Effects of Macrolide Treatment during the Hospitalization of Children with Childhood Wheezing Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Selection
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
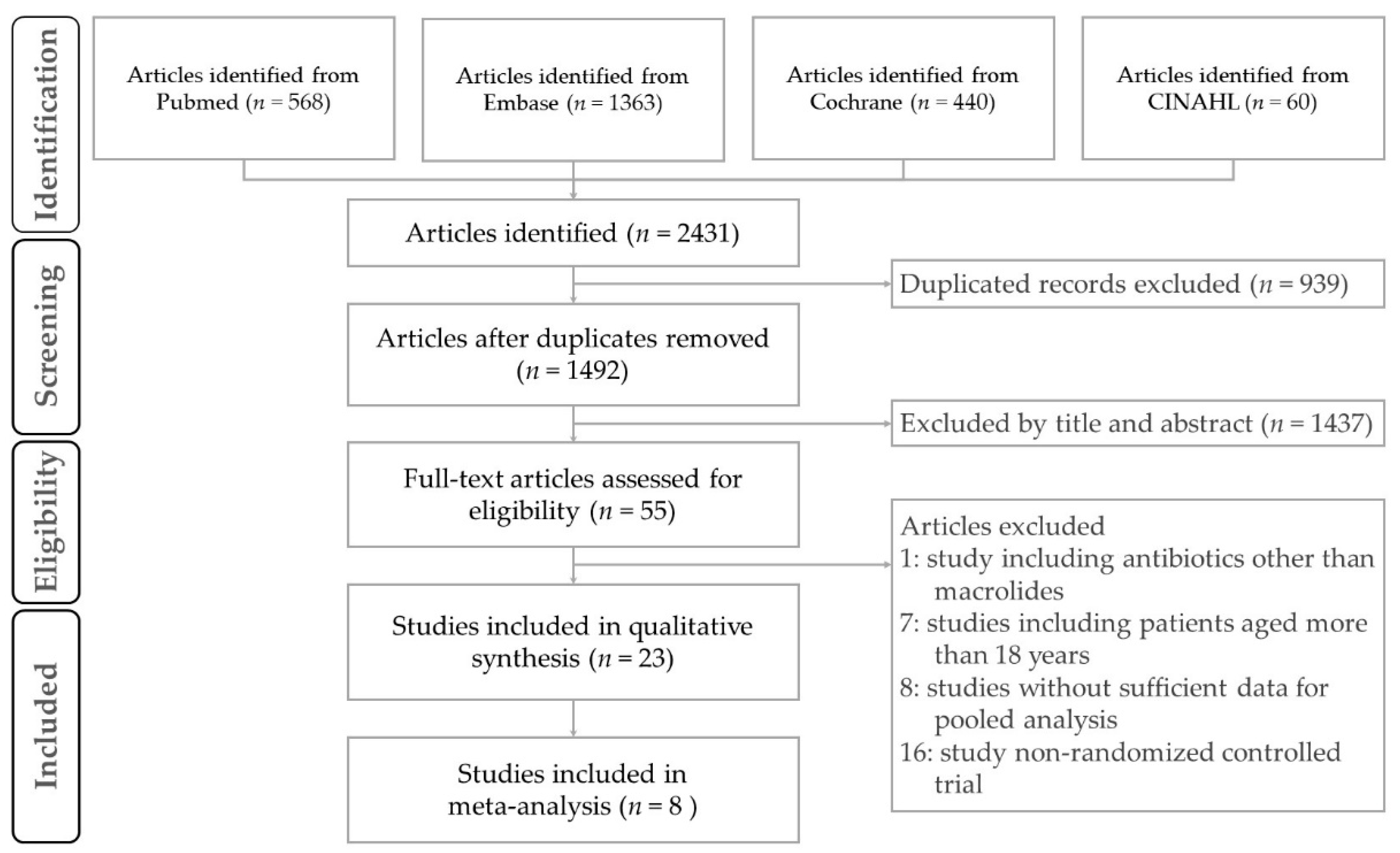
3. Results
3.1. Description of Studies and Quality Assessment
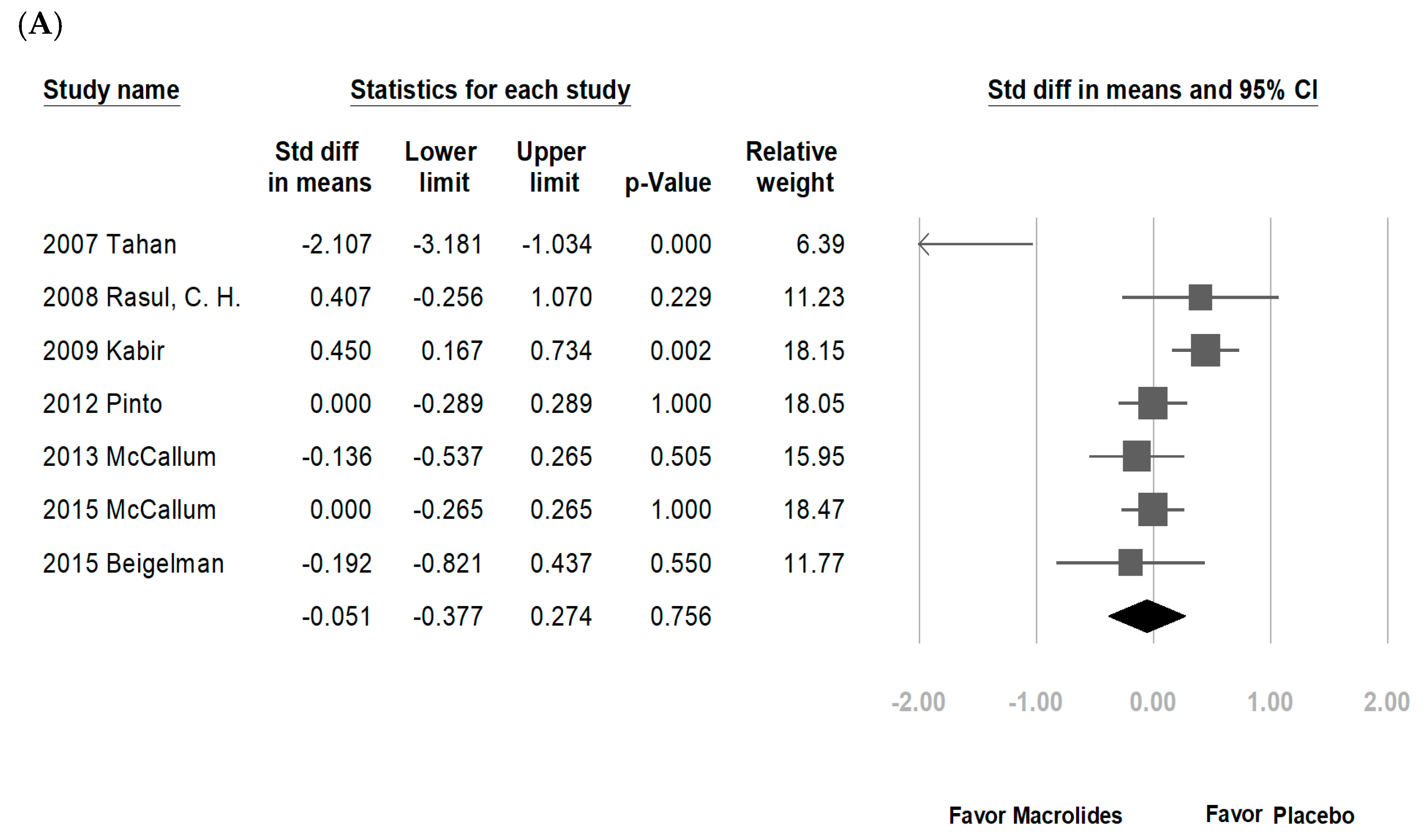
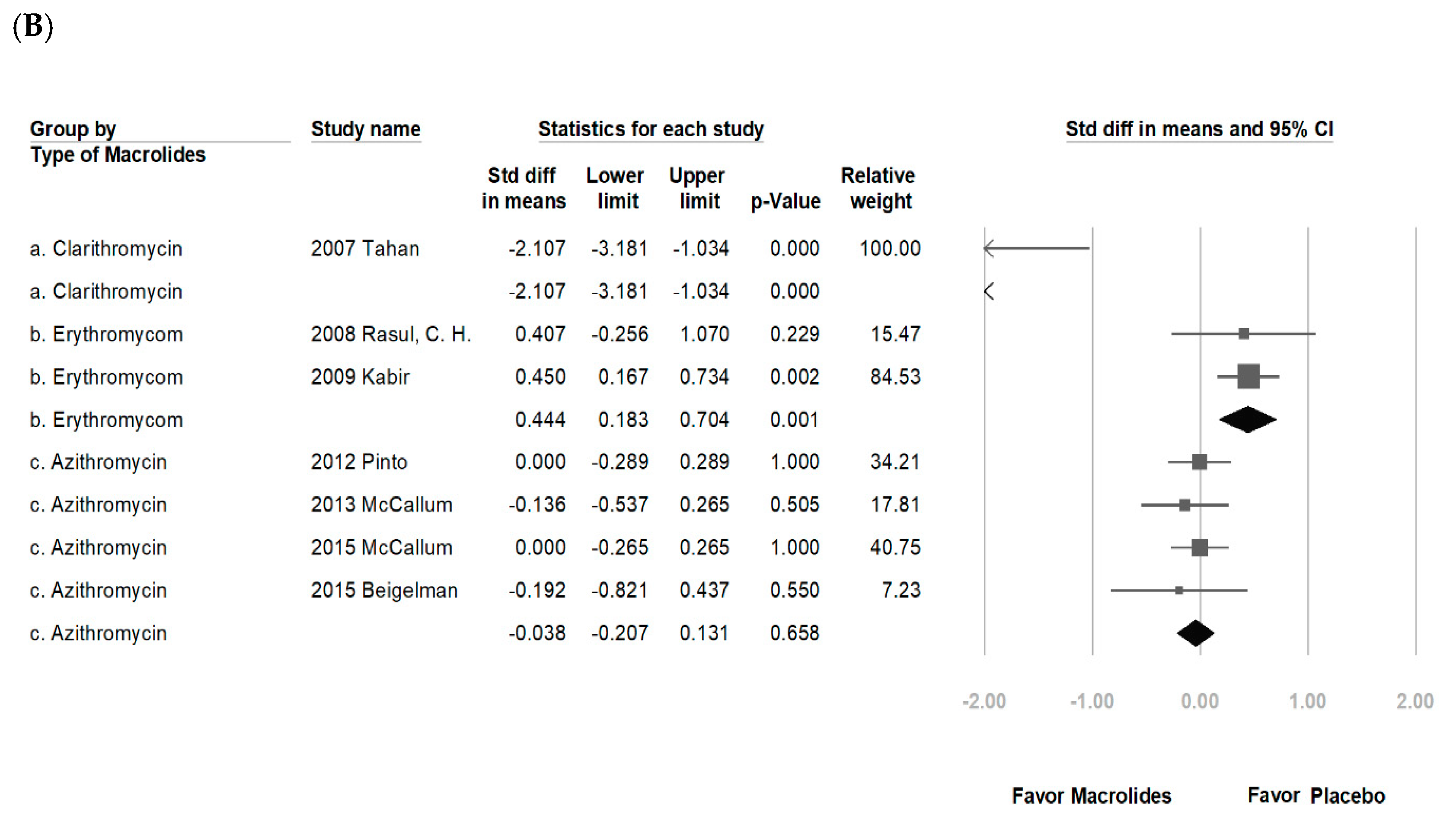
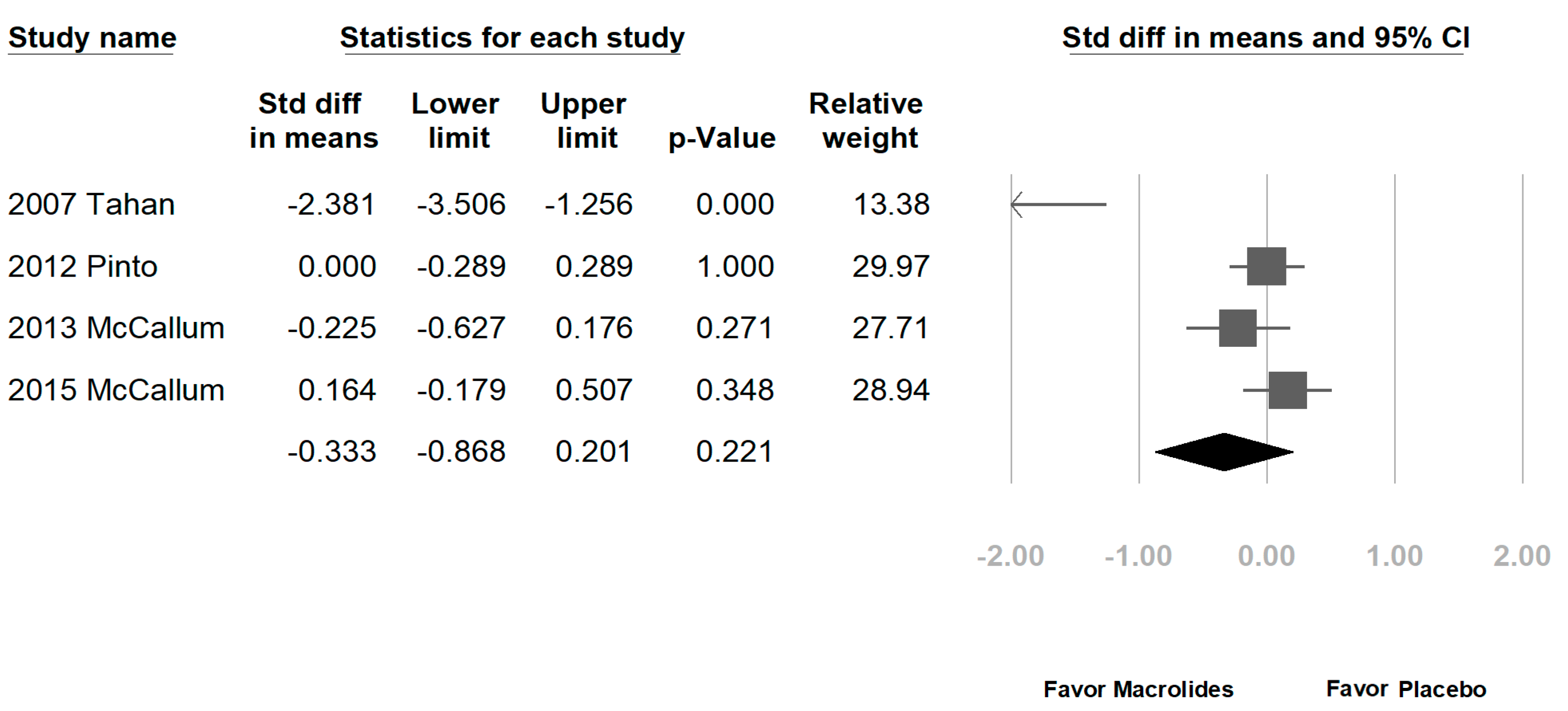
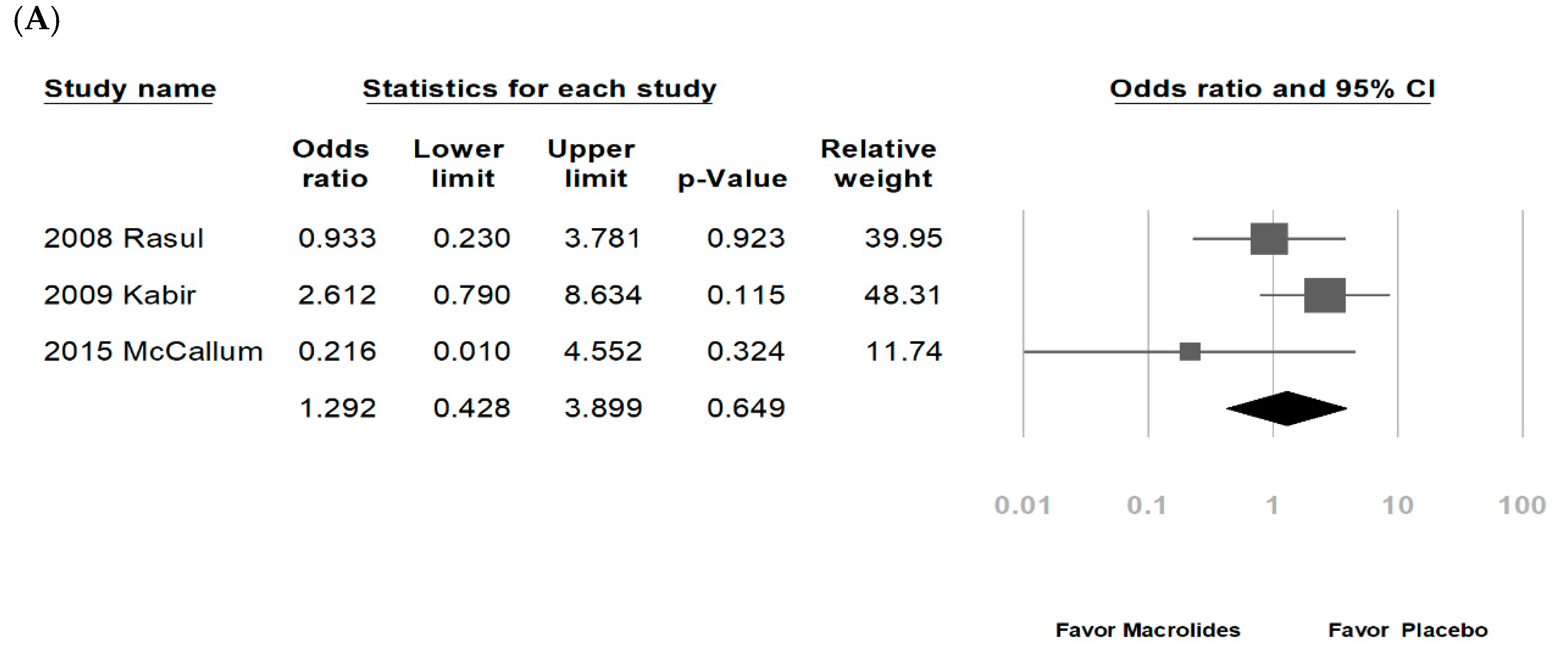
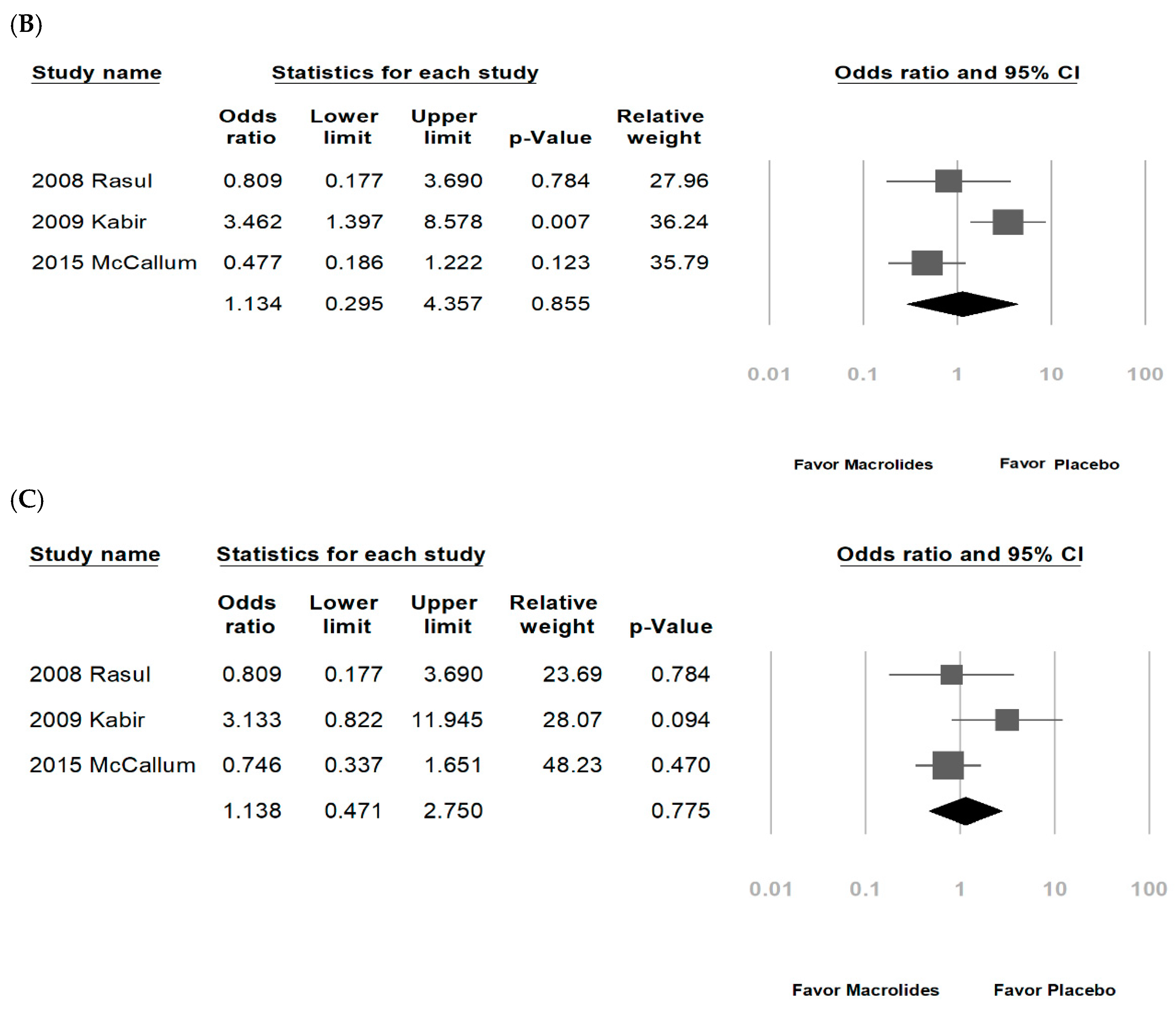
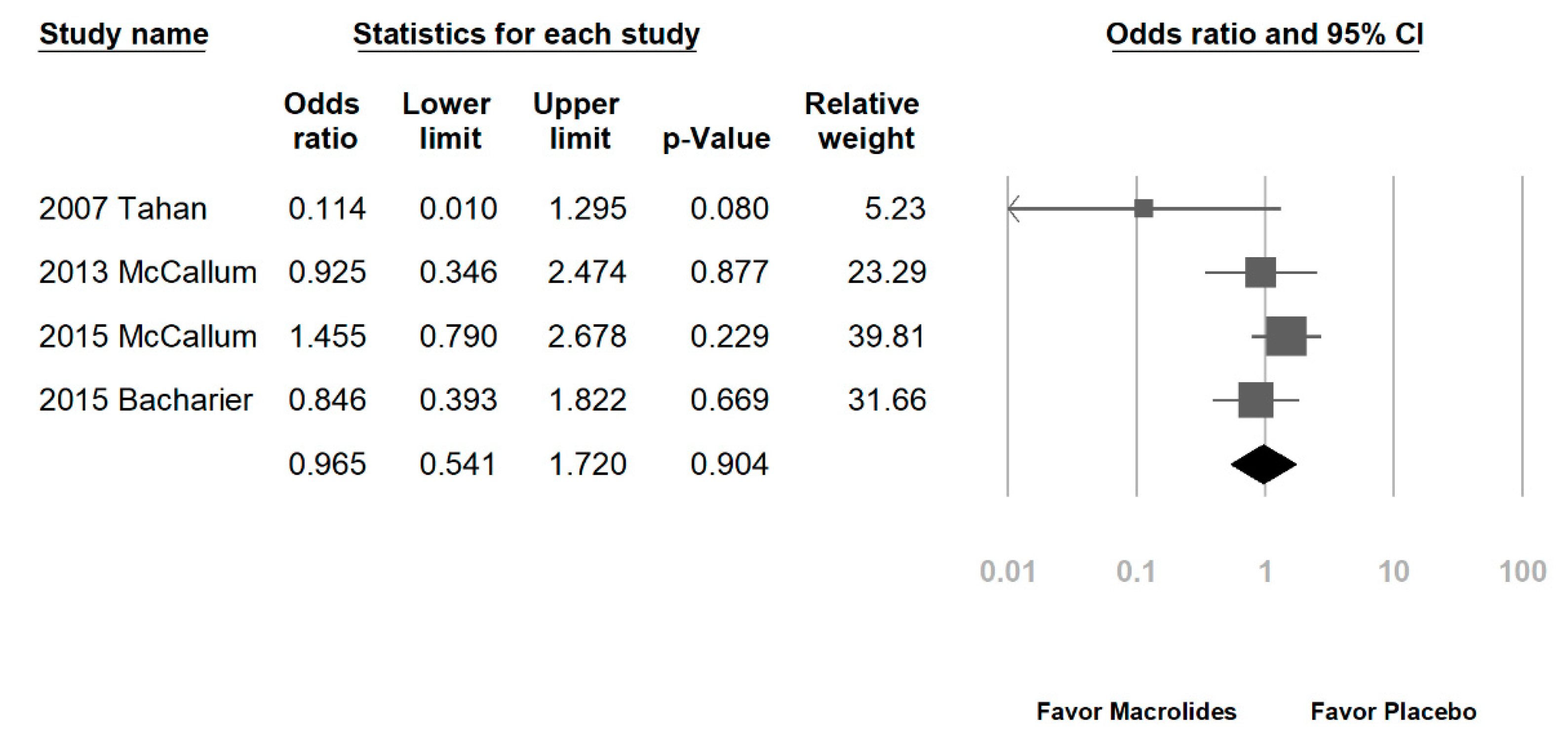
3.2. Data Synthesis and Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: Isaac phases one and three repeat multicountry cross-sectional surveys. Lancet (London, England) 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Subbarao, P.; Mandhane, P.J.; Sears, M.R. Asthma: Epidemiology, etiology and risk factors. CMAJ Can. Med. Assoc. J. 2009, 181, E181–E190. [Google Scholar] [CrossRef] [PubMed]
- Backman, K.; Piippo-Savolainen, E.; Ollikainen, H.; Koskela, H.; Korppi, M. Increased asthma risk and impaired quality of life after bronchiolitis or pneumonia in infancy. Pediatr. Pulmonol. 2014, 49, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Muglia, C.; Oppenheimer, J. Wheezing in infancy: An overview of recent literature. Curr. Allergy Asthma Rep. 2017, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Asher, I.; Pearce, N. Global burden of asthma among children. Int. J. Tuberc. Lung Dis. 2014, 18, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.B.; Hsia, R.Y. Children’s emergency department use for asthma, 2001–2010. Acad. Pediatr. 2015, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Spurling, G.K.; Fonseka, K.; Doust, J.; Del Mar, C. Antibiotics for bronchiolitis in children. Cochrane Database Syst. Rev. 2007, Cd005189. [Google Scholar]
- Steel, H.C.; Theron, A.J.; Cockeran, R.; Anderson, R.; Feldman, C. Pathogen- and host-directed anti-inflammatory activities of macrolide antibiotics. Med. Inflamm. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, J.; de Graaff, C.S.; van der Werf, T.S.; Boersma, W.G. Immunomodulatory effects of macrolide antibiotics—Part 1: Biological mechanisms. Respiration 2011, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ziesenitz, V.C.; Curtis, N.; Ritz, N. The immunomodulatory effects of macrolides—A systematic review of the underlying mechanisms. Front. Immunol. 2018, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Chatila, T.A. Innate immunity in asthma. N. Eng. J. Med. 2016, 375, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, S.W.; Park, C.S.; Kim, B.I.; Kang, H.; Koh, Y.Y. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J. Allergy Clin. Immunol. 2003, 112, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kew, K.M.; Undela, K.; Kotortsi, I.; Ferrara, G. Macrolides for chronic asthma. Cochrane Database Syst. Rev. 2015, Cd002997. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, S.; Lorrot, M.; Marc, E.; Moulin, F.; Boutonnat-Faucher, B.; Heilbronner, C.; Iniguez, J.-L.; Chaussain, M.; Nicand, E.; Raymond, J.; et al. Mycoplasma pneumoniae and asthma in children. Clin. Infect. Dis. 2004, 38, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J. The role of mycoplasma pneumoniae infection in asthma. Allergy Asthma Immunol. Res. 2012, 4, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.D.; Hill, M.R.; Brennor, M.; Sanks, R.; Szefler, S.J. Effect of low-dose troleandomycin on glucocorticoid pharmacokinetics and airway hyperresponsiveness in severely asthmatic children. Ann. Allergy 1990, 65, 37–45. [Google Scholar] [PubMed]
- Kamada, A.K.; Hill, M.R.; Ikle, D.N.; Brenner, A.M.; Szefler, S.J. Efficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthma. J. Allergy Clin. Immunol. 1993, 91, 873–882. [Google Scholar] [CrossRef]
- Fonseca-Aten, M.; Okada, P.J.; Bowlware, K.L.; Chavez-Bueno, S.; Mejias, A.; Rios, A.M.; Katz, K.; Olsen, K.; Ng, S.; Jafri, H.S.; et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: A double-blind, randomized, placebo-controlled trial. Ann. Allergy Asthma Immunol. 2006, 97, 457–463. [Google Scholar] [CrossRef]
- Piacentini, G.L.; Peroni, D.G.; Bodini, A.; Pigozzi, R.; Costella, S.; Loiacono, A.; Boner, A.L. Azithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: A preliminary report. Allergy Asthma Proc. 2007, 28, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Tahan, F.; Ozcan, A.; Koc, N. Clarithromycin in the treatment of RSV bronchiolitis: A double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 2007, 29, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rasul, C.H.; Kabir, A.R.M.L.; Rashid, A.K.M.M.; Mahboob, A.A.; Hassan, M.A. Role of antibiotic in the outcome of bronchiolitis. Pak. J. Med. Sci. 2008, 24, 707–711. [Google Scholar]
- Strunk, R.C.; Bacharier, L.B.; Phillips, B.R.; Szefler, S.J.; Zeiger, R.S.; Chinchilli, V.M.; Martinez, F.D.; Lemanske, R.F., Jr.; Taussig, L.M.; Mauger, D.T.; et al. Azithromycin or montelukast as inhaled corticosteroid-sparing agents in moderate-to-severe childhood asthma study. J. Allergy Clin. Immunol. 2008, 122, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.R.M.L.; Mollah, A.H.; Anwar, K.S.; Rahman, A.K.M.F.; Amin, R.; Rahman, M.E. Management of bronchiolitis without antibiotics: A multicentre randomized control trial in Bangladesh. Acta Paediatr. 2009, 98, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Koutsoubari, I.; Papaevangelou, V.; Konstantinou, G.N.; Makrinioti, H.; Xepapadaki, P.; Kafetzis, D.; Papadopoulos, N.G. Effect of clarithromycin on acute asthma exacerbations in children: An open randomized study. Pediatr. Allergy Immunol. 2012, 23, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.A.; Pitrez, P.M.; Luisi, F.; de Mello, P.P.; Gerhardt, M.; Ferlini, R.; Barbosa, D.C.; Daros, I.; Jones, M.H.; Stein, R.T.; et al. Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: A randomized, double-blinded, and placebo-controlled clinical trial. J. Pediatr. 2012, 161, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- McCallum, G.B.; Morris, P.S.; Chatfield, M.D.; Maclennan, C.; White, A.V.; Sloots, T.P.; Mackay, I.M.; Chang, A.B. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: A randomised, placebo-controlled trial. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chiong-Manaysay, K.; Andaya, A. Effectiveness of macrolide (clarithromycin) treatment on pediatric patients with mild-moderate persistent asthma: A randomised controlled study. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 291. [Google Scholar]
- Youssef, D.; Elbhedy, R.; Salah, H. P83-asthma inflammatory subtype specific treatment; a randomised clinical study. Clin. Transl. Allergy 2014, 4, 135. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Guilbert, T.W.; Mauger, D.T.; Boehmer, S.; Beigelman, A.; Fitzpatrick, A.M.; Jackson, D.J.; Baxi, S.N.; Benson, M.; Burnham, C.A.D.; et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses a randomized clinical trial. JAMA 2015, 314, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Beigelman, A.; Bacharier, L.B.; Baty, J.; Buller, R.; Mason, S.; Schechtman, K.B.; Sajol, G.; Isaacson-Schmid, M.; Castro, M.; Storch, G.A. Does azithromycin modify viral load during severe respiratory syncytial virus bronchiolitis? J. Allergy Clin. Immunol. 2015, 136, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Beigelman, A.; Isaacson-Schmid, M.; Sajol, G.; Baty, J.; Rodriguez, O.M.; Leege, E.; Lyons, K.; Schweiger, T.L.; Zheng, J.; Schechtman, K.B.; et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2015, 135, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- McCallum, G.B.; Morris, P.S.; Grimwood, K.; Maclennan, C.; White, A.V.; Chatfield, M.D.; Sloots, T.P.; Mackay, I.M.; Smith-Vaughan, H.; McKay, C.C.; et al. Three-weekly doses of azithromycin for indigenous infants hospitalized with bronchiolitis: A multicentre, randomized, placebo-controlled trial. Front. Pediatr. 2015, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- D’Azevedo Silveira, V.; Roza, C.A.; Luisi, F.; Pitrez, P.M.; Stein, R.T.; Pinto, L.A. Azithromycin therapy in infants with bronchiolitis reduces recurrent wheezing 3 months after hospitalization: A randomized, placebo-controlled trial. Pediatr. Pulm. 2016, 51, S9. [Google Scholar]
- Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Pedersen, T.M.; Vinding, R.K.; Schoos, A.M.M.; Wolsk, H.M.; Thorsteinsdóttir, S.; Hallas, H.W.; et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 19–26. [Google Scholar] [CrossRef]
- Wan, K.S.; Liu, Y.C.; Huang, C.S.; Su, Y.M. Effects of low-dose clarithromycin added to fluticasone on inflammatory markers and pulmonary function among children with asthma: A randomized clinical trial. Allergy Rhinol. 2016, 7, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bacharier, L.B.; Isaacson-Schmid, M.; Baty, J.; Schechtman, K.B.; Sajol, G.; Wylie, K.; Storch, G.A.; Castro, M.; Beigelman, A. Azithromycin therapy during respiratory syncytial virus bronchiolitis: Upper airway microbiome alterations and subsequent recurrent wheeze. J. Allergy Clin. Immunol. 2016, 138, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Mandhane, P.J.; Paredes Zambrano De Silbernagel, P.; Nwe Aung, Y.; Williamson, J.; Lee, B.E.; Spier, S.; Noseworthy, M.; Craig, W.R.; Johnson, D.W. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: A placebo-controlled randomized trial. PLoS ONE 2017, 12, e0182411. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.A.; Jones, M.H.; Pitrez, P.M.; Stein, R.T. Azithromycin administered at the time of severe bronchiolitis has a protective effect on subsequent wheezing in infants. Pediatr. Pulm. 2017, 52, S165–S166. [Google Scholar]
- Jartti, T.; Gern, J.E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 2017, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Unger, S.A.; Bogaert, D. The respiratory microbiome and respiratory infections. J. Infect. 2017, 74, S84–S88. [Google Scholar] [CrossRef]
- Hu, J.J.; Lu, C.Y.; Chang, L.Y.; Huang, C.H.; Chou, C.C.; Huang, F.Y.; Lee, C.Y.; Huang, L.M. Survey of pertussis in patients with prolonged cough. J. Microbiol. Immunol. Infect. 2006, 39, 54–58. [Google Scholar] [PubMed]
- Marchello, C.; Dale, A.P.; Thai, T.N.; Han, D.S.; Ebell, M.H. Prevalence of atypical pathogens in patients with cough and community-acquired pneumonia: A meta-analysis. Ann. Fam. Med. 2016, 14, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.A.; Arredondo, S.M.; Bono, M.R.; Gaggero, A.A.; Diaz, P.V. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics 2006, 117, e878–e886. [Google Scholar] [CrossRef] [PubMed]
- Kips, J.C. Cytokines in asthma. Eur. Respir. J. 2001, 34, 24s–33s. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.L.; Albert, R.K. Chronic macrolide therapy in inflammatory airways diseases. Chest 2010, 138, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.; Porter, J.D.; Edwards, M.R.; Johnston, S.L. The role of macrolides in asthma: Current evidence and future directions. Lancet Respir. Med. 2014, 2, 657–670. [Google Scholar] [CrossRef]
- Blyth, C.C.; Gerber, J.S. Macrolides in children with community-acquired pneumonia: Panacea or placebo? J. Pediatr. Infect. Dis. Soc. 2018, 7, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Buller, R.; Bledsoe, S.; Storch, G.A. Rising rates of macrolide-resistant mycoplasma pneumoniae in the central united states. Pediatr. Infect. Dis. J. 2012, 31, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Macrolide-resistant mycoplasma pneumoniae: Its role in respiratory infection. J. Antimicrob. Chemother. 2013, 68, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.M.; Lewis, W.D.; Smith, E.A.; Tracy, L.R.; Moerschel, S.K. Pertussis: A reemerging infection. Am. Fam. Physician 2013, 88, 507–514. [Google Scholar] [PubMed]
- Bacharier, L.B.; Cohen, R.; Schweiger, T.; Yin-Declue, H.; Christie, C.; Zheng, J.; Schechtman, K.B.; Strunk, R.C.; Castro, M. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012, 130, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Yoshida, M.; Suzuki, T.; Hatachi, Y.; Sasaki, T.; Yasuda, H.; Nakayama, K.; Nishimura, H.; Nagatomi, R.; Kubo, H.; et al. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antivir. Res. 2009, 83, 191–200. [Google Scholar] [CrossRef] [PubMed]
| Study Author, Year [Ref] | Country | Study Population (M%: F%) | Severity, Diagnosis | Exclude Bacterial Infection? | Detect Pathogens? | Age | Macrolide Used | Dose, Interval | Duration | Concomitant Medication | Outcome Measure | Benefits of Macrolide? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ball, 1990 [22] | USA | 15 (60:40) | Severe asthma | N | NR | 8–18 years | Troleando-mycin | 250 mg QD × 2 days the QOD | 14 days | Methyl-prednisolone |
| Y; increase steroid dose reduction and decrease bronchial hyperresonsiveness to methacholine |
| Kamada, 1993 [23] | USA | 18 (36:64) | Severe asthma | NR | NR | 6–17 years | Troleando-mycin | 250 mg QD or QOD | 12 weeks | Prednisolone, bronchodilator, theophylline, ICS |
| Y; increase steroid dose reduction |
| Fonseca-Aten, 2006 [24] | USA | 43 (74:26) | Recurrent wheezing, ED | Y; Exclude patients with bacterial infection | Y; evidences of M pneumoniae or C peumoniae: 53% | 4–17 years | Clarithromycin | 15 mg/kg/day, BID | 5 days | β2-agonist and/or ICS |
| Y; decrease nasopharyngeal cytokine levels |
| Piacentini, 2007 [25] | Italy | 16 (75:25) | Hospitalized, asthma | Y; None with airway infection in the month before and during study | NR | 13.9 years | Azithromycin | 10 mg/kg QD for 3 consecutive days/week | 8 weeks | ICS, β2-agonist as needed |
| Y; decrease bronchial hyperresponsiveness and sputum neutrophil percentage |
| Tahan, 2007 [26] * | Turkey | 21 (57:43) | Hospitalized, RSV bronchiolitis | NR | Y; RSV | ≤7 months | Clarithromycin | 15 mg/kg QD | 3 weeks | β2-agonist |
| Y; decrease LOS, DOD |
| Rasul, 2008 [27] * | Bangladesh | 60 (72:28) | Hospitalized, bronchiolitis | N | NR | 0–2 years (80% below 6 months) | Erythromycin | NR | ND | O2 |
| N; no statistically significant differences found |
| Strunk, 2008 [28] | USA | 55 (58:42) | moderate to severe persistent asthma | NR | Y; no M pneumoniae, C pneumoniae was detected by PCR | 6–18 years | Azithromycin | 250 mg or 500 mg QD | ND | ICS |
| N; no differences in time to inadequate asthma control |
| Kabir, 2009 [29] * | Bangladesh | 295 (73:27) | Hospitalized bronchiolitis | Y; Exclude patients with antibiotics use | NR | <24 months | Erythromycin | 10 mg/kg/dose 6 hourly | ND | Inhaled bronchodilator, O2 |
| N; no differences among groups |
| Koutsoubari, 2012 [30] | Greece | 40 (45:55) | Intermittent/mild persistent asthma | NR | Y; 18 rhinovirus, 3 adenovirus, 2 M pneumoniae, 2 parainfluenza, 1 RSV, and no C pneumoniae | 6–14 years | Clarithromycin | 15 mg/kg | 3 weeks | ICS |
| Y; increase symptom-free days and improve asthma control |
| Pinto, 2012 [31] * | Brazil | 184 (60:40) | Hospitalized, bronchiolitis | Y; exclude Chlamydia spp or Bordetella pertussis respiratory infection | Y; RSV, influenza, and parainfluenza | ≤2 months | Azithromycin | 10 mg/kg/day | 7 days | Antibiotics, Steroid, bronchodilator |
| N; no differences in LOS, DOD, detected viruses |
| McCallum, 2013 [32] * | Australian/New Zealand | 96 (68:32) | Hospitalized, bronchiolitis | Y; exclude patients with macrolide treatment or diagnoses of pneumonia | Y; RSV, rhinovirus, metapneumovirus, corona virus, and bacteria | ≤18 months | Azithromycin | 30 mg/kg | Single dose | Antibiotics |
| N; no differences in LOS, DOD, and re-admission rates |
| Chiong-Manaysay, 2014 [33] | Philippines | 23 | FEV1 <80% before treatment | NR | Y; 1 (4.8%) positive for M pneumoniae | Children | Clarithromycin | 15 mg/kg/day bid | 3 weeks | NR |
| Y; improved asthma control and FEV1 |
| Youssef D, 2014 [34] | Greece | 80 (44:36) | Persistent asthma | NR | NR | 11.5 years | Clarithromycin | 15 mg/kg bid | 8 weeks | ICS, β2-agonist |
| N; significant decrease of neutrophils |
| Bacharier, 2015 [35] * | USA | 443 (62:38) | recurrent, severe wheezing | Y; exclude patients received antibiotics within the past month for any indication | Y; viral pathogens were detected in 47% of children in the azithromycin group and 43%in the placebo group | 12–71 months | Azithromycin | 12 mg/kg/day | 5 days | β2-agonist |
| Y, lower risk to progress to severe LRTI |
| Beigelman, 2015 [36] * | USA | 39 (59:41) | Hospitalized, RSV bronchiolitis | Y; treatment with any antibiotics within past 2 weeks (4 weeks for macrolide antibiotics) | Y; RSV | 1–18 months | Azithromycin | 10 mg/kg/day × 7 days then 5 mg/kg/day × 7 days | 14 days | Antibiotic |
| Y; decrease of nasal lavage IL-8 levels but not serum IL-8 levels. ≥2 additional wheezing episodes after treatment: not different |
| Beigelman, 2015 [37] | USA | 39 (59:41) | Hospitalized, RSV bronchiolitis | Y; exclude patients with treatment with any antibiotics within past 2 weeks (4 weeks for macrolide antibiotics) | Y; RSV | 1–18 months | Azithromycin | 10 mg/kg/day × 7 days then 5mg/kg/day × 7 days | 14 days | Antibiotic |
| N; azithromycin-treated group had lower RSV clearance |
| McCallum, 2015 [38] * | Australia/New Zealand | 219 (62:38) | Hospitalized, bronchiolitis | Y; exclude patients received macrolides within last seven-days, or a primary pneumonia; non-macrolide antibiotics: 43% | Y; RSV (42%), rhinovirus (37%), adenovirus (7%), etc. | ≤24 months | Azithromycin | 30 mg/kg/dose weekly | 3 weeks | Non-macrolide antibiotics |
| Y; no differences of LOS, DOD and readmission. Nasopharyngeal bacteria were less common in azithromycin group |
| D’Azevedo Silveira, 2016 [39] | Brazil | 91 | Hospitalized, bronchiolitis | NR | NR | <12 months | Azithromycin | ND | 7 days | NR |
| Y; readmission was not different but azithromycin group had lower recurrent wheezing |
| Stokholm, 2016 [40] | Denmark | 72 (65:35) | recurrent asthma-like symptoms, troublesome lung symptoms ≥3 days | Y; exclude patients with signs of pneumonia | Y; any virus (43%), any bacteria (67%, H influenzae, S pneumoniae, M catarrhalis); not modify treatment effects | 1–3 years | Azithromycin | 10 mg/kg/day | 3 days | ICS, Montelukast |
| Y; azithromycin shortened the symptomatic period and the duration of β2 agonist use. Time to next episode was not different. |
| Wan, 2016 [41] | Taiwan | 56 (63:37) | Mild persistent asthma | NR | Y; positive M pneumoniae: 58.3% for IgG and 41.7% for IgM in the study group, and 65.0% for IgG and 35.0% for IgM in the control group | 5–16 years | Clarithromycin | 5 mg/kg/day | 4 weeks | ICS |
| Y; improve pulmonary function and decrease eosinophilic inflammation and disease severity |
| Zhou, 2016 [42] | USA | 39 (59:41) | Hospitalized, RSV bronchiolitis | Y; exclude patients with treatment with any antibiotics within past 2 weeks (4 weeks for macrolide antibiotics) | Y; RSV and bacteria (Moraxella) | 1–18 months | Azithromycin | 10 mg/kg/day × 7 days then 5 mg/kg/day × 7 days | 14 days | Antibiotic |
| Y; the relative abundance of Moraxella decreased significantly |
| Mandhane, 2017 [43] | Canada | 222 (72:28) | Wheezing, ED | Y; exclude patients with antibiotics use in the past 30 days | NR | 12–60 months | Azithromycin | 10 mg/kg/day at day 1 then 5 mg/kg/day × 4 days (day 2–5) | 5 days | ICS, β2-agonist |
| N |
| Pinto, 2017 [44] | Brazil | 83 | Hospitalized, bronchiolitis | NR | Y; RSV | <12 months | Azithromycin | ND | 7 days | NR |
| Y; subsequent wheezing was significant reduced. The readmission rate was not different. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Yeh, T.-L.; Liu, S.-J.; Lin, H.-H.; Cheng, Y.-J.; Hung, H.-H.; Tsai, M.-C.; Liu, J.-M.; Lei, W.-T. Effects of Macrolide Treatment during the Hospitalization of Children with Childhood Wheezing Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2018, 7, 432. https://doi.org/10.3390/jcm7110432
Lin C-Y, Yeh T-L, Liu S-J, Lin H-H, Cheng Y-J, Hung H-H, Tsai M-C, Liu J-M, Lei W-T. Effects of Macrolide Treatment during the Hospitalization of Children with Childhood Wheezing Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2018; 7(11):432. https://doi.org/10.3390/jcm7110432
Chicago/Turabian StyleLin, Chien-Yu, Tzu-Lin Yeh, Shu-Jung Liu, Hsin-Hui Lin, Yu-Jyun Cheng, Hua-His Hung, Mu-Chieh Tsai, Jui-Ming Liu, and Wei-Te Lei. 2018. "Effects of Macrolide Treatment during the Hospitalization of Children with Childhood Wheezing Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 7, no. 11: 432. https://doi.org/10.3390/jcm7110432
APA StyleLin, C.-Y., Yeh, T.-L., Liu, S.-J., Lin, H.-H., Cheng, Y.-J., Hung, H.-H., Tsai, M.-C., Liu, J.-M., & Lei, W.-T. (2018). Effects of Macrolide Treatment during the Hospitalization of Children with Childhood Wheezing Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 7(11), 432. https://doi.org/10.3390/jcm7110432







