Nox4 Overexpression as a Poor Prognostic Factor in Patients with Oral Tongue Squamous Cell Carcinoma Receiving Surgical Resection
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Population
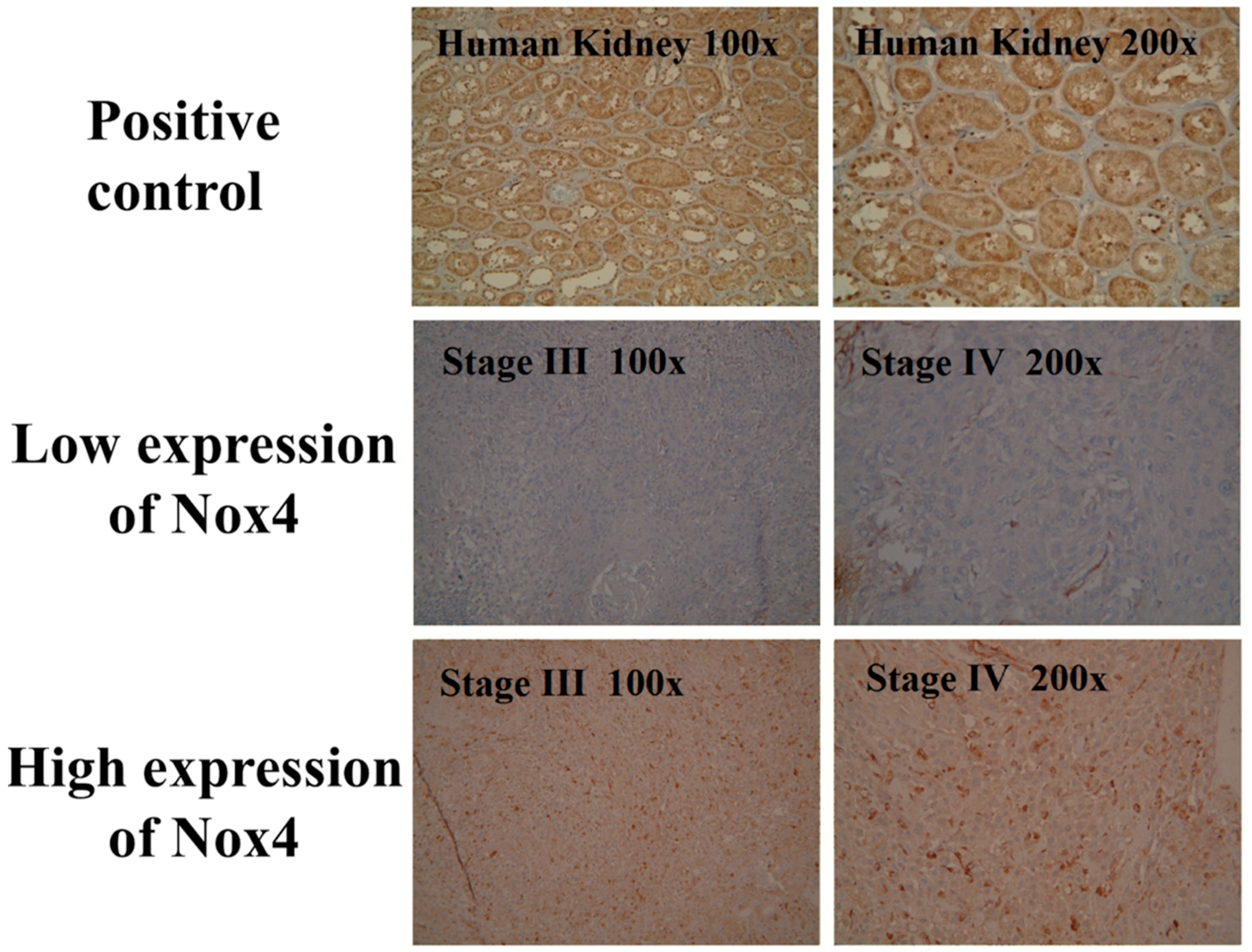
2.2. Immunohistochemistry
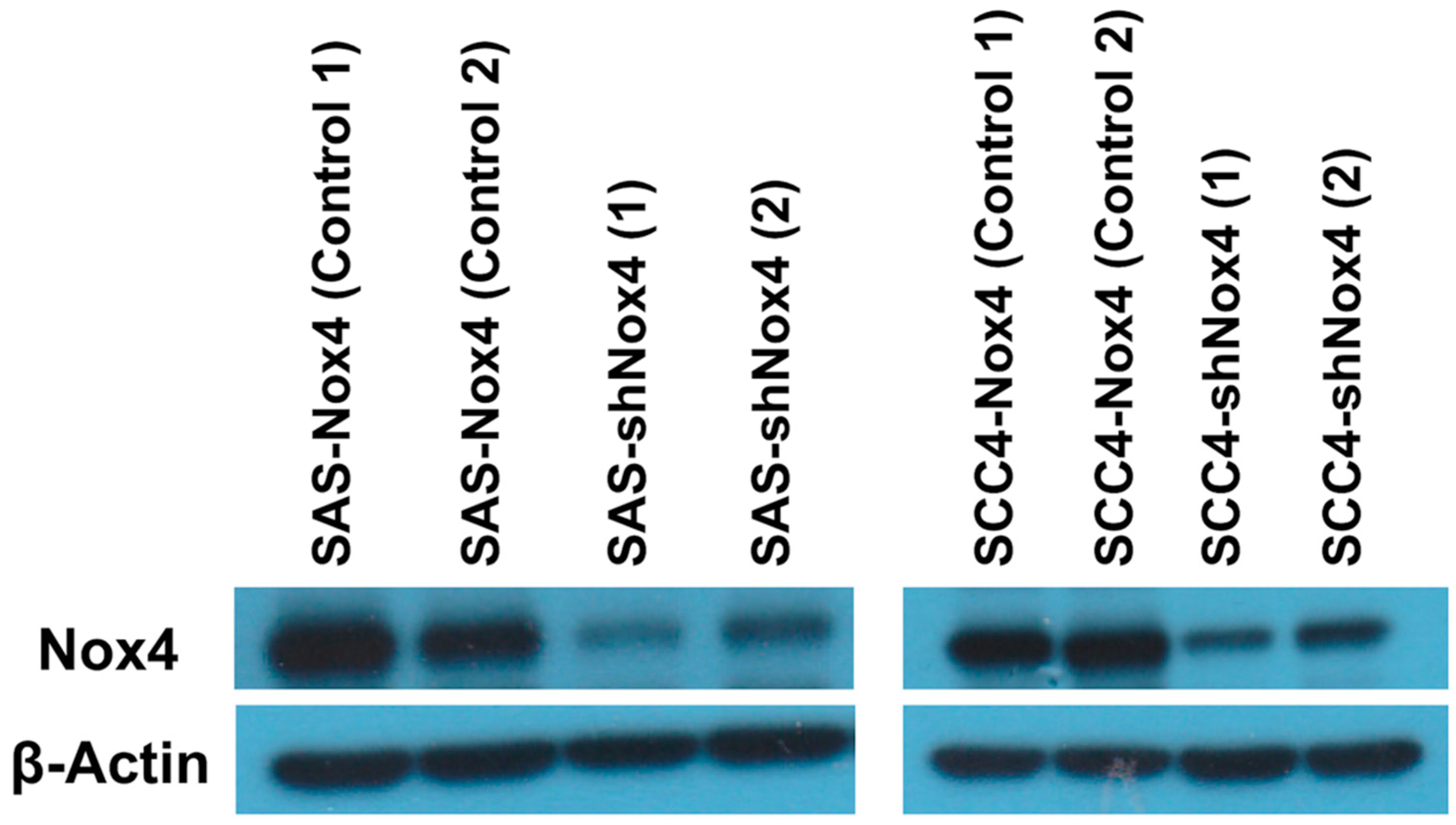
2.3. Western Blot Analysis
2.4. Cell Culture and Transfection
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. Patient Population
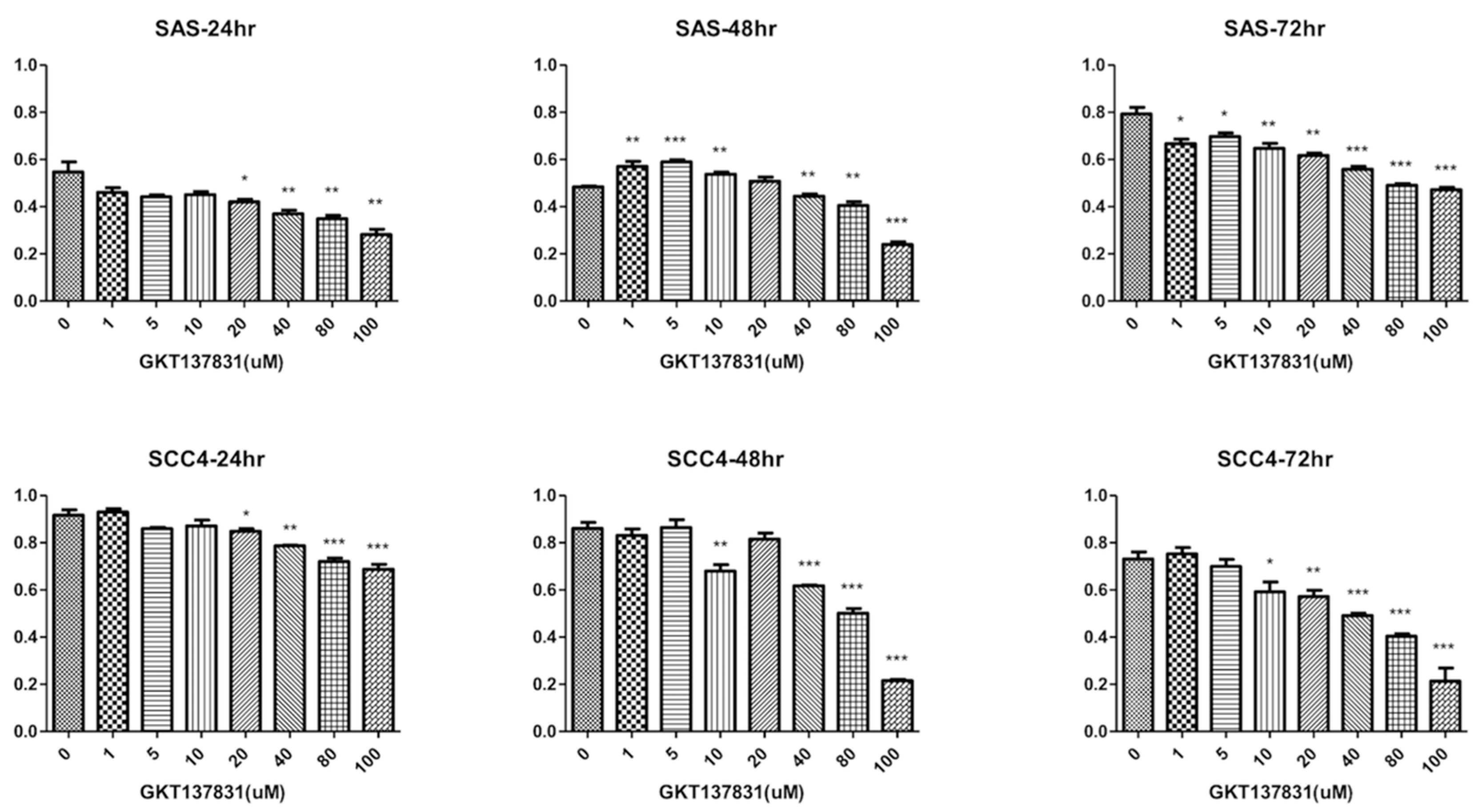
3.2. Silencing Nox4 Expression Reduces Tumor Cell Proliferation in Vitro
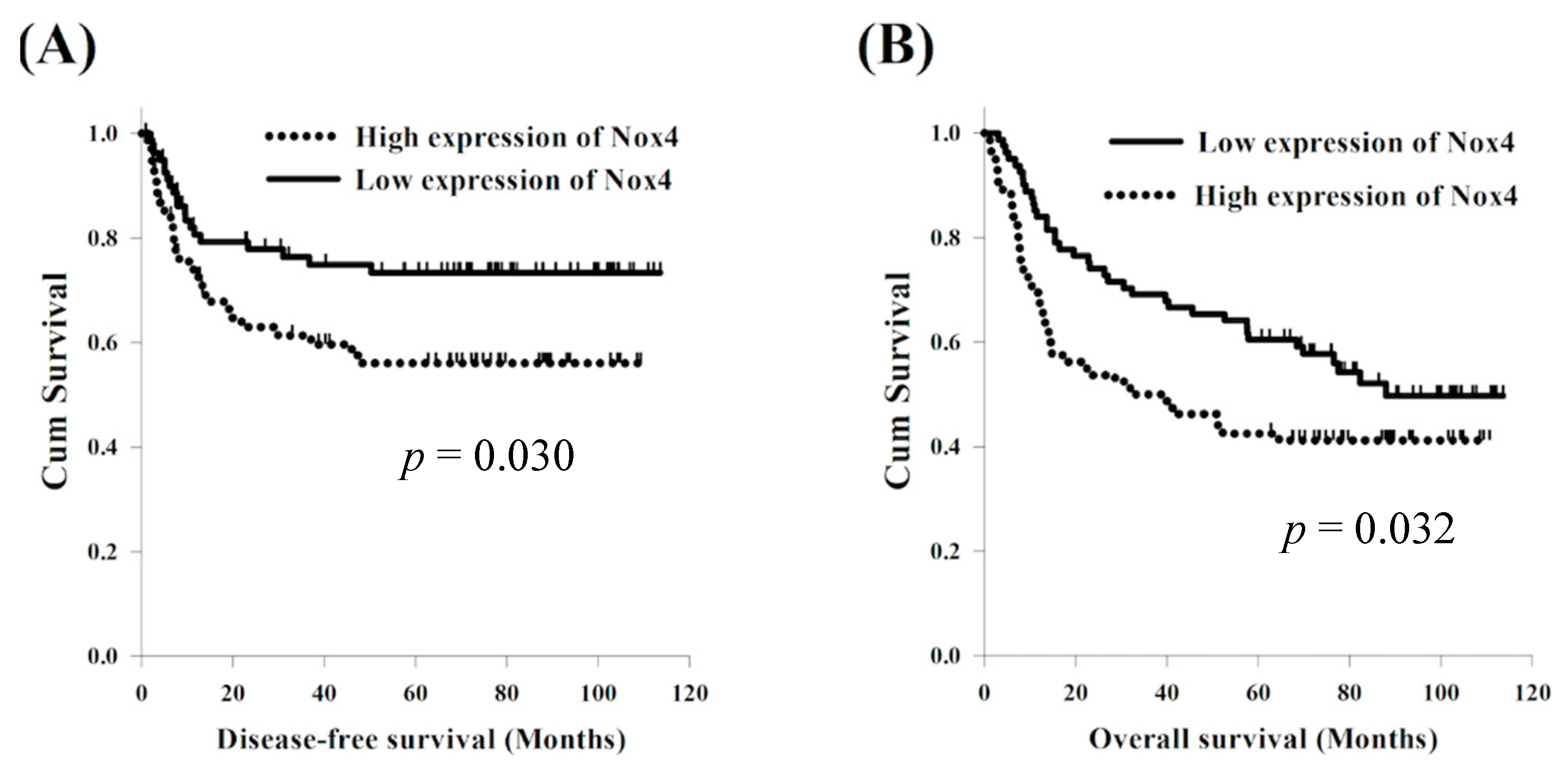
3.3. Expression of Nox4 and Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HNSCC | Head and neck squamous cell carcinoma |
| OTSCC | Oral tongue squamous cell carcinoma |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NOXs | Nicotinamide adenine dinucleotide phosphate oxidases |
| ROS | Reactive oxygen species |
| DMEM | Dulbecco′s modified Eagle′s medium |
| DFS | Disease-free survival |
| OS | Overall survival |
| IL-6 | Interleukin-6 |
| EGFR | Epidermal growth factor receptor |
| CAFs | Cancer-associated fibroblasts |
| ENE | Extranodal extension |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry Annual Report 2015; National Department of Health: Taipei, China, 2015.
- Camisasca, D.R.; Silami, M.A.; Honorato, J.; Dias, F.L.; de Faria, P.A.; Lourenco Sde, Q. Oral squamous cell carcinoma: Clinicopathological features in patients with and without recurrence. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Lindenblatt Rde, C.; Martinez, G.L.; Silva, L.E.; Faria, P.S.; Camisasca, D.R.; Lourenco Sde, Q. Oral squamous cell carcinoma grading systems—Analysis of the best survival predictor. J. Oral. Pathol. Med. 2012, 41, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Guo, S.; Chen, X. The human Nox4: Gene, structure, physiological function and pathological significance. J. Drug Target. 2015, 23, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Wu, Y.; Meitzler, J.L.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Antony, S.; Doroshow, J.H. NADPH oxidases and cancer. Clin. Sci. 2015, 128, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.M.; Watts, T.N.; Buechler, S.; Hummon, A.B. Proteomic and functional investigation of the colon cancer relapse-associated genes NOX4 and ITGA3. J. Proteome Res. 2014, 13, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Lanza-Jacoby, S. Metformin decreases growth of pancreatic cancer cells by decreasing reactive oxygen species: Role of NOX4. Biochem. Biophys. Res. Commun. 2015, 465, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Jung, Y.S.; Kim, J.Y.; Kim, H.M.; Lim, I.K. Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene 2016, 35, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, E.C.; Edderkaoui, M.; Pandol, S.J.; Gukovsky, I.; Gukovskaya, A.S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem. 2004, 279, 34643–34654. [Google Scholar] [CrossRef] [PubMed]
- Crosas-Molist, E.; Bertran, E.; Sancho, P.; Lopez-Luque, J.; Fernando, J.; Sanchez, A.; Fernandez, M.; Navarro, E.; Fabregat, I. The NADPH oxidase NOX4 inhibits hepatocyte proliferation and liver cancer progression. Free Radic. Biol. Med. 2014, 69, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.P.; Nayak, B.; Shanmugasundaram, K.; Friedrichs, W.; Sudarshan, S.; Eid, A.A.; DeNapoli, T.; Parekh, D.J.; Gorin, Y.; Block, K. Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS ONE 2012, 7, e30712. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.V.; Love-Homan, L.; Sobhakumari, A.; Feddersen, C.R.; Koch, A.T.; Goel, A.; Simons, A.L. EGFR inhibition induces proinflammatory cytokines via NOX4 in HNSCC. Mol. Cancer Res. MCR 2013, 11, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Helfinger, V.; Henke, N.; Harenkamp, S.; Walter, M.; Epah, J.; Penski, C.; Mittelbronn, M.; Schroder, K. The NADPH Oxidase Nox4 mediates tumour angiogenesis. Acta Physiol. 2016, 216, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lan, T.; Zhang, C.; Zeng, C.; Hou, J.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget 2015, 6, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Sanders, Y.Y.; Liu, H.; Liu, G.; Thannickal, V.J. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic. Biol. Med. 2015, 79, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lan, T.; Hou, J.; Li, J.; Fang, R.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation of PI3K/Akt signaling. Oncotarget 2014, 5, 4392–4405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Grandi, C.; Alloisio, M.; Moglia, D.; Podrecca, S.; Sala, L.; Salvatori, P.; Molinari, R. Prognostic significance of lymphatic spread in head and neck carcinomas: Therapeutic implications. Head Neck Surg. 1985, 8, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kalnins, I.K.; Leonard, A.G.; Sako, K.; Razack, M.S.; Shedd, D.P. Correlation between prognosis and degree of lymph node involvement in carcinoma of the oral cavity. Am. J. Surg. 1977, 134, 450–454. [Google Scholar] [CrossRef]
- Schuller, D.E.; McGuirt, W.F.; McCabe, B.F.; Young, D. The prognostic significance of metastatic cervical lymph nodes. Laryngoscope 1980, 90, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Snow, G.B.; Annyas, A.A.; van Slooten, E.A.; Bartelink, H.; Hart, A.A. Prognostic factors of neck node metastasis. Clin. Otolaryngol. Allied. Sci. 1982, 7, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Yang, L.; Fu, S.W.; Lin, W.F.; Gao, Y.J.; Chen, H.Y.; Ge, Z.Z. Overexpression of NOX4 predicts poor prognosis and promotes tumor progression in human colorectal cancer. Oncotarget 2017, 8, 33586–33600. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.T.; Lin, X.L.; Wu, S.; Liang, Q.; Yang, L.; Gao, Y.J.; Ge, Z.Z. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell. Signal. 2018, 46, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ota, A.; Ono, T.; Nakaoka, T.; Wahiduzzaman, M.; Karnan, S.; Konishi, H.; Furuhashi, A.; Hayashi, T.; Yamada, Y.; et al. Inhibition of Nox1 induces apoptosis by attenuating the AKT signaling pathway in oral squamous cell carcinoma cell lines. Oncol. Rep. 2016, 36, 2991–2998. [Google Scholar] [CrossRef] [PubMed]
- Sobhakumari, A.; Schickling, B.M.; Love-Homan, L.; Raeburn, A.; Fletcher, E.V.; Case, A.J.; Domann, F.E.; Miller, F.J., Jr.; Simons, A.L. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol. Appl. Pharmacol. 2013, 272, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G.; Minguet, S.; Navarro-Lerida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibanez, T.; Pellinen, T.; Echarri, A. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011, 146, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.; Suchak, K.; Moutasim, K.A.; Vallath, S.; Hopper, C.; Jerjes, W.; Upile, T.; Kalavrezos, N.; Violette, S.M.; Weinreb, P.H. Stromal features are predictive of disease mortality in oral cancer patients. J. Pathol. 2011, 223, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Mellone, M.; Ford, K.; Thirdborough, S.M.; Mellows, T.; Frampton, S.J.; Smith, D.M.; Harden, E.; Szyndralewiez, C.; Bullock, M. Targeting the Myofibroblastic Cancer-Associated Fibroblast Phenotype through Inhibition of NOX4. J. Natl. Cancer Inst. 2018, 110, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Lo Muzio, L.; Santarelli, A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th Edition Oral Cavity Squamous Cell Carcinoma Staging—Is It an Improvement on the AJCC 7th Edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | |
|---|---|
| Age | 53 years old (26–86) |
| Sex | |
| Male | 148 (92%) |
| Female | 13 (8%) |
| Cigarette smoking | |
| Absent | 29 (18%) |
| Present | 132 (82%) |
| Alcohol consumption | |
| Absent | 32 (20%) |
| Present | 129 (80%) |
| T status | |
| 1 | 46 (29%) |
| 2 | 53 (33%) |
| 3 | 13 (8%) |
| 4 | 49 (30%) |
| N status | |
| 0 | 93 (58%) |
| 1 | 22 (14%) |
| 2 | 44 (27%) |
| 3 | 2 (1%) |
| Tumor stage | |
| I | 36 (22%) |
| II | 34 (21%) |
| III | 23 (14%) |
| IVA | 62 (39%) |
| IVB | 6 (4%) |
| Nox4 expression | |
| High | 80 (51%) |
| Low | 81 (49%) |
| Characteristics | |||
|---|---|---|---|
| High expression of Nox4 (N = 80) | Low expression of Nox4 (N = 81) | p-Value | |
| Age | 53 years old (32–86) | 51 years old (26–70) | |
| Sex | |||
| Male | 75 (94%) | 73 (90%) | 0.40 |
| Female | 5 (6%) | 8 (10%) | |
| Cigarette smoking | |||
| Absent | 14 (17%) | 15 (18%) | 0.87 |
| Present | 66 (83%) | 66 (82%) | |
| Alcohol consumption | |||
| Absent | 16 (20%) | 16 (20%) | 0.97 |
| Present | 64 (80%) | 65 (80%) | |
| T status | |||
| 1–2 | 47 (59%) | 52 (64%) | 0.48 |
| 3–4 | 33 (41%) | 29 (36%) | |
| N status | |||
| 0 | 46 (58%) | 47 (58%) | 0.95 |
| 1–3 | 34 (42%) | 34 (42%) | |
| Tumor stage | |||
| I–III | 35 (44%) | 35 (43%) | 0.95 |
| IVA–IVB | 45 (56%) | 46 (57%) | |
| Characteristics | No. of Patients | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median DFS (months) | p-Value | HR (95% CI) | p-Value | Median OS (months) | p-Value | HR (95% CI) | p-Value | ||
| Age | |||||||||
| <60 years | 127 (79%) | 77.5 | <0.001 * | NR | 0.001 * | ||||
| ≥60 years | 34 (21%) | 10.7 | 13.4 | ||||||
| Sex | |||||||||
| Male | 393 (97%) | 46.0 | 0.24 | 57.7 | 0.14 | ||||
| Female | 11 (3%) | NR | NR | ||||||
| Cigarette smoking | |||||||||
| Absent | 29 (18%) | NR | 0.047 * | NR | 0.026 * | ||||
| Present | 132 (82%) | 40.2 | 52.1 | ||||||
| Alcohol consumption | |||||||||
| Absent | 32 (20%) | 57.4 | 0.55 | NR | 0.42 | ||||
| Present | 129 (80%) | 41.2 | 64.5 | ||||||
| T status | |||||||||
| 1 + 2 | 99 (62%) | 77.5 | 0.004 * | NR | 0.001 * | 0.59 (0.38–0.92) | 0.019 * | ||
| 3 + 4 | 62 (38%) | 12.1 | 15.5 | ||||||
| N status | |||||||||
| 0 | 93 (58%) | NR | <0.001 * | 0.47 (0.27–0.83) | 0.009 * | NR | <0.001 * | 0.47 (0.30–0.73) | 0.001 * |
| 1 + 2 + 3 | 68 (42%) | 13.4 | 15.5 | ||||||
| Tumor stage | |||||||||
| I–III | 93 (58%) | NR | 0.025 * | NR | <0.001 * | ||||
| IVA–IVB | 68 (42%) | NR | 28.6 | ||||||
| Nox4 expression | |||||||||
| High | 80 (49%) | 21.9 | 0.030 * | 33.0 | 0.032 * | ||||
| Low | 81 (51%) | 77.5 | 0.50 (0.28–0.89) | 0.018 * | 87.9 | 0.57 (0.37–0.88) | 0.011 * | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Chien, C.-Y.; Fang, F.-M.; Huang, T.-L.; Su, Y.-Y.; Luo, S.-D.; Huang, C.-C.; Lin, W.-C.; Li, S.-H. Nox4 Overexpression as a Poor Prognostic Factor in Patients with Oral Tongue Squamous Cell Carcinoma Receiving Surgical Resection. J. Clin. Med. 2018, 7, 497. https://doi.org/10.3390/jcm7120497
Chen Y-H, Chien C-Y, Fang F-M, Huang T-L, Su Y-Y, Luo S-D, Huang C-C, Lin W-C, Li S-H. Nox4 Overexpression as a Poor Prognostic Factor in Patients with Oral Tongue Squamous Cell Carcinoma Receiving Surgical Resection. Journal of Clinical Medicine. 2018; 7(12):497. https://doi.org/10.3390/jcm7120497
Chicago/Turabian StyleChen, Yen-Hao, Chih-Yen Chien, Fu-Min Fang, Tai-Lin Huang, Yan-Ye Su, Sheng-Dean Luo, Chao-Cheng Huang, Wei-Che Lin, and Shau-Hsuan Li. 2018. "Nox4 Overexpression as a Poor Prognostic Factor in Patients with Oral Tongue Squamous Cell Carcinoma Receiving Surgical Resection" Journal of Clinical Medicine 7, no. 12: 497. https://doi.org/10.3390/jcm7120497
APA StyleChen, Y.-H., Chien, C.-Y., Fang, F.-M., Huang, T.-L., Su, Y.-Y., Luo, S.-D., Huang, C.-C., Lin, W.-C., & Li, S.-H. (2018). Nox4 Overexpression as a Poor Prognostic Factor in Patients with Oral Tongue Squamous Cell Carcinoma Receiving Surgical Resection. Journal of Clinical Medicine, 7(12), 497. https://doi.org/10.3390/jcm7120497








