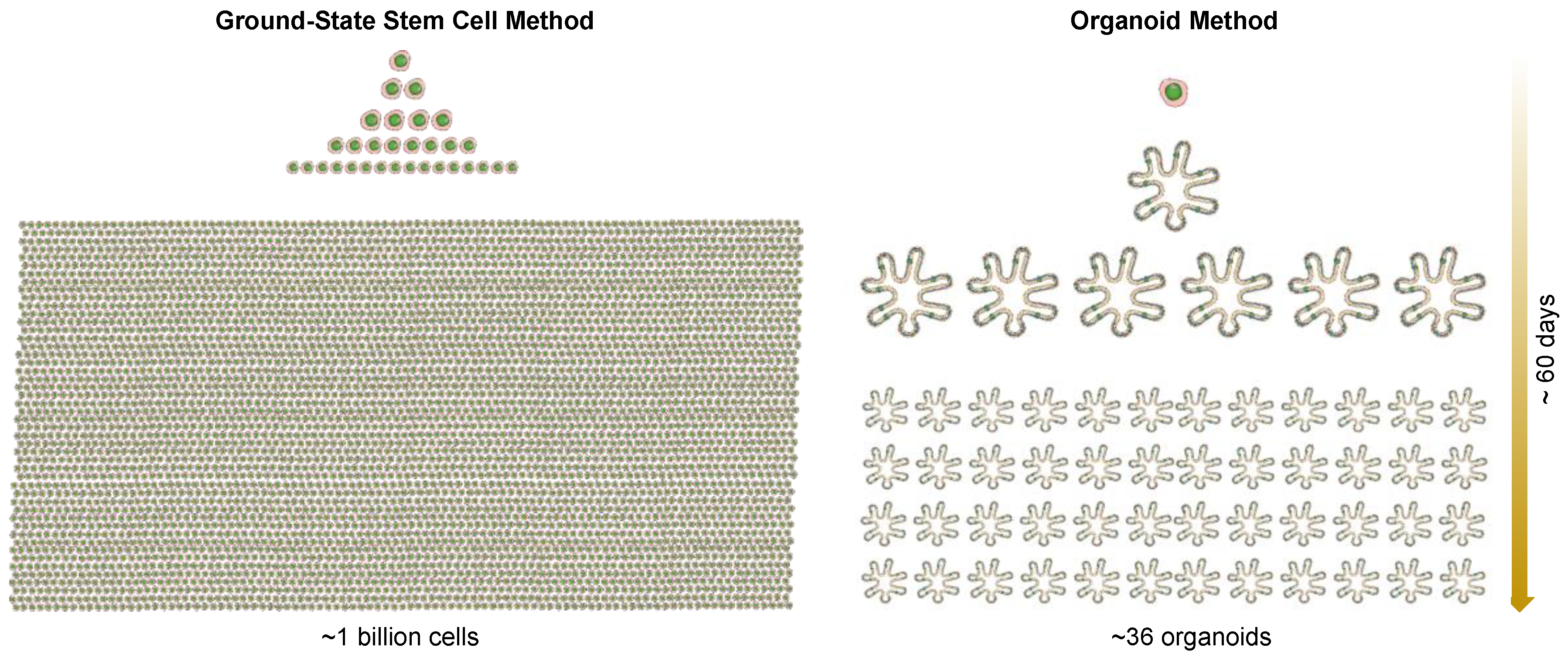
3. Ground-State Stem Cells versus Organoids
4. Biobanking of Ground-State Stem Cells and Personalized Regenerative Medicine
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, A.M.; Dzierzak, E.A. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development 1993, 118, 1343–1351. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8269860 (accessed on 11 November 2018).
- Helgason, C.D.; Sauvageau, G.; Lawrence, H.J.; Largman, C.; Humphries, R.K. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood 1996, 87, 2740–2749. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8639890 (accessed on 11 November 2018).
- Bonde, S.; Dowden, A.M.; Chan, K.-M.; Tabayoyong, W.B.; Zavazava, N. HOXB4 But Not BMP4 Confers Self-Renewal Properties to ES-Derived Hematopoietic Progenitor Cells. Transplantation 2008, 86, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Dabelsteen, S.; Easley, K.; Rheinwald, J.G.; Green, H. Immortalized keratinocyte lines derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Amabile, G.; Welner, R.S.; Nombela-Arrieta, C.; D’Alise, A.M.; di Ruscio, A.; Ebralidze, A.K.; Kraytsberg, Y.; Ye, M.; Kocher, O.; Neuberg, D.S.; et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood 2013, 121, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yamazaki, S.; Yamaguchi, T.; Okabe, M.; Masaki, H.; Takaki, S.; Otsu, M.; Nakauchi, H. Generation of engraftable hematopoietic stem cells from induced pluripotent stem cells by way of teratoma formation., Molecular Therapy. J. Am. Soc. Gene Ther. 2013, 21, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975, 6, 331–343. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1052771 (accessed on 11 November 2018). [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; de Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. p63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell 2007, 129, 523–536. [Google Scholar] [CrossRef]
- Kumar, P.A.; Hu, Y.; Yamamoto, Y.; Hoe, N.B.; Wei, T.S.; Mu, D.; Sun, Y.; Joo, L.S.; Dagher, R.; Zielonka, E.M.; et al. Distal Airway Stem Cells Yield Alveoli In Vitro and during Lung Regeneration following H1N1 Influenza Infection. Cell 2011, 147, 525–538. [Google Scholar] [CrossRef]
- Matsuura, R.; Kogo, H.; Ogaeri, T.; Miwa, T.; Kuwahara, M.; Kanai, Y.; Nakagawa, T.; Kuroiwa, A.; Fujimoto, T.; Torihashi, S. Crucial Transcription Factors in Endoderm and Embryonic Gut Development Are Expressed in Gut-Like Structures from Mouse ES Cells. Stem Cells 2006, 24, 624–630. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Fordham, R.P.; Yui, S.; Hannan, N.R.F.; Soendergaard, C.; Madgwick, A.; Schweiger, P.J.; Nielsen, O.H.; Vallier, L.; Pedersen, R.A.; Nakamura, T.; et al. Transplantation of Expanded Fetal Intestinal Progenitors Contributes to Colon Regeneration after Injury. Cell Stem Cell 2013, 13, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Farin, H.F.; van Es, J.H.; Clevers, H.; Langer, R.; Karp, J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods 2014, 11, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yamamoto, Y.; Wilson, L.H.; Zhang, T.; Howitt, B.E.; Farrow, M.A.; Kern, F.; Ning, G.; Hong, Y.; Khor, C.C.; et al. Cloning and variation of ground state intestinal stem cells. Nature 2015, 522, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Wang, X.; Bertrand, D.; Kern, F.; Zhang, T.; Duleba, M.; Srivastava, S.; Khor, C.C.; Hu, Y.; Wilson, L.H.; et al. Mutational spectrum of Barrett’s stem cells suggests paths to initiation of a precancerous lesion. Nat. Commun. 2016, 7, 10380. [Google Scholar] [CrossRef] [PubMed]
- Duleba, M.; Qi, Y.; Mahalingam, R.; Flynn, K.; Rinaldi, F.; Liew, A.-A.; Neupane, R.; Vincent, M.; Crum, C.P.; Ho, K.Y.; et al. An Efficient Method for Cloning Gastrointestinal Stem Cells from Patients via Endoscopic Biopsies. Gastroenterology 2018. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.-A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature 2015, 517, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Llames, S.; García-Pérez, E.; Meana, Á.; Larcher, F.; del Río, M. Feeder Layer Cell Actions and Applications. Tissue Eng. Part B Rev. 2015, 21, 345–353. [Google Scholar] [CrossRef]
- Boyce, S.T.; Ham, R.G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J. Investig. Dermatol. 1983, 81, 33s–40s. [Google Scholar] [CrossRef]
- de Corte, P.; Verween, G.; Verbeken, G.; Rose, T.; Jennes, S.; de Coninck, A.; Roseeuw, D.; Vanderkelen, A.; Kets, E.; Haddow, D.; et al. Feeder layer- and animal product-free culture of neonatal foreskin keratinocytes: Improved performance, usability, quality and safety. Cell Tissue Bank. 2012, 13, 175–189. [Google Scholar] [CrossRef]
- Lenihan, C.; Rogers, C.; Metcalfe, A.D.; Martin, Y.H. The effect of isolation and culture methods on epithelial stem cell populations and their progeny—Toward an improved cell expansion protocol for clinical application. Cytotherapy 2014, 16, 1750–1759. [Google Scholar] [CrossRef]
- de Luca, M.; Pellegrini, G.; Green, H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regener. Med. 2006, 1, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Green, H. The birth of therapy with cultured cells. BioEssays 2008, 30, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Kakitani, M.; Zhao, J.; Oshima, T.; Tang, T.; Binnerts, M.; Liu, Y.; Boyle, B.; Park, E.; Emtage, P.; et al. Mitogenic Influence of Human R-Spondin1 on the Intestinal Epithelium. Science 2005, 309, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, O.; Brivanlou, A.H. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007, 3, 7–17. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17873377 (accessed on 11 November 2018). [CrossRef] [PubMed]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [PubMed]
- International Stem Cell Initiative; Amps, K.; Andrews, P.W.; Anyfantis, G.; Armstrong, L.; Avery, S.; Baharvand, H.; Baker, J.; Baker, D.; Munoz, M.B.; et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011, 29, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.; Hirst, A.J.; Baker, D.; Lim, C.Y.; Alagaratnam, S.; Skotheim, R.I.; Lothe, R.A.; Pera, M.F.; Colman, A.; Robson, P.; et al. BCL-XL mediates the strong selective advantage of a 20q11.21 amplification commonly found in human embryonic stem cell cultures. Stem Cell Rep. 2013, 1, 379–386. [Google Scholar] [CrossRef]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10648628 (accessed on 11 November 2018). [CrossRef]
- Lee, G.; Hynes, R.; Kirschner, M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell 1984, 36, 729–740. Available online: http://www.ncbi.nlm.nih.gov/pubmed/6697394 (accessed on 11 November 2018).
- Bissell, D.M.; Arenson, D.M.; Maher, J.J.; Roll, F.J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J. Clin. Investig. 1987, 79, 801–812. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Aggeler, J.; Ram, T.G.; Bissell, M.J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 1989, 105, 223–235. [Google Scholar]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, S.Y.; Maes, S.; Martinello, T.; Aerts, D.; Chiers, K.; Mariën, T.; Patruno, M.; Franco-Obregón, A.; Spaas, J.H. Equine Epidermis: A Source of Epithelial-Like Stem/Progenitor Cells with In Vitro and In Vivo Regenerative Capacities. Stem Cells Dev. 2014, 23, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ootani, A.; Li, X.; Sangiorgi, E.; Ho, Q.T.; Ueno, H.; Toda, S.; Sugihara, H.; Fujimoto, K.; Weissman, I.L.; Capecchi, M.R.; et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009, 15, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Scoville, D.; He, X.C.; Mahe, M.M.; Box, A.; Perry, J.M.; Smith, N.R.; Lei, N.Y.; Davies, P.S.; Fuller, M.K.; et al. Isolation and Characterization of Intestinal Stem Cells Based on Surface Marker Combinations and Colony-Formation Assay. Gastroenterology 2013, 145, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. Available online: http://www.ncbi.nlm.nih.gov/pubmed/293669 (accessed on 11 November 2018). [CrossRef] [PubMed]
- O’Connor, N.; Mulliken, J.; Banks-Schlegel, S.; Kehinde, O.; Green, H. Grafting of Burns with Cultured Epithelium Prepared from Autologous Epidermal Cells. Lancet 1981, 317, 75–78. [Google Scholar] [CrossRef]
- Gallico, G.G.; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent Coverage of Large Burn Wounds with Autologous Cultured Human Epithelium. N. Engl. J. Med. 1984, 311, 448–451. [Google Scholar] [CrossRef]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; de Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- Buchman, A.L.; Scolapio, J.; Fryer, J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003, 124, 1111–1134. [Google Scholar] [CrossRef]
- Hong, S.N.; Dunn, J.C.Y.; Stelzner, M.; Martín, M.G. Concise Review: The Potential Use of Intestinal Stem Cells to Treat Patients with Intestinal Failure. Stem Cells Transl. Med. 2017, 6, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.Y.; Söderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, W.; Duleba, M.; Yamamoto, Y.; Vincent, M.; McKeon, F. Biobanking Organoids or Ground-State Stem Cells? J. Clin. Med. 2018, 7, 555. https://doi.org/10.3390/jcm7120555
Xian W, Duleba M, Yamamoto Y, Vincent M, McKeon F. Biobanking Organoids or Ground-State Stem Cells? Journal of Clinical Medicine. 2018; 7(12):555. https://doi.org/10.3390/jcm7120555
Chicago/Turabian StyleXian, Wa, Marcin Duleba, Yusuke Yamamoto, Matthew Vincent, and Frank McKeon. 2018. "Biobanking Organoids or Ground-State Stem Cells?" Journal of Clinical Medicine 7, no. 12: 555. https://doi.org/10.3390/jcm7120555
APA StyleXian, W., Duleba, M., Yamamoto, Y., Vincent, M., & McKeon, F. (2018). Biobanking Organoids or Ground-State Stem Cells? Journal of Clinical Medicine, 7(12), 555. https://doi.org/10.3390/jcm7120555



