Distinct Expression and Clinical Significance of Zinc Finger AN-1-Type Containing 4 in Oral Squamous Cell Carcinomas
Abstract
1. Introduction
2. Experimental Section
2.1. Patients and Tissue Specimens
2.2. Tissue Microarray Construction
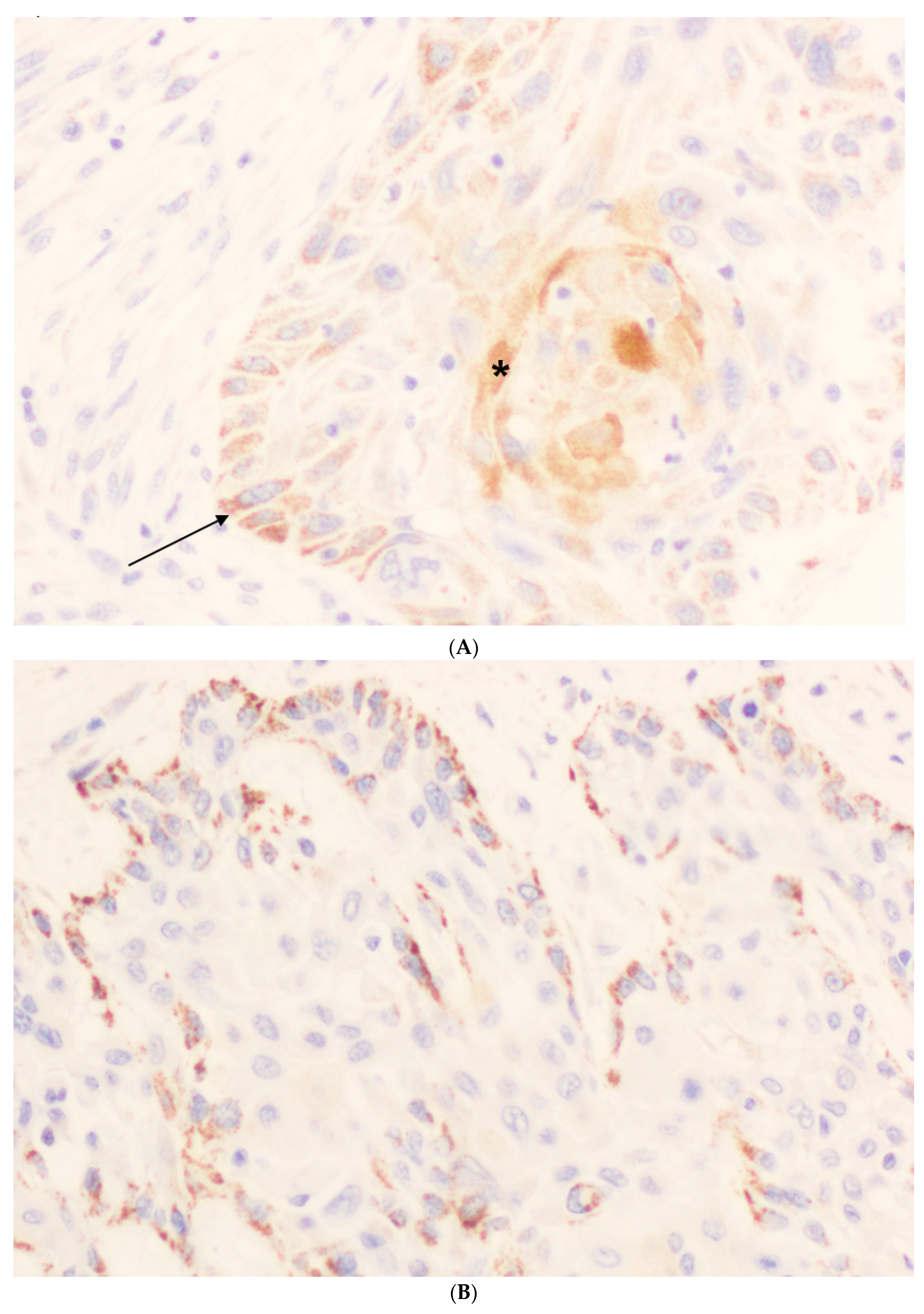
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Immunohistochemical Analysis of ZFAND4 Expression in OSCC Tissue Specimens
3.3. Associations of ZFAND4 with Clinicopathologic Characteristics
3.4. ZFAND4 Expression and Patients’ Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rautava, J.; Luukkaa, M.; Heikinheimo, K.; Alin, J.; Grenman, R.; Happonen, R.P. Squamous cell carcinomas arising from different types of oral epithelia differ in their tumor and patient characteristics and survival. Oral Oncol. 2007, 43, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Kirita, T.; Kuniyasu, H. Update of molecular pathobiology in oral cancer: A review. Int. J. Clin. Oncol. 2014, 19, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Kurihara, M.; Nishiguchi, Y.; Fujiwara, R.; Kirita, T.; Kuniyasu, H. NEDD 4 binding protein 2-like 1 promotes cancer cell invasion in oral squamous cell carcinoma. Virchows Arch. 2016, 469, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kurihara-Shimomura, M.; Sasahira, T.; Nakamura, H.; Nakashima, C.; Kuniyasu, H.; Kirita, T. Zinc finger AN1-type containing 4 is a novel marker for predicting metastasis and poor prognosis in oral squamous cell carcinoma. J. Clin. Pathol. 2018, 71, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, F.; Pang, E.J.; Zhang, Z.Q.; Jin, B.W.; Dong, W.F. MicroRNA-182 inhibits proliferation through targeting oncogenic ANUBL1 in gastric cancer. Oncol. Rep. 2015, 33, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar] [PubMed]
- Kandoth, C.; McLellan, M.D.; Vandin, F.; Ye, K.; Nium, B.; Lu, C.; Xie, M.; Zhang, Q.; McMichael, J.F.; Wyczalkowski, M.A.; et al. Mutational landscape and significance across 12 major cancer types. Nature 2013, 502, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Frederick, M.J.; Pickering, C.R.; Bettegowda, C.; Chang, K.; Li, R.J.; Fakhry, C.; Xie, T.X.; Zhang, J.; Wang, J.; et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011, 333, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.R.; Zhang, J.; Yoo, S.Y.; Bengtsson, L.; Moorthy, S.; Neskey, D.M.; Zhao, M.; Ortega Alves, M.V.; Chang, K.; Drummond, J.; et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013, 3, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]

| Variable | Number (%) |
|---|---|
| Age (year) (mean ± SD; median; range) | 58.6 ± 14.3; 57; 28–91 |
| Gender | |
| Men | 81 (66) |
| Women | 42 (34) |
| Tobacco use | |
| Smoker | 83 (67) |
| Non-smoker | 40 (33) |
| Alcohol use | |
| Drinker | 68 (55) |
| Non-drinker | 55 (45) |
| Location of oral squamous oral cell carcinoma | |
| Tongue | 51 (41) |
| Floor of the mouth | 35 (28) |
| Other sites within te oral cavity | 37 (31) |
| Tumor status | |
| pT1 | 27 (22) |
| pT2 | 53 (43) |
| pT3 | 15 (12) |
| pT4 | 28 (23) |
| Nodal status | |
| pN0 | 74 (60) |
| pN1 | 25 (20) |
| pN2 | 24 (20) |
| Clinical stage | |
| Stage I | 20 (16) |
| Stage II | 31 (25) |
| Stage III | 25 (20) |
| Stage IV | 47 (39) |
| G status | |
| G1 | 78 (63) |
| G2 | 41 (33) |
| G3 | 4 (4) |
| Second primary carcinoma | |
| No | 104 (85) |
| Yes | 19 (15) |
| Local recurrence | |
| No | 69 (56) |
| Yes | 54 (44) |
| Clinical status at the end of the follow-up | |
| Live and without recurrence | 51 (41) |
| Dead of index cancer | 53 (43) |
| Lost or died of other causes (censored) | 19 (16) |
| Variable | Number of Cases | High ZFAND4 Expression in Undifferentiated Cells (%) | p | High ZFAND4 Expression in Differentiated Cells (%) |
|---|---|---|---|---|
| Gender | 0.98 | |||
| Men | 81 | 50 (62) | 38 (47) | |
| Women | 42 | 26 (62) | 17 (40) | |
| Tobacco use | 0.77 | |||
| Smoker | 83 | 52 (63) | 34 (41) | |
| Non-smoker | 40 | 24 (60) | 21 (52) | |
| Alcohol use | 0.99 | |||
| Drinker | 68 | 42 (62) | 31 (46) | |
| Non-drinker | 55 | 34 (62) | 24 (44) | |
| pT | 0.44 | |||
| pT1 | 27 | 19 (70) | 16 (59) | |
| pT2 | 53 | 33 (62) | 25 (47) | |
| pT3 | 15 | 10 (67) | 4 (27) | |
| pT4 | 28 | 14 (50) | 10 (36) | |
| pN | 0.75 | |||
| pN0 | 74 | 45 (61) | 34 (46) | |
| pN1 | 25 | 17 (68) | 13 (52) | |
| pN2 | 24 | 14 (58) | 8 (33) | |
| Clinical stage | 0.69 | |||
| Stage I | 20 | 13 (65) | 9 (45) | |
| Stage II | 31 | 20 (64) | 16 (52) | |
| Stage III | 25 | 17 (68) | 11 (44) | |
| Stage IV | 47 | 26 (55) | 19 (40) | |
| G status | 0.14 | |||
| G1 (Well) | 78 | 52 (67) | 40 (51) | |
| G2 + G3 (Moderate + poor) | 45 | 24 (53) | 15 (33) | |
| Tumor location | 0.005 | |||
| Tongue | 51 | 39 (76) | 23 (45) | |
| Rest | 72 | 37 (51) | 32 (44) | |
| Tumor location | 0.5 | |||
| Floor of the mouth | 35 | 20 (57) | 16 (46) | |
| Rest | 88 | 56 (64) | 39 (44) | |
| Tumor recurrence | 0.61 | |||
| No | 69 | 44 (64) | 36 (52) | |
| Yes | 54 | 32 (59) | 19 (35) | |
| Second primary carcinoma | 0.89 | |||
| No | 104 | 64 (61) | 48 (46) | |
| Yes | 19 | 12 (63) | 7 (37) | |
| Clinical status at the end of the follow-up | 0.73 | |||
| Live and without recurrence | 51 | 32 (63) | 26 (51) | |
| Dead of index cancer | 53 | 31 (58) | 20 (38) | |
| Lost or died of other causes | 19 | 13 (68) | 9 (47) |
| Variable | Number of Cases | High ZFAND4 Expression in Undifferentiated Cells (%) | p | High ZFAND4 Expression in Differentiated Cells (%) | p |
|---|---|---|---|---|---|
| Gender | 0.95 | 0.33 | |||
| Men | 81 | 42 (52) | 26 (32) | ||
| Women | 42 | 22 (52) | 10 (24) | ||
| Tobacco use | 0.75 | 0.58 | |||
| Smoker | 83 | 44 (53) | 23 (28) | ||
| Non-smoker | 40 | 20 (50) | 13 (32) | ||
| Alcohol use | 0.89 | 0.21 | |||
| Drinker | 68 | 35 (51) | 23 (34) | ||
| Non-drinker | 55 | 29 (53) | 13 (24) | ||
| pT | 0.29 | 0.33 | |||
| pT1 | 27 | 16 (59) | 11 (41) | ||
| pT2 | 53 | 27 (51) | 15 (28) | ||
| pT3 | 15 | 10 (67) | 2 (13) | ||
| pT4 | 28 | 11 (39) | 8 (29) | ||
| pN | 0.85 | 0.02 | |||
| pN0 | 74 | 37 (50) | 24 (32) | ||
| pN1 | 25 | 14 (56) | 10 (40) | ||
| pN2 | 24 | 13 (54) | 2 (8) | ||
| Clinical stage | 0.46 | 0.81 | |||
| Stage I | 20 | 10 (50) | 6 (30) | ||
| Stage II | 31 | 17 (55) | 11 (35) | ||
| Stage III | 25 | 16 (64) | 9 (36) | ||
| Stage IV | 47 | 21 (45) | 10 (21) | ||
| G status | 0.36 | 0.08 | |||
| G1 (Well) | 78 | 43 (55) | 27 (35) | ||
| G2 + G3 (Moderate + poor) | 45 | 21 (47) | 9 (20) | ||
| Tumor location | 0.1 | 0.43 | |||
| Tongue | 51 | 31 (61) | 13 (25) | ||
| Rest | 72 | 33 (46) | 23 (32) | ||
| Tumor location | 0.75 | 0.44 | |||
| Floor of the mouth | 35 | 19 (54) | 12 (34) | ||
| Rest | 88 | 45 (51) | 24 (27) | ||
| Tumor recurrence | 0.97 | 0.47 | |||
| No | 69 | 36 (52) | 22 (32) | ||
| Yes | 54 | 28 (52) | 14 (26) | ||
| Second primary carcinoma | 0.29 | 0.75 | |||
| No | 104 | 52 (50) | 31 (30) | ||
| Yes | 19 | 12 (63) | 5 (26) | ||
| Clinical status at the end of the follow-up | 0.85 | 0.59 | |||
| Live and without recurrence | 51 | 26 (51) | 17 (33) | ||
| Dead of index cancer | 53 | 27 (51) | 15 (28) | ||
| Lost or died of other causes | 19 | 11 (58) | 4 (21) |
| ZFAND4 Expression | Censored Patients (%) | Mean Survival Time (95% CI) | HR (95% CI) | p |
|---|---|---|---|---|
| Undifferentiated cells | 0.7 | |||
| calculated by using the mean Allred score | ||||
| Low | 33 (56) | 107.72 (86.32–129.12) | Reference | |
| High | 37 (58) | 135.20 (108.87–161.52) | 0.90 (0.52–1.55) | |
| Differentiated cells | 0.8 | |||
| calculated by using the mean Allred score | ||||
| Low | 49 (56) | 126.62 (104.38–148.85) | Reference | |
| High | 21 (58) | 135.11 (99.60–170.62) | 0.93 (0.51–1.69) | |
| Undifferentiated cells | 0.53 | |||
| calculated by using the maximum Allred score | ||||
| Low | 25 (53) | 104.14 (80.46–127.82) | Reference | |
| High | 45 (59) | 136.44 (111.81–161.06) | 0.84 (0.48–1.45) | |
| Differentiated cells | 0.25 | |||
| calculated by using the maximum Allred score | ||||
| Low | 35 (51) | 105.55 (85.11–126.00) | Reference | |
| High | 35 (64) | 144.08 (114.95–173.21) | 0.72 (0.41–1.26) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez-Canto, J.; Suárez-Sánchez, F.J.; Domínguez-Iglesias, F.; Hernández-Vallejo, G.; García-Pedrero, J.M.; De Vicente, J.C. Distinct Expression and Clinical Significance of Zinc Finger AN-1-Type Containing 4 in Oral Squamous Cell Carcinomas. J. Clin. Med. 2018, 7, 534. https://doi.org/10.3390/jcm7120534
Suárez-Canto J, Suárez-Sánchez FJ, Domínguez-Iglesias F, Hernández-Vallejo G, García-Pedrero JM, De Vicente JC. Distinct Expression and Clinical Significance of Zinc Finger AN-1-Type Containing 4 in Oral Squamous Cell Carcinomas. Journal of Clinical Medicine. 2018; 7(12):534. https://doi.org/10.3390/jcm7120534
Chicago/Turabian StyleSuárez-Canto, Julián, Faustino Julián Suárez-Sánchez, Francisco Domínguez-Iglesias, Gonzalo Hernández-Vallejo, Juana M. García-Pedrero, and Juan C. De Vicente. 2018. "Distinct Expression and Clinical Significance of Zinc Finger AN-1-Type Containing 4 in Oral Squamous Cell Carcinomas" Journal of Clinical Medicine 7, no. 12: 534. https://doi.org/10.3390/jcm7120534
APA StyleSuárez-Canto, J., Suárez-Sánchez, F. J., Domínguez-Iglesias, F., Hernández-Vallejo, G., García-Pedrero, J. M., & De Vicente, J. C. (2018). Distinct Expression and Clinical Significance of Zinc Finger AN-1-Type Containing 4 in Oral Squamous Cell Carcinomas. Journal of Clinical Medicine, 7(12), 534. https://doi.org/10.3390/jcm7120534





