Anti-Mycobacterial Antibodies in Paired Cerebrospinal Fluid and Serum Samples from Japanese Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Serum and CSF Samples
2.3. Antigens
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Determination of Intrathecal IgG Synthesis
2.6. Magnetic Resonance Imaging (MRI)
2.7. Visual Evoked Potential (VEP)
2.8. Statistical Analysis
3. Results
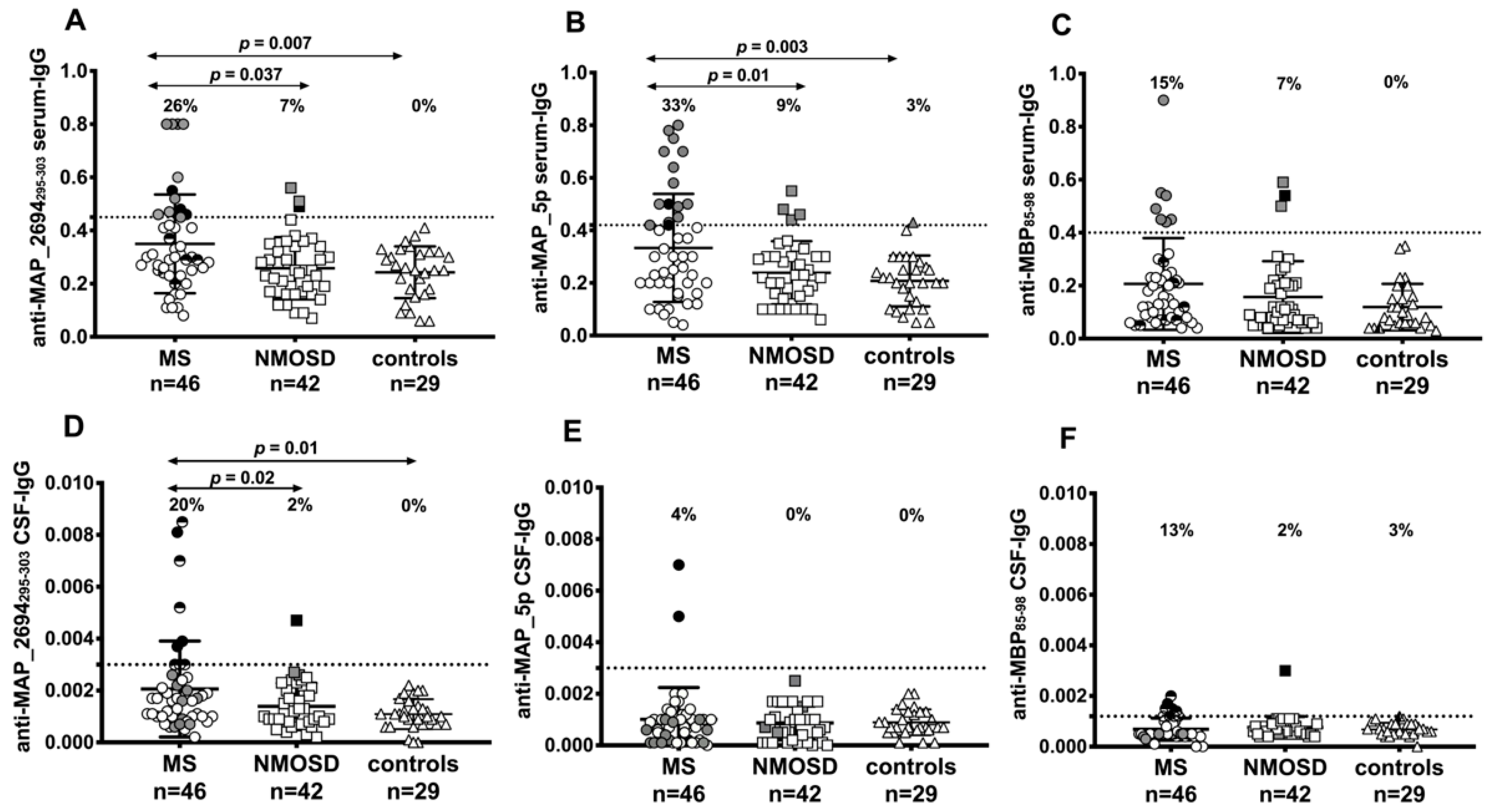
3.1. Various Specific IgG Antibodies are Present in Paired Serum and CSF Samples of Patients with MS or NMOSD
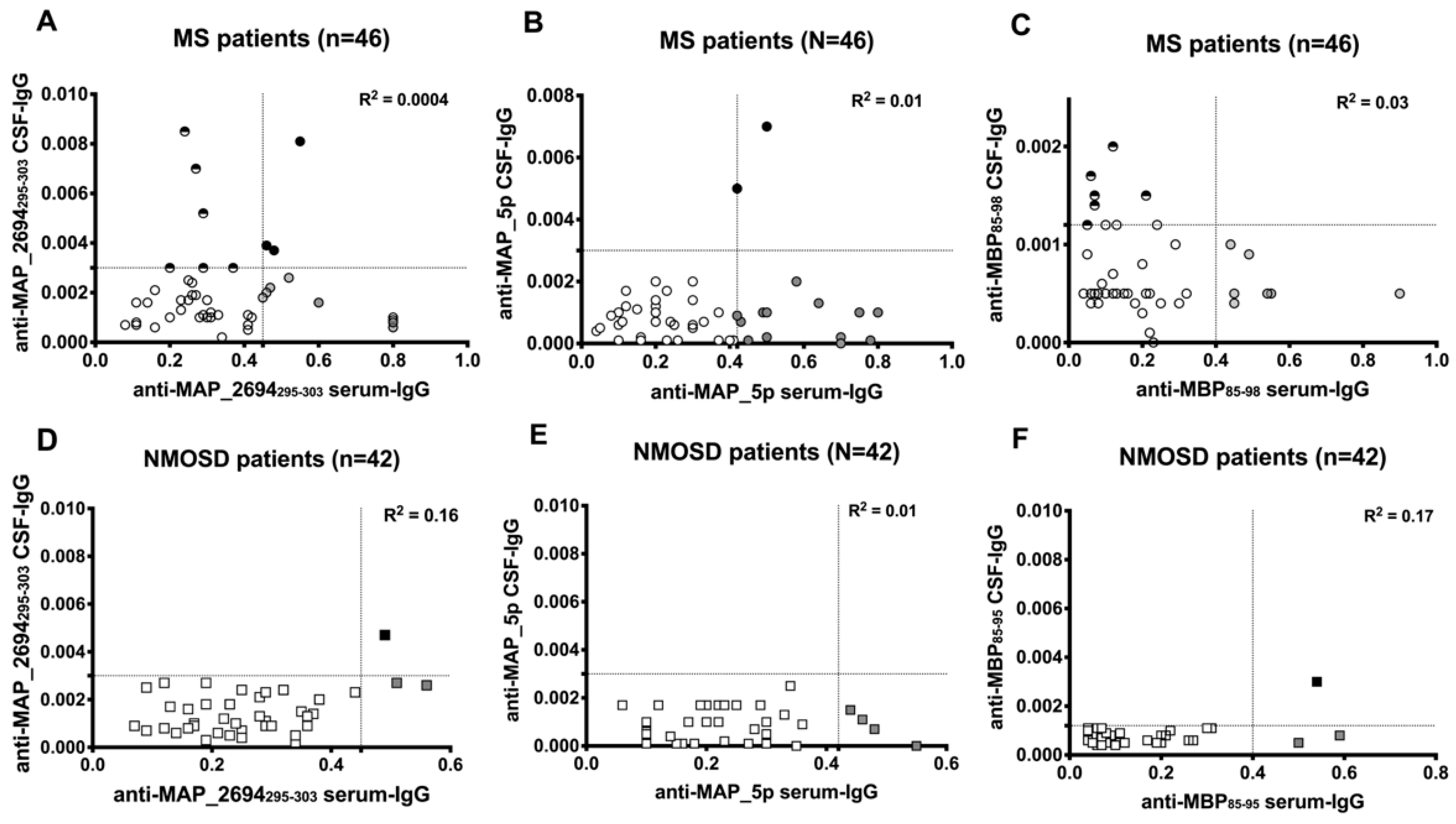
3.2. Synthesis of Intrathecal Anti-Mycobacterial Antibodies in Patient with MS
3.3. Correlation Between Antibody Positivity and Clinical Characteristics of Patients with MS
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hohlfeld, R.; Dornmair, K.; Meinl, E.; Wekerle, H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2016, 15, 317–331. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Ioannidis, J.P.; Tzoulaki, I. Environmental risk factors and multiple sclerosis: An umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015, 14, 263–273. [Google Scholar] [CrossRef]
- Delgado-Garcia, M.; Matesanz, F.; Alcina, A.; Fedetz, M.; García-Sánchez, M.I.; Ruiz-Peña, J.L.; Fernández, Ó.; Pinto Medel, M.J.; Leyva, L.; Arnal, C.; et al. A new risk variant for multiple sclerosis at the immunoglobulin heavy chain locus associates with intrathecal IgG, IgM index and oligoclonal bands. Mult. Scler. 2015, 21, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
- Mameli, G.; Cocco, E.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein Barr Virus and Mycobacterium avium subsp. paratuberculosis peptides are recognized in sera and cerebrospinal fluid of MS patients. Sci. Rep. 2016, 6, 22401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Melis, P.; Genna, V.; Cocco, E.; Marrosu, M.G.; Pieroni, E. Antigenic peptide molecular recognition by the DRB1-DQB1 haplotype modulates multiple sclerosis susceptibility. Mol. Biosyst. 2014, 10, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Cocco, E.; Paccagnini, D.; Masala, S.; Ahmed, N.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Association of Mycobacterium avium subsp. paratuberculosis with multiple sclerosis in Sardinian patients. PLoS ONE 2011, 6, e18482. [Google Scholar] [CrossRef]
- Frau, J.; Cossu, D.; Coghe, G.; Lorefice, L.; Fenu, G.; Melis, M.; Paccagnini, D.; Sardu, C.; Murru, M.R; Tranquilli, S.; et al. Mycobacterium avium subsp. paratuberculosis and multiple sclerosis in Sardinian patients: Epidemiology and clinical features. Mult. Scler. 2013, 19, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Masala, S.; Frau, J.; Mameli, G.; Marrosu, M.G.; Cocco, E.; Sechi, L.A. Antigenic epitopes of MAP2694 homologous to T-cell receptor gamma-chain are highly recognized in multiple sclerosis Sardinian patients. Mol. Immunol. 2014, 57, 138–140. [Google Scholar] [CrossRef]
- Kumar, A.; Sechi, L.A.; Caboni, P.; Marrosu, M.G.; Atzori, L.; Pieroni, E. Dynamical insights into the differential characteristics of Mycobacterium avium subsp. paratuberculosis peptide binding to HLA-DRB1 proteins associated with multiple sclerosis. New J. Chem. 2015, 39, 1355–1366. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Sechi, L.A.; Otsubo, S.; Tomizawa, Y.; Momotani, E.; Hattori, N.; Yokoyama, K.; Sechi, L.A.; Otsubo, S.; Tomizawa, Y.; Momotani, E.; Hattori, N. Humoral response against host-mimetic homologous epitopes of Mycobacterium avium subsp. paratuberculosis in Japanese multiple sclerosis patients. Sci. Rep. 2016, 6, 29227. [Google Scholar] [CrossRef] [PubMed]
- Biet, F.; Bay, S.; Thibault, V.C.; Euphrasie, D.; Grayon, M.; Ganneau, C.; Lanotte, P.; Daffé, M.; Gokhale, R.; Etienne, G.; et al. Lipopentapeptide induces a strong host humoral response and distinguishes Mycobacterium avium subsp. paratuberculosis from M. avium subsp. avium. Vaccine 2008, 26, 257–268. [Google Scholar] [CrossRef]
- Niegowska, M.; Rapini, N.; Biet, F.; Piccinini, S.; Bay, S.; Lidano, R.; Bitti, M.L.; Sechi, L.A. Seroreactivity against Specific L5P Antigen from Mycobacterium avium subsp. paratuberculosis in Children at Risk for T1D. PLoS ONE 2016, 11, e0161516. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Wucherpfennig, K.W.; Cannella, B.; Hansen, B.E.; Svejgaard, A.; Pyrdol, J.; Ditzel, H.; Raine, C.; Engberg, J.; Fugger, L. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85-99 complex. J. Exp. Med. 2000, 191, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Cossu, D.; Cocco, E.; Masala, S.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Epstein-Barr virus and Mycobacterium avium subsp. paratuberculosis peptides are cross recognized by anti-myelin basic protein antibodies in multiple sclerosis patients. J. Neuroimmunol. 2014, 270, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuck, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Reiber, H.; Lange, P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: Sensitive and specific detection of antibody synthesis in brain. Clin. Chem. 1991, 37, 1153–1160. [Google Scholar]
- Takemura, M.Y.; Hori, M.; Yokoyama, K.; Hamasaki, N.; Suzuki, M.; Kamagata, K.; Kamiya, K.; Suzuki, Y.; Kyogoku, S.; Masutani, Y.; et al. Alterations of the optic pathway between unilateral and bilateral optic nerve damage in multiple sclerosis as revealed by the combined use of advanced diffusion kurtosis imaging and visual evoked potentials. Magn. Reson. Imaging 2017, 39, 24–30. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neuro.l Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef]
- Nakashima, I.; Fujihara, K.; Okita, N.; Takase, S.; Itoyama, Y. Clinical and MRI study of brain stem and cerebellar involvement in Japanese patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1999, 67, 153–157. [Google Scholar] [CrossRef]
- Quintana, F.J.; Farez, M.F.; Izquierdo, G.; Lucas, M.; Cohen, I.R.; Weiner, H.L. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology 2012, 78, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Yokoyama, K.; Tomizawa, Y.; Momotani, E.; Hattori, N. Altered humoral immunity to mycobacterial antigens in Japanese patients affected by inflammatory demyelinating diseases of the central nervous system. Sci. Rep. 2017, 7, 3179. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Yokoyama, K.; Hattori, N. Conflicting role of Mycobacterium species in multiple sclerosis. Front. Neurol. 2017, 8, 216. [Google Scholar] [CrossRef]
- Kostic, M.; Stojanovic, I.; Marjanovic, G.; Zivkovic, N.; Cvetanovic, A. Deleterious versus protective autoimmunity in multiple sclerosis. Cell. Immunol. 2015, 296, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Novakova, L.; Axelsson, M.; Malmeström, C.; Zetterberg, H.; Lycke, J.; Cardell, S.L. High Interferon-gamma uniquely in delta1 T cells correlates with markers of inflammation and axonal damage in early multiple sclerosis. Front. Immunol. 2017, 8, 260. [Google Scholar] [CrossRef]
- Cornaby, C.; Gibbons, L.; Mayhew, V.; Sloan, C.S.; Welling, A.; Poole, B.D. B cell epitope spreading: Mechanisms and contribution to autoimmune diseases. Immunol. Lett. 2015, 163, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Haregewoin, A.; Soman, G.; Hom, R.C.; Finberg, R.W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature 1989, 340, 309–312. [Google Scholar] [CrossRef]
- Lehmann-Horn, K.; Wang, S.Z.; Sagan, S.A.; Zamvil, S.S.; von Büdingen, H.C. B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight 2016, 1, e87234. [Google Scholar] [CrossRef] [PubMed]
- Pollok, K.; Mothes, R.; Ulbricht, C.; Liebheit, A.; Gerken, J.D.; Uhlmann, S.; Paul, F.; Niesner, R.; Radbruch, H.; Hauser, A.E. The chronically inflamed central nervous system provides niches for long-lived plasma cells. Acta Neuropathol. Commun. 2017, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. [Google Scholar] [CrossRef]

| CSF Parameters | MS (n = 46) | NMOSD (n = 42) | Controls (n = 29) | p |
|---|---|---|---|---|
| Oligoclonal IgG band positive | 34 (74%) | 11(26%) | 7 (24%) | <0.0001 * <0.0001 ** |
| IgG index ≥0.7 (%) | 33 (72%) | 7 (17%) | 8 (27%) | <0.0001 * 0.0003 ** |
| Albumin-Quotient (QAlb) ≥ 7 × 10−3 (%) | 10 (22%) | 17 (40%) | 6 (21%) | NS NS |
| AI-IgG MAP_2694295–303 >1.5 (mean) | 8 (4.9) | 0 | 0 | 0.006 * 0.02 ** |
| AI-IgG MAP_5p >1.5 (mean) | 2 (2.8) | 0 | 0 | NS NS |
| AI-IgG MBP85–98 >1.5 (mean) | 5 (5.7) | 1 (1.5) | 0 | NS NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoyama, K.; Cossu, D.; Hoshino, Y.; Tomizawa, Y.; Momotani, E.; Hattori, N. Anti-Mycobacterial Antibodies in Paired Cerebrospinal Fluid and Serum Samples from Japanese Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder. J. Clin. Med. 2018, 7, 522. https://doi.org/10.3390/jcm7120522
Yokoyama K, Cossu D, Hoshino Y, Tomizawa Y, Momotani E, Hattori N. Anti-Mycobacterial Antibodies in Paired Cerebrospinal Fluid and Serum Samples from Japanese Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder. Journal of Clinical Medicine. 2018; 7(12):522. https://doi.org/10.3390/jcm7120522
Chicago/Turabian StyleYokoyama, Kazumasa, Davide Cossu, Yasunobu Hoshino, Yuji Tomizawa, Eiichi Momotani, and Nobutaka Hattori. 2018. "Anti-Mycobacterial Antibodies in Paired Cerebrospinal Fluid and Serum Samples from Japanese Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder" Journal of Clinical Medicine 7, no. 12: 522. https://doi.org/10.3390/jcm7120522
APA StyleYokoyama, K., Cossu, D., Hoshino, Y., Tomizawa, Y., Momotani, E., & Hattori, N. (2018). Anti-Mycobacterial Antibodies in Paired Cerebrospinal Fluid and Serum Samples from Japanese Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorder. Journal of Clinical Medicine, 7(12), 522. https://doi.org/10.3390/jcm7120522






