Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Qualification of Horses
2.2. ASC Isolation and Culture
2.3. Extraction and Culture of Peripheral Blood Mononuclear Cells (PBMC)
2.4. Flow Cytometry Analysis
2.4.1. ASC-PBMC Co-Culture
2.4.2. ASC-RAW264.7 Co-Culture
2.5. Nitric Oxide (NO) and Superoxide Dismutase (SOD) Levels Analysis
2.6. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. ELISA Tests
2.8. Statistical Analysis
3. Results
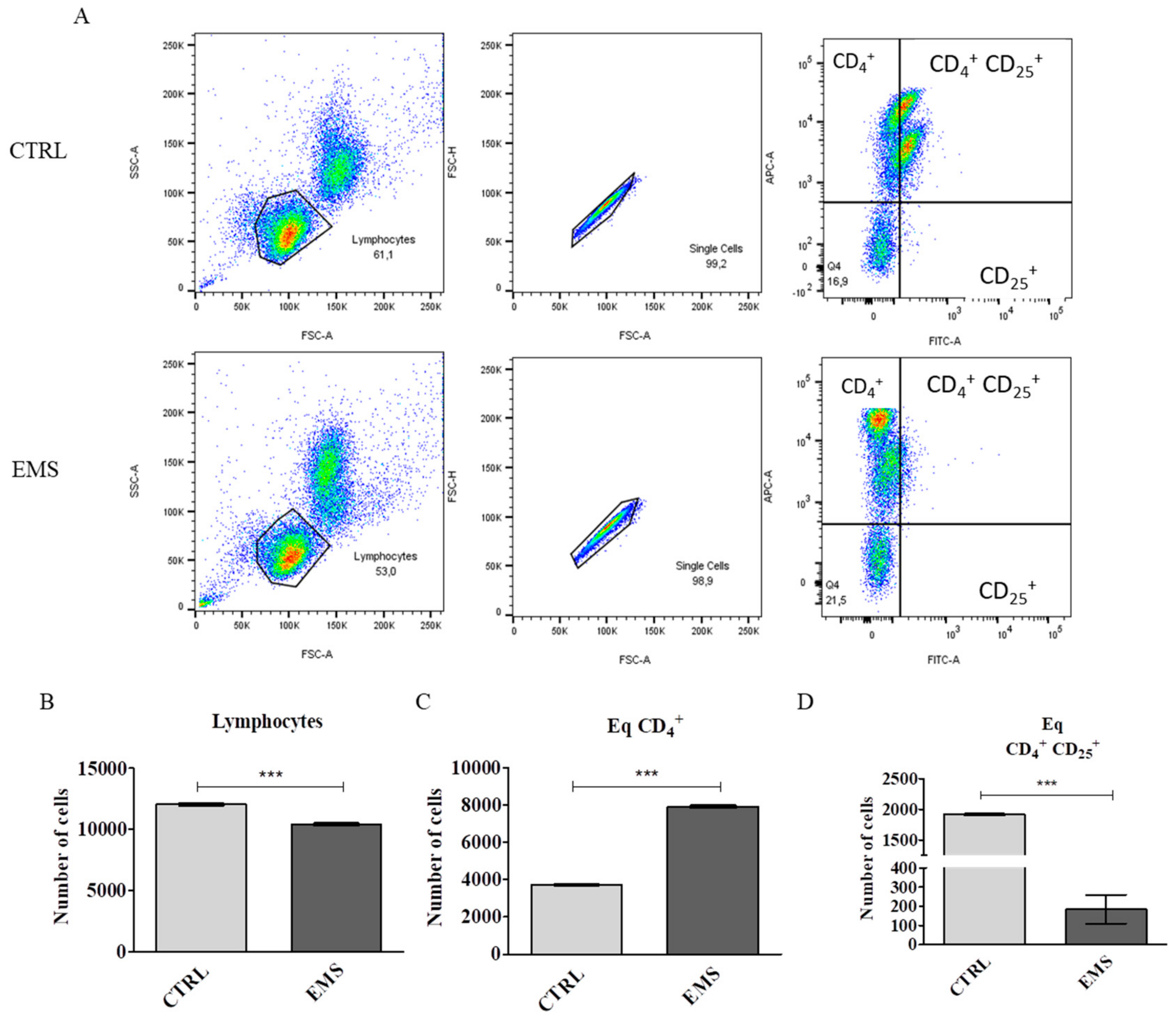
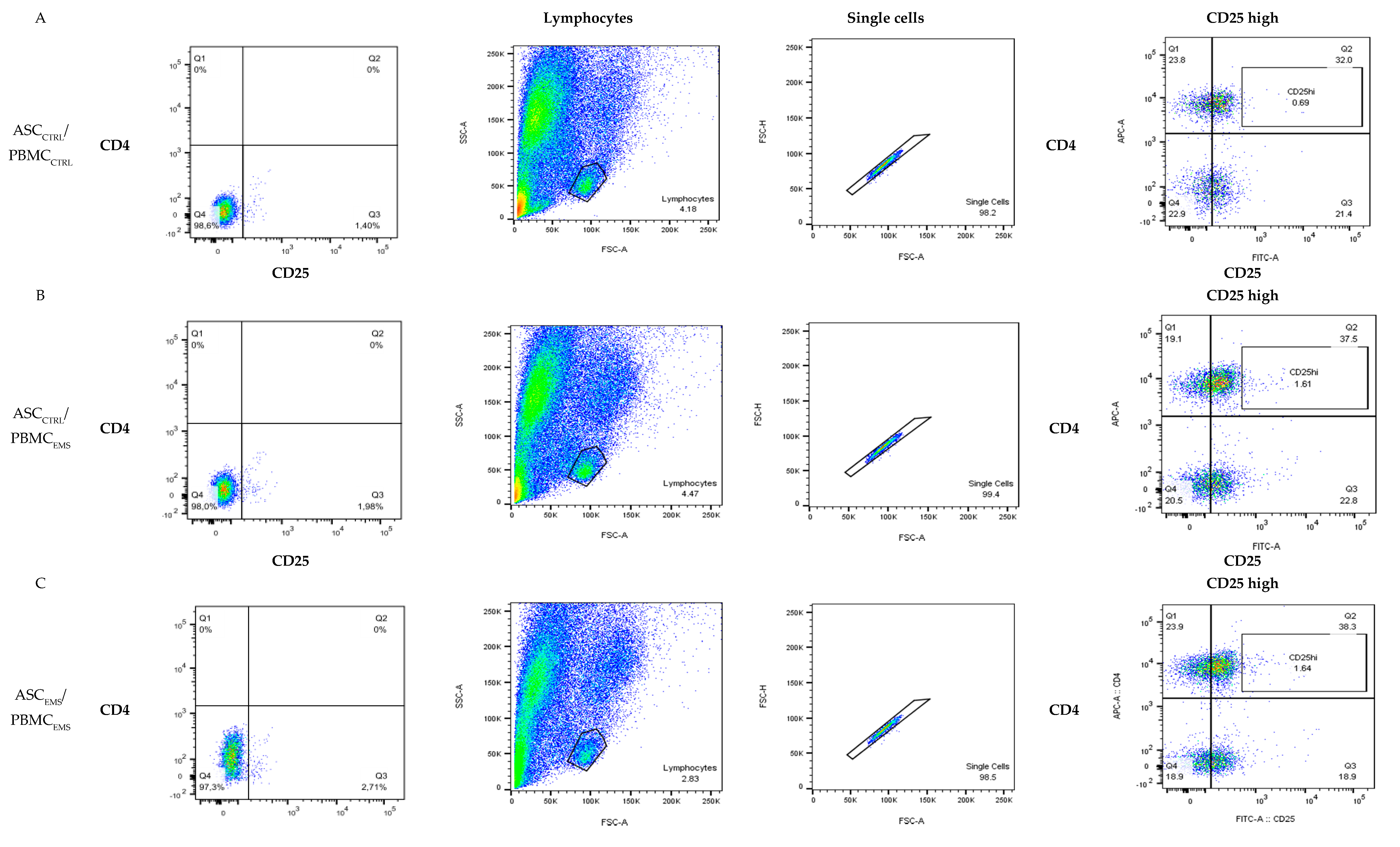
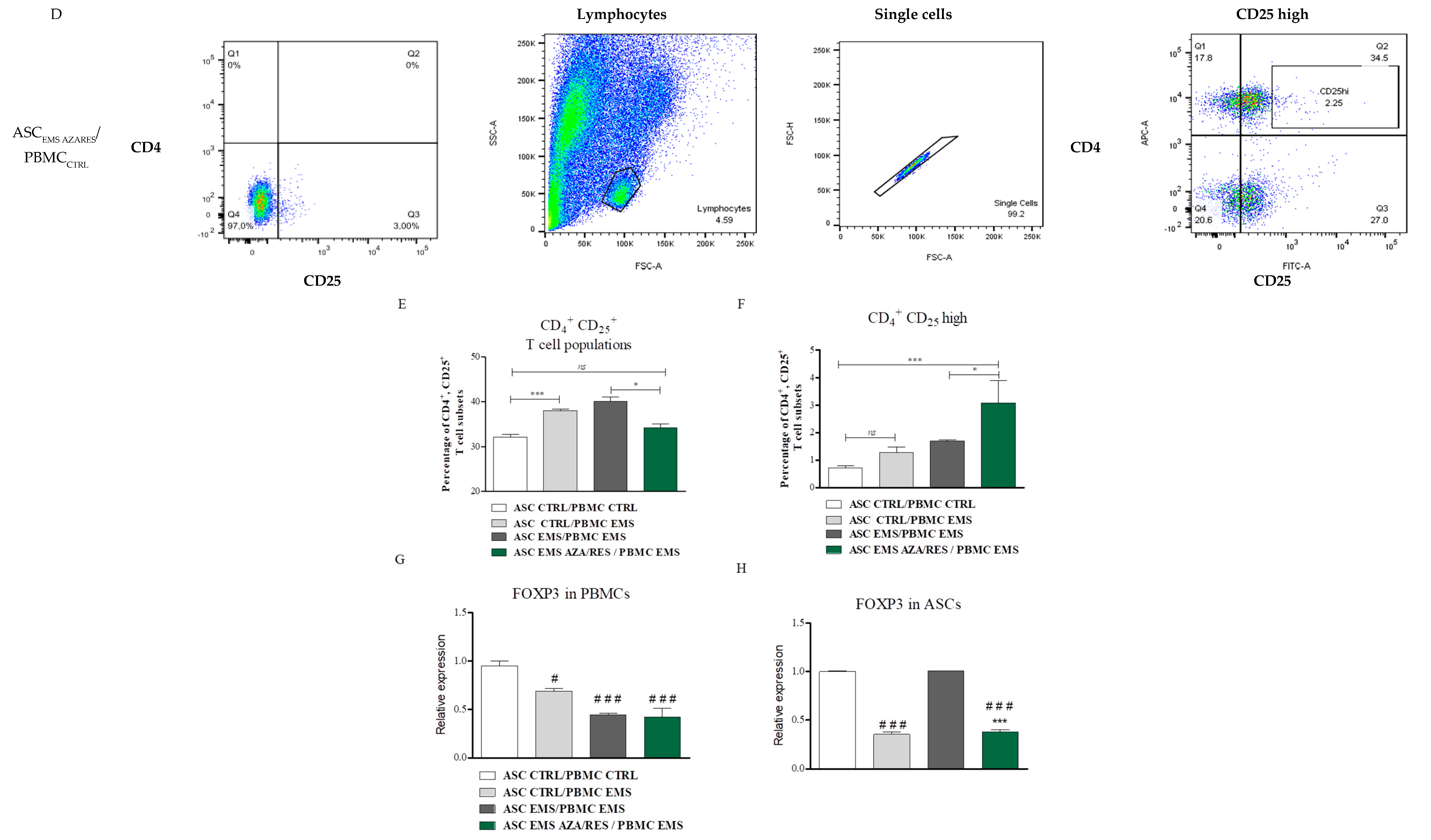
3.1. Flow Cytometry Analysis of PBMCs
3.2. Gene Expression After ASC-PBMC Co-Culture
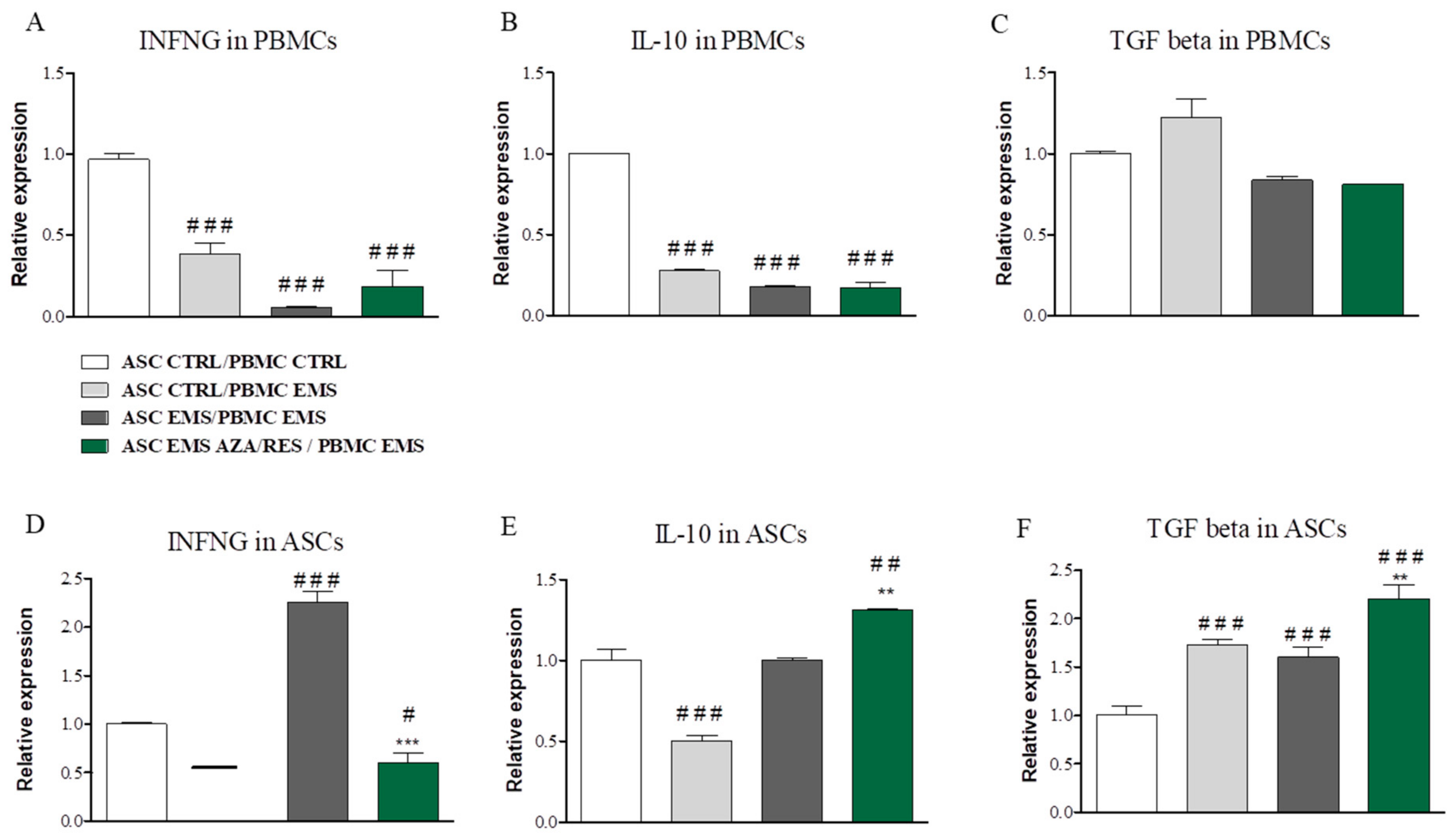
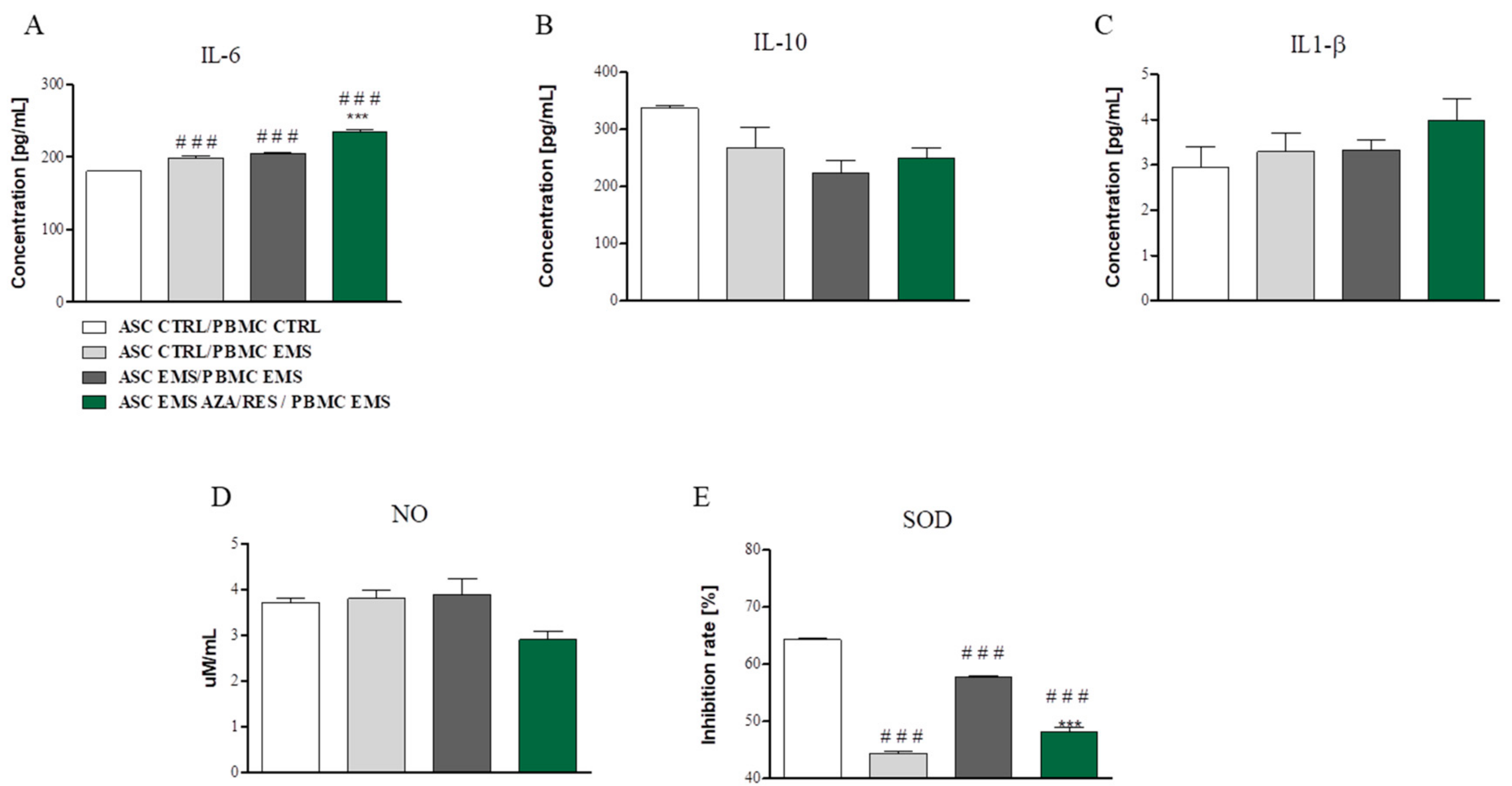
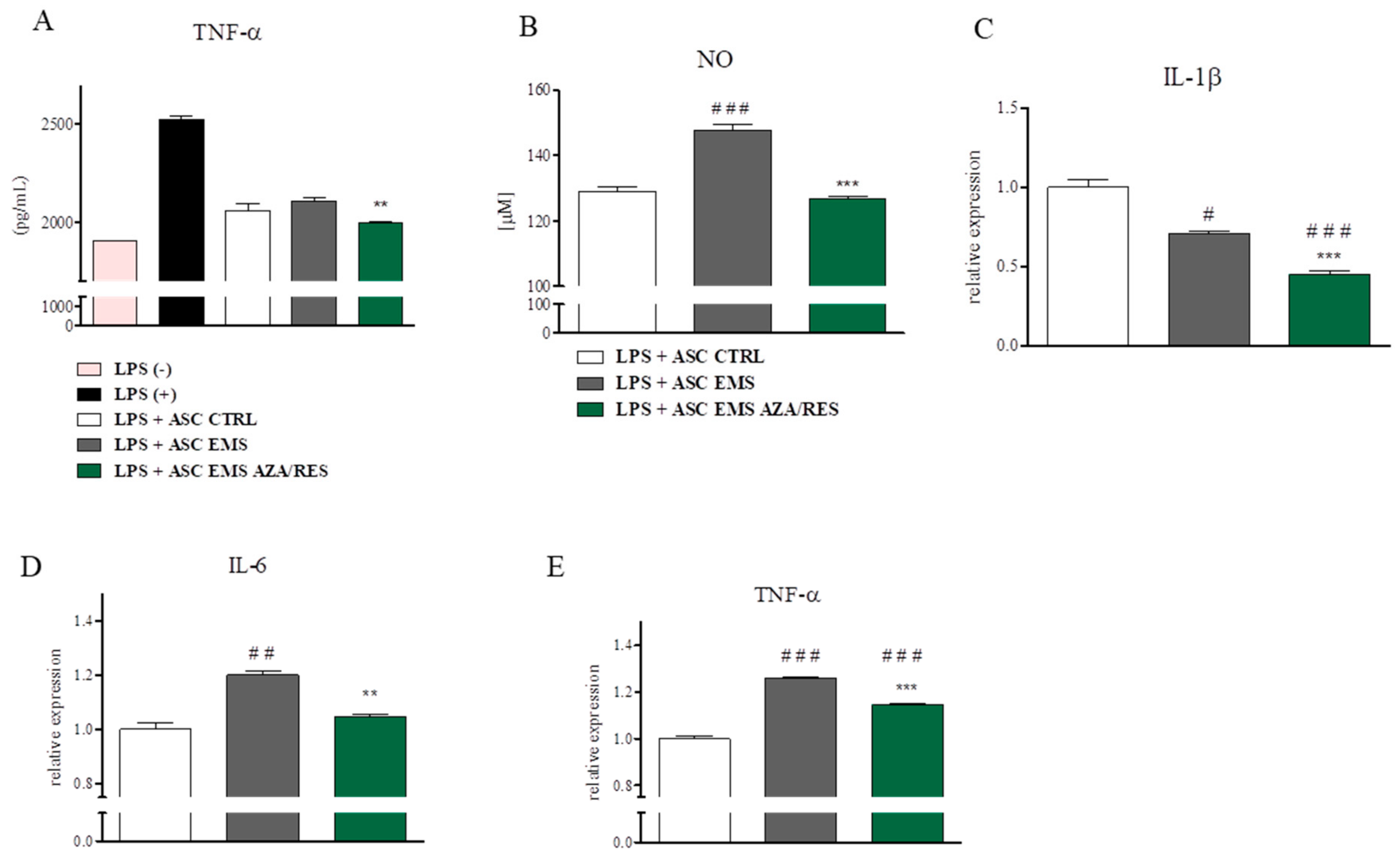
3.3. Cytokines, NO, and SOD Amount after ASC-PBMC Co-Culture
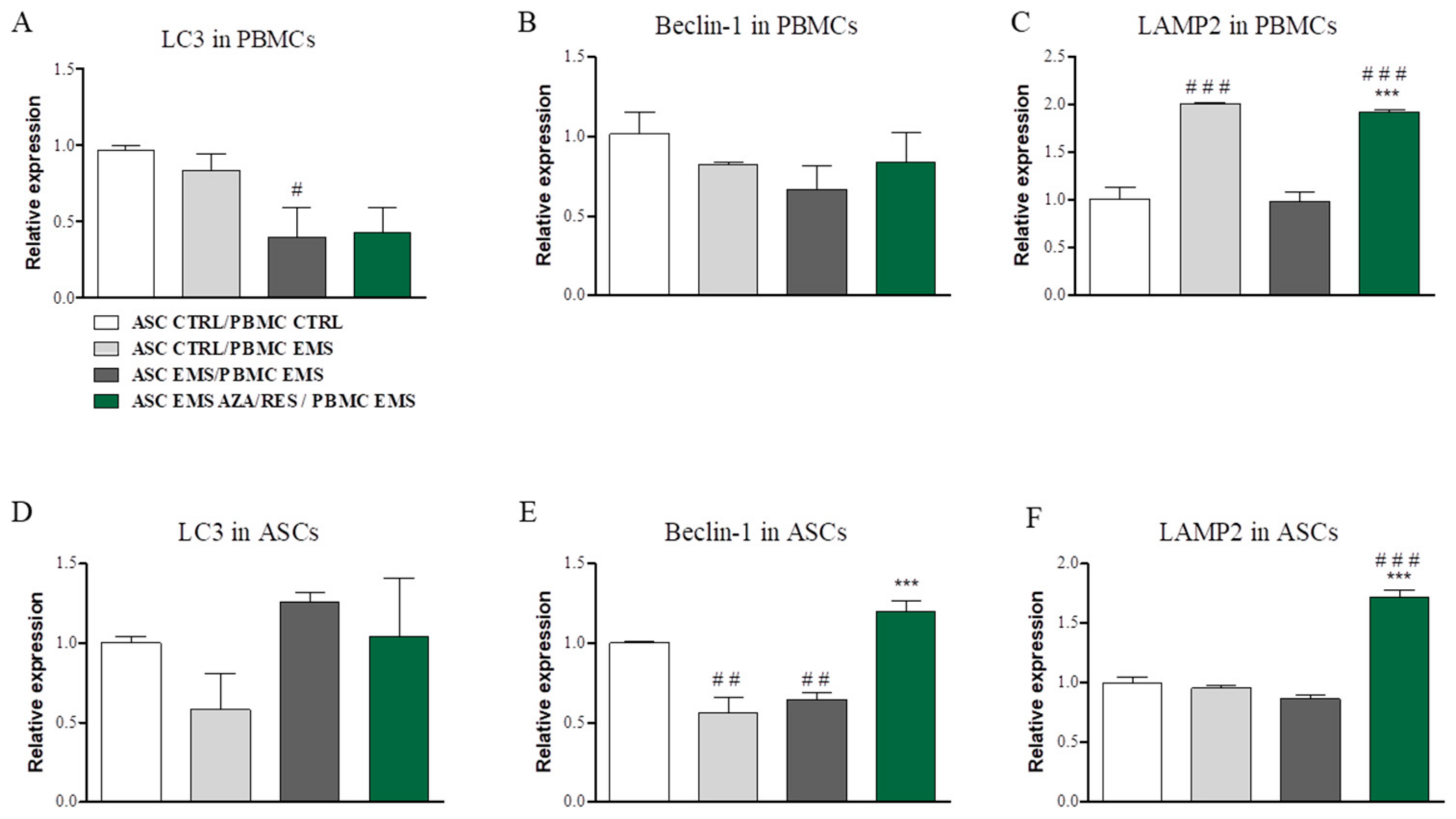
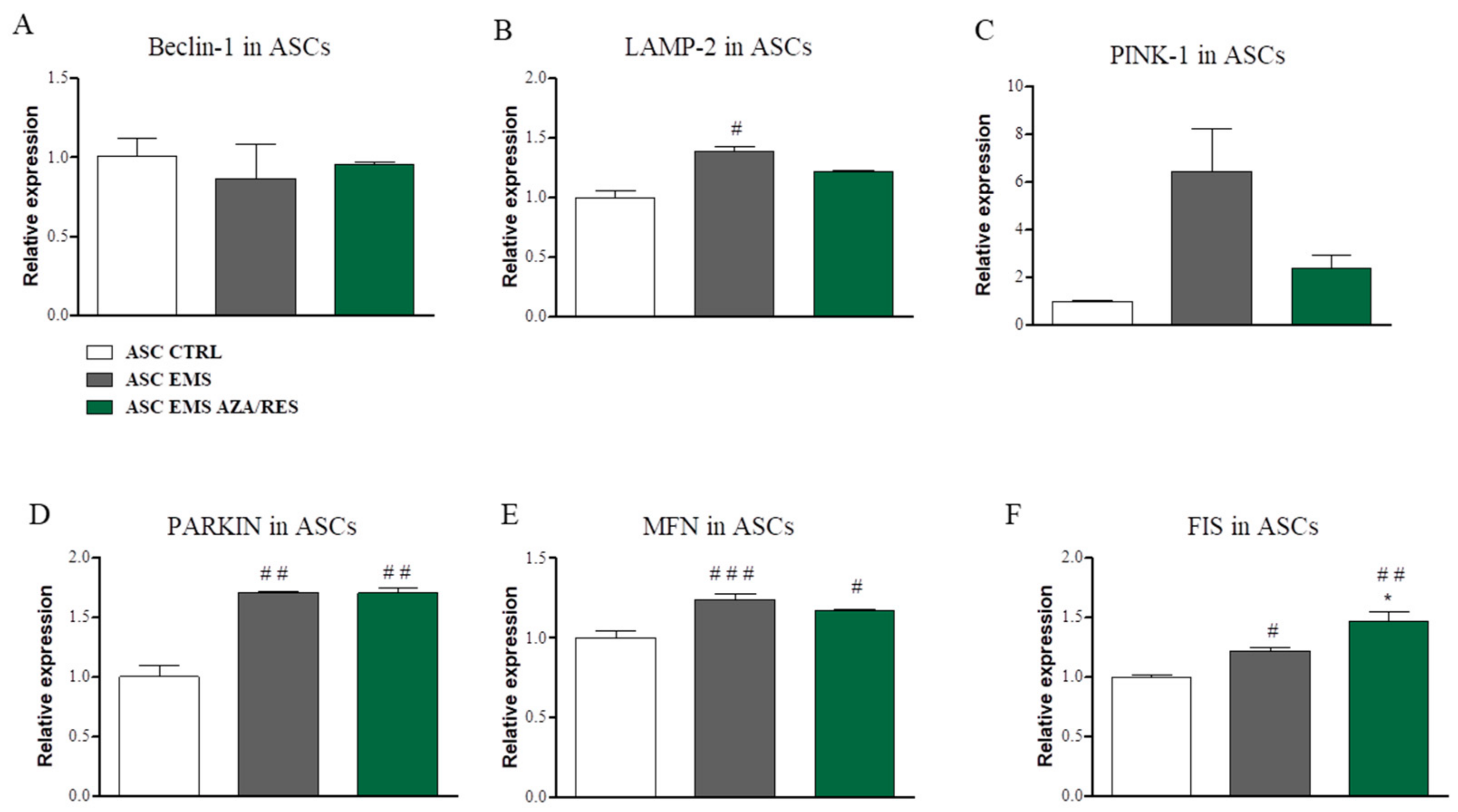
3.4. Evaluation of Autophagy-Related Genes after ASC-PBMC Co-Culture
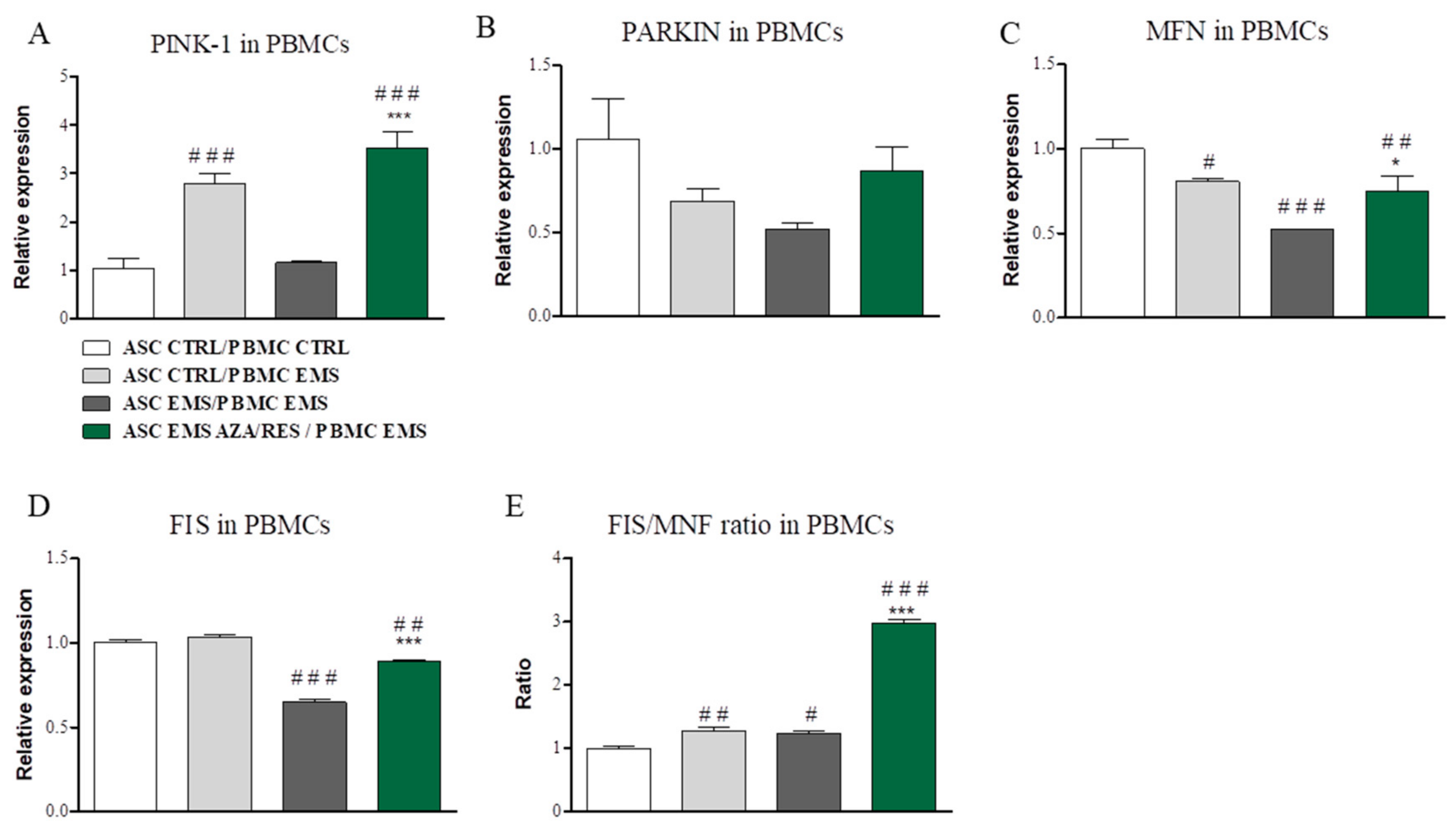
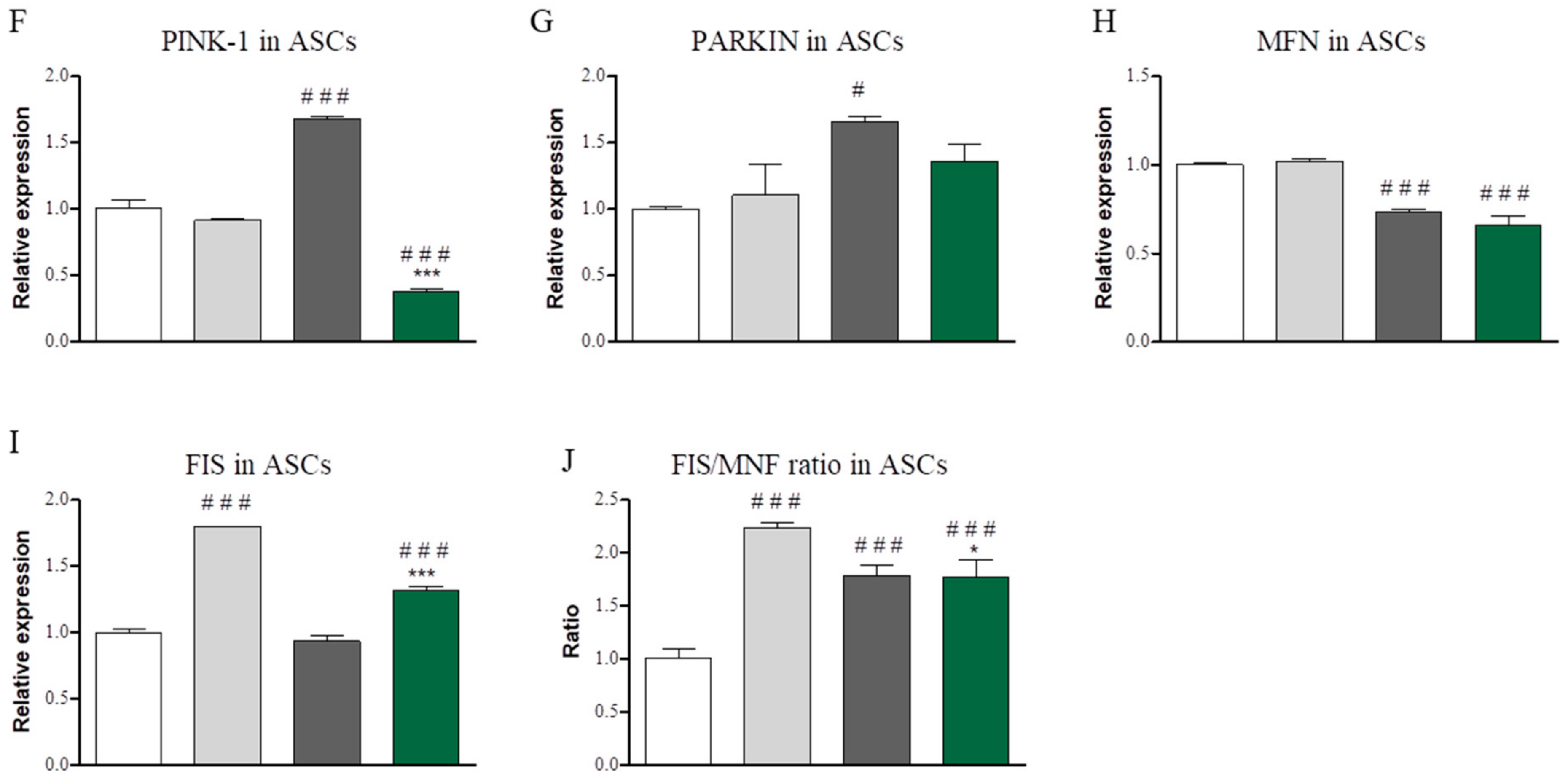
3.5. Evaluation of Mitophagy-Related Genes after Co ASC-PBMC Co-Culture
3.6. Co-Culture of ASC with RAW264.7 Macrophages
3.7. Auto- and Mitophagy after ASC-RAW264.7 Co-Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, H.; Côté, P.; Shearer, H.M.; Wong, J.J.; Sutton, D.A.; Randhawa, K.A.; Varatharajan, S.; Southerst, D.; Mior, S.A.; Ameis, A.; et al. Effectiveness of passive physical modalities for shoulder pain: Systematic review by the Ontario protocol for traffic injury management collaboration. Phys. Ther. 2015, 95, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.T.; Lacefield, K.; C’de Baca, J.; Blankenship, A.; Qualls, C. Effectiveness of group-delivered cognitive therapy and treatment length in women veterans with PTSD. Behav. Sci. 2014, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Spinetti, E.; de Mello, W.; Trindade, L.S.; Taub, D.D.; Taichman, R.S.; Balduino, A. Human bone marrow mesenchymal progenitors: Perspectives on an optimized in vitro manipulation. Front. Cell Dev. Biol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.d.V.; Telles, P.D.d.S.; Nascimento, I.L.O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Tang, K.C.; Patel, A.P.; Bonilla, L.M.; Pierobon, N.; Ponzio, N.M.; Rameshwar, P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood 2006, 107, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.-F.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Szlapka-Kosarzewska, J.; Weiss, C. Excessive endoplasmic reticulum stress correlates with impaired mitochondrial dynamics, mitophagy and apoptosis, in liver and adipose tissue, but not in Muscles in EMS horses. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, T.C.; Tipton, T.; McFarlane, D. Neutrophil and cytokine dysregulation in hyperinsulinemic obese horses. Vet. Immunol. Immunopathol. 2012, 145, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.A.; Geor, R.J.; Mudge, M.C.; McCutcheon, L.J.; Hinchcliff, K.W.; Belknap, J.K. Proinflammatory cytokine and chemokine gene expression profiles in subcutaneous and visceral adipose tissue depots of insulin-resistant and insulin-sensitive light breed horses. J. Vet. Intern. Med. 2010, 24, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Vick, M.M.; Murphy, B.A.; Sessions, D.R.; Reedy, S.E.; Kennedy, E.L.; Horohov, D.W.; Cook, R.F.; Fitzgerald, B.P. Effects of systemic inflammation on insulin sensitivity in horses and inflammatory cytokine expression in adipose tissue. Am. J. Vet. Res. 2008, 69, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine metabolic syndrome affects viability, senescence, and stress factors of equine adipose-derived mesenchymal stromal stem cells: New insight into EqASCs isolated from EMS horses in the context of their aging. Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Śmieszek, A.; Szłapka, J. Macroautophagy and Selective Mitophagy Ameliorate Chondrogenic Differentiation Potential in Adipose Stem Cells of Equine Metabolic Syndrome: New Findings in the Field of Progenitor Cells Differentiation. Available online: https://www.hindawi.com/journals/omcl/2016/3718468/ (accessed on 27 July 2017).
- Marycz, K.; Kornicka, K.; Marędziak, M.; Golonka, P.; Nicpoń, J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J. Cell. Mol. Med. 2016, 20, 2384–2404. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Marycz, K.; Marędziak, M.; Tomaszewski, K.A.; Nicpoń, J. The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J. Cell. Mol. Med. 2017, 21, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Nawrocka, D.; Lis-Bartos, A.; Marędziak, M.; Marycz, K. Polyurethane–polylactide-based material doped with resveratrol decreases senescence and oxidative stress of adipose-derived mesenchymal stromal stem cell (ASCs). RSC Adv. 2017, 7, 24070–24084. [Google Scholar] [CrossRef]
- Kuballa, P.; Nolte, W.M.; Castoreno, A.B.; Xavier, R.J. Autophagy and the immune system. Annu. Rev. Immunol. 2012, 30, 611–646. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Weiss, C.; Śmieszek, A.; Kornicka, K. Evaluation of oxidative stress and mitophagy during adipogenic differentiation of adipose-derived stem cells isolated from equine metabolic syndrome (EMS) horses. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Van der Weerd, K.; Dik, W.A.; Schrijver, B.; Schweitzer, D.H.; Langerak, A.W.; Drexhage, H.A.; Kiewiet, R.M.; van Aken, M.O.; van Huisstede, A.; van Dongen, J.J.M.; et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes 2012, 61, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Yan, X.; Shinmura, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yamamoto, T.; Anzai, A.; Isobe, S.; Yoshida, N.; et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Investig. 2016, 126, 4626–4639. [Google Scholar] [CrossRef] [PubMed]
- Basinska, K.; Marycz, K.; Śmieszek, A.; Nicpoń, J. The production and distribution of IL-6 and TNF-α in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J. Vet. Sci. 2015, 16, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Rudensky, A.Y. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007, 8, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.-M.; Brandhorst, G.; Czepluch, F.; Lankeit, M.; Eberle, C.; Herzberg, S.; Faustin, V.; Riggert, J.; Oellerich, M.; Hasenfuss, G.; et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity 2013, 21, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Sundin, M.; D’arcy, P.; Johansson, C.C.; Barrett, A.J.; Lönnies, H.; Sundberg, B.; Nava, S.; Kiessling, R.; Mougiakakos, D.; Le Blanc, K. Multipotent mesenchymal stromal cells express FoxP3: A marker for the immunosuppressive capacity? J. Immunother. 2011, 34, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.A.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.; Fiorina, P.; Adra, C.N.; Atkinson, M.; Sayegh, M.H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 2008, 57, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, S.; Akasu, F.; Kasuga, Y.; Snow, K.; Keystone, E.; Volpé, R. In vitro production of interferon-gamma by peripheral blood from patients with Graves’ disease, Hashimoto’s thyroiditis and rheumatoid arthritis. Clin. Exp. Immunol. 1990, 82, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, C.; Wang, P.; Xu, W.; Zhang, A.; Li, Q.; Xu, X. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Hum. Immunol. 2014, 75, 289–296. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Liu, G.; Wei, Y. Association between IL-6 and related risk factors of metabolic syndrome and cardiovascular disease in young rats. Int. J. Clin. Exp. Med. 2015, 8, 13491–13499. [Google Scholar] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Mazloom, H.; Sadeghi, A.; Emamgholipour, S.; Golestani, A.; Noorbakhsh, F.; Khoshniatnikoo, M.; Meshkani, R. Evidence for the link between defective autophagy and inflammation in peripheral blood mononuclear cells of type 2 diabetic patients. J. Physiol. Biochem. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Kang, D.; Kanki, T. The physiological role of mitophagy: New insights into phosphorylation events. Int. J. Cell Biol. 2012, 2012, 354914. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Yin, X.-M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Deen, N.; Zamani, S.; Hasnat, M.A. Mitophagy and the release of inflammatory cytokines. Mitochondrion 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.-P.; Fu, J.-S.; Bai, L.; Guo, L. Resveratrol augments therapeutic efficiency of mouse bone marrow mesenchymal stem cell-based therapy in experimental autoimmune encephalomyelitis. Int. J. Dev. Neurosci. 2016, 49, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-H.; Ma, Q.-Y.; Wang, L.-C.; Sha, H.-C.; Wu, S.-L.; Zhang, M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm. Res. 2005, 54, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Li, X.; Zhou, Q.; Bai, J.; Shi, Y.; Le, G. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res. 2013, 33, 971–981. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Sequence 5′-3′ | Amplicon Length (bp) | Accession no. |
|---|---|---|---|---|
| INFNG | F: | CACCAGCAAGCTGGAAGACT | 163 | NM_001081949.1 |
| R: | CCGGCCTCGAAATGGATTCT | |||
| FOXP3 | F: | AGATGCTGGCCGAGGTCAAC | 149 | XM_023633195.1 |
| R: | TGCGGAACTCGAACTCATCC | |||
| IL-10 | F: | ATAAGAGCAAGGCAGTGGAGC | 77 | NM_001082490.1 |
| R: | ACTCATGGCTTTGTAGACACC | |||
| TGF beta | F: | ATTCCTGGCGCTACCTCAGT | 197 | NM_001081849.1 |
| R: | GCTGGAACTGAACCCGTTGAT | |||
| LC3 | F: | TTCTGAGACACAGTCGGAGC | 128 | XM_001493613.6 |
| R: | CTTTGTTCGAAGGTGTGGCG | |||
| Beclin–1 | F: | GATGCGTTATGCCCAGATGC | 233 | XM_014833759.1 |
| R: | AACGGCAGCTCCTCTGAAAT | |||
| LAMP-2 | F: | GCACCCCTGGGAAGTTCTTA | 147 | XM_014831347.1 |
| R: | ATCCAGCGAACACTCTTGGG | |||
| PINK-1 | F: | GCACAATGAGCCAGGAGCTA | 298 | XM_014737247.1 |
| R: | GGGGTATTCACGCGAAGGTA | |||
| PARKIN | F: | TCCCAGTGGAGGTCGATTCT | 218 | XM_014858374.1 |
| R: | CCCTCCAGGTGTGTTCGTTT | |||
| MFN | F: | AAGTGGCATTTTTCGGCAGG | 217 | XM_001495170.5 |
| R: | TCCATATGAAGGGCATGGGC | |||
| FIS | F: | GGTGCGAAGCAAGTACAACG | 118 | XM_001504462.4 |
| R: | GTTGCCCACAGCCAGATAGA | |||
| GAPDH | F: | GATGCCCCAATGTTTGTGA | 250 | XM_014866500.1 |
| R: | AAGCAGGGATGATGTTCTGG | |||
| TNF-α | F: | ACAGAAAGCATGATCCGCGA | 295 | NM_013693.3 |
| R: | CTTGGTGGTTTGCTACGACG | |||
| IL-1β | F: | TGCCACCTTTTGACAGTGATG | 138 | NM_008361.4 |
| R: | TGATGTGCTGCTGCGAGATT | |||
| IL-6 | F: | GAGGATACCACTCCCAACAGACC | 141 | NM_001314054.1 |
| R: | AAGTGCATCATCGTTGTTCATACA | |||
| GAPDH | F: | TGCACCACCAACTGCTTAG | 177 | XM_017321385.1 |
| R: | GGATGCAGGGATGATGTTC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornicka, K.; Śmieszek, A.; Węgrzyn, A.S.; Röcken, M.; Marycz, K. Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals. J. Clin. Med. 2018, 7, 383. https://doi.org/10.3390/jcm7110383
Kornicka K, Śmieszek A, Węgrzyn AS, Röcken M, Marycz K. Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals. Journal of Clinical Medicine. 2018; 7(11):383. https://doi.org/10.3390/jcm7110383
Chicago/Turabian StyleKornicka, Katarzyna, Agnieszka Śmieszek, Agnieszka Sławomira Węgrzyn, Michael Röcken, and Krzysztof Marycz. 2018. "Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals" Journal of Clinical Medicine 7, no. 11: 383. https://doi.org/10.3390/jcm7110383
APA StyleKornicka, K., Śmieszek, A., Węgrzyn, A. S., Röcken, M., & Marycz, K. (2018). Immunomodulatory Properties of Adipose-Derived Stem Cells Treated with 5-Azacytydine and Resveratrol on Peripheral Blood Mononuclear Cells and Macrophages in Metabolic Syndrome Animals. Journal of Clinical Medicine, 7(11), 383. https://doi.org/10.3390/jcm7110383





