Use of Psychotropic Drugs among Children and Adolescents with Autism Spectrum Disorders in Denmark: A Nationwide Drug Utilization Study
Abstract
1. Introduction
2. Experimental Section
2.1. Data Sources
2.2. Study Population
2.3. Study Drugs
2.4. Analysis
2.4.1. Characteristics of Study Population
2.4.2. One-Year Prevalence Proportion of Medication Use from 2010 to 2017
2.4.3. Early Discontinuation and Persistence Rate
2.4.4. Age and Sex Stratified Prevalence Proportion of Medication Use in 2017
2.4.5. Age-Stratified Prevalence Proportion of Medication Use by Birth Year
2.4.6. Drug Use According to Psychiatric Comorbidities
2.4.7. Age at First ASD Diagnosis and First Prescription
2.4.8. Most Commonly Used Drugs in 2017
2.4.9. Sensitivity Analyses
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Birth Year | Calendar Year | |||||||
|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| 1992 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| 1993 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 |
| 1995 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 1996 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 1997 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 1998 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 1999 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 2000 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| 2001 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 2002 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 2003 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 2004 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 2005 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 2006 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 2007 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2008 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 2009 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 2010 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 2011 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
Appendix B
| Psychiatric Comorbidities | ICD-10 Codes |
|---|---|
| ADHD | F90, F98.8C |
| Intellectual disability | F70-F79 |
| Other psychiatric comorbidity | All F-codes with the exception of codes used to define ASD (F84.0, F84.1, F84.5, F84.8, F84.9 *) and codes used to define ADHD and intellectual disability |
| No psychiatric comorbidity | Absence of ADHD, intellectual disability and other psychiatric comorbidity as defined above |
References
- Lai, M.-C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Lauritsen, M.B. Autism spectrum disorders. Eur. Child Adolesc. Psychiatry 2013, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Elsabbagh, M.; Divan, G.; Koh, Y.-J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders: Global epidemiology of autism. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Atladottir, H.O.; Gyllenberg, D.; Langridge, A.; Sandin, S.; Hansen, S.N.; Leonard, H.; Gissler, M.; Reichenberg, A.; Schendel, D.E.; Bourke, J.; et al. The increasing prevalence of reported diagnoses of childhood psychiatric disorders: A descriptive multinational comparison. Eur. Child Adolesc. Psychiatry 2014, 24, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Jobski, K.; Höfer, J.; Hoffmann, F.; Bachmann, C. Use of psychotropic drugs in patients with autism spectrum disorders: A systematic review. Acta Psychiatr. Scand. 2017, 135, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Findling, R.L. An update on pharmacotherapy for autism spectrum disorder in children and adolescents. Curr. Opin. Psychiatry 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Earle, J.F. An introduction to the psychopharmacology of children and adolescents with autism spectrum disorder. J. Child Adolesc. Psychiatr. Nurs. 2016, 29, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Rogdaki, M.; Findon, J.L.; Wichers, R.H.; Charman, T.; King, B.H.; Loth, E.; McAlonan, G.M.; McCracken, J.T.; Parr, J.R.; et al. Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British association for psychopharmacology. J. Psychopharmacol. (Oxf.) 2018, 32, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.; Ong, R.C.; Bolognani, F. Psychiatric comorbidities and use of psychotropic medications in people with autism spectrum disorder in the United States: Psychotropic medications in autism in the US. Autism Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Coury, D.L.; Anagnostou, E.; Manning-Courtney, P.; Reynolds, A.; Cole, L.; McCoy, R.; Whitaker, A.; Perrin, J.M. Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics 2012, 130, S69–S76. [Google Scholar] [CrossRef] [PubMed]
- Accordino, R.E.; Kidd, C.; Politte, L.C.; Henry, C.A.; McDougle, C.J. Psychopharmacological interventions in autism spectrum disorder. Expert Opin. Pharmacother. 2016, 17, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish national patient registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Mors, O.; Perto, G.P.; Mortensen, P.B. The Danish psychiatric central research register. Scand. J. Public Health 2011, 39, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Schmidt, S.A.J.; Wallach-Kildemoes, H.; Sørensen, H.T.; Hallas, J.; Schmidt, M. Data resource profile: The Danish national prescription registry. Int. J. Epidemiol. 2016, 46, 798–798f. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B. The Danish civil registration system. Scand. J. Public Health 2011, 39, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, M.B.; Jørgensen, M.; Madsen, K.M.; Lemcke, S.; Toft, S.; Grove, J.; Schendel, D.E.; Thorsen, P. Validity of childhood autism in the Danish psychiatric central register: Findings from a cohort sample born 1990–1999. J. Autism Dev. Disord. 2010, 40, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Pratt, N.; Hansen, M.R.; Hallas, J.; Pottegård, A. Using the “proportion of patients covered” and the Kaplan-Meier survival analysis to describe treatment persistence. Pharmacoepidemiol. Drug Saf. 2018, 27, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, L.C.; Daasnes, C.; Thaulow, I.; Brønnum-Hansen, H. Introduction to Danish (nationwide) registers on health and social issues: Structure, access, legislation, and archiving. Scand. J. Public Health 2011, 39, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, C.J.; Manthey, T.; Kamp-Becker, I.; Glaeske, G.; Hoffmann, F. Psychopharmacological treatment in children and adolescents with autism spectrum disorders in Germany. Res. Dev. Disabil. 2013, 34, 2551–2563. [Google Scholar] [CrossRef] [PubMed]
- Schubart, J.R.; Camacho, F.; Leslie, D. Psychotropic medication trends among children and adolescents with autism spectrum disorder in the Medicaid program. Autism 2014, 18, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Esbensen, A.J.; Greenberg, J.S.; Seltzer, M.M.; Aman, M.G. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J. Autism Dev. Disord. 2009, 39, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Woods, C.; Stevenson, M.; Davis, D.W.; Radmacher, P.; Smith, M. Psychotropic medication use in children with autism in the kentucky medicaid population. Clin. Pediatr. (Phila.) 2012, 51, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Bjerregaard, B.K.; Glintborg, D.; Hallas, J.; Moreno, S.I. The use of medication against attention deficit hyperactivity disorder in Denmark: A drug use study from a national perspective. Eur. J. Clin. Pharmacol. 2012, 68, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, S.; Nielsen, H.S.; Simonsen, M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: A danish register-based study. J. Child Adolesc. Psychopharmacol. 2013, 23, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Udviklingen i Antal Brugere af Lægemidler Med Melatonin for Unge under 25 år i Perioden 2007–2014. Available online: https://laegemiddelstyrelsen.dk/da/udgivelser/2015/bivirkningsindberetninger-fra-medicinbrugere-og-paaroerende-i-danmark-og-sammenligning-med-indberetninger-fra-sundhedsprofessionelle/~/media/0C604F0EF3F543B480EF025A03080397.ashx (accessed on 9 October 2018). (In Danish).
- Pottegård, A.; Bjerregaard, B.K.; Glintborg, D.; Kortegaard, L.S.; Hallas, J.; Moreno, S.I. The use of medication against attention deficit/hyperactivity disorder in Denmark: A drug use study from a patient perspective. Eur. J. Clin. Pharmacol. 2013, 69, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Bjerregaard, B.K.; Kortegaard, L.S.; Zoëga, H. Early discontinuation of ADHD drug treatment: A Danish nationwide drug utilization study. Basic Clin. Pharmacol. Toxicol. 2015, 116, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Sturman, N.; Deckx, L.; van Driel, M.L. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J. Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr. Psychiatry Rep. 2010, 12, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Autism, P. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 2005, 62, 1266–1274. [Google Scholar] [CrossRef]
- Frazier, T.W.; Shattuck, P.T.; Narendorf, S.C.; Cooper, B.P.; Wagner, M.; Spitznagel, E.L. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2011, 21, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.S.; Rasmussen, L.; Hellfritzsch, M.; Thomsen, P.H.; Nørgaard, M.; Laursen, T. Trends in off-label prescribing of sedatives, hypnotics and antidepressants among children and adolescents-A Danish, nationwide register-based study. Basic Clin. Pharmacol. Toxicol. 2017, 120, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.S.; Hellfritzsch, M.; Sørensen, M.J.; Rasmussen, H.; Thomsen, P.H.; Laursen, T. Off-label prescribing of psychotropic drugs in a Danish child and adolescent psychiatric outpatient clinic. Eur. Child Adolesc. Psychiatry 2016, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Braüner, J.V.; Johansen, L.M.; Roesbjerg, T.; Pagsberg, A.K. Off-label prescription of psychopharmacological drugs in child and adolescent psychiatry. J. Clin. Psychopharmacol. 2016, 36, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders. Curr. Clin. Pharmacol. 2014, 9, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Ivanenko, A.; Johnson, K. Sleep in children with autistic spectrum disorder. Sleep Med. 2010, 11, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Vejledning om Medikamentel Behandling af Børn og Unge Med Psykiske Lidelser. Available online: https://www.retsinformation.dk/Forms/R0710.aspx?id=146409 (accessed on 9 October 2018). (In Danish).
- Douglas-Hall, P.; Curran, S.; Bird, V.; Taylor, D. Aripiprazole: A review of its use in the treatment of irritability associated with autistic disorder patients aged 6–17. J. Cent. Nerv. Syst. Dis. 2011, 3, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Almandil, N.B.; Liu, Y.; Murray, M.L.; Besag, F.M.C.; Aitchison, K.J.; Wong, I.C.K. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: A systematic review and meta-analysis. Pediatr. Drugs 2013, 15, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, S.; Pringsheim, T. Using antipsychotics for behavioral problems in children. Expert Opin. Pharmacother. 2018, 19, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Brignell, A.; Randall, M.; Silove, N.; Hazell, P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Iyer, T.; Menon, V. The influence of sex and age on prevalence rates of comorbid conditions in autism: Influence of sex & age on autism comorbidities. Autism Res. 2017, 10, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ramtekkar, U.P.; Reiersen, A.M.; Todorov, A.A.; Todd, R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Duvekot, J.; van der Ende, J.; Verhulst, F.C.; Slappendel, G.; van Daalen, E.; Maras, A.; Greaves-Lord, K. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism 2017, 21, 646–658. [Google Scholar] [CrossRef] [PubMed]
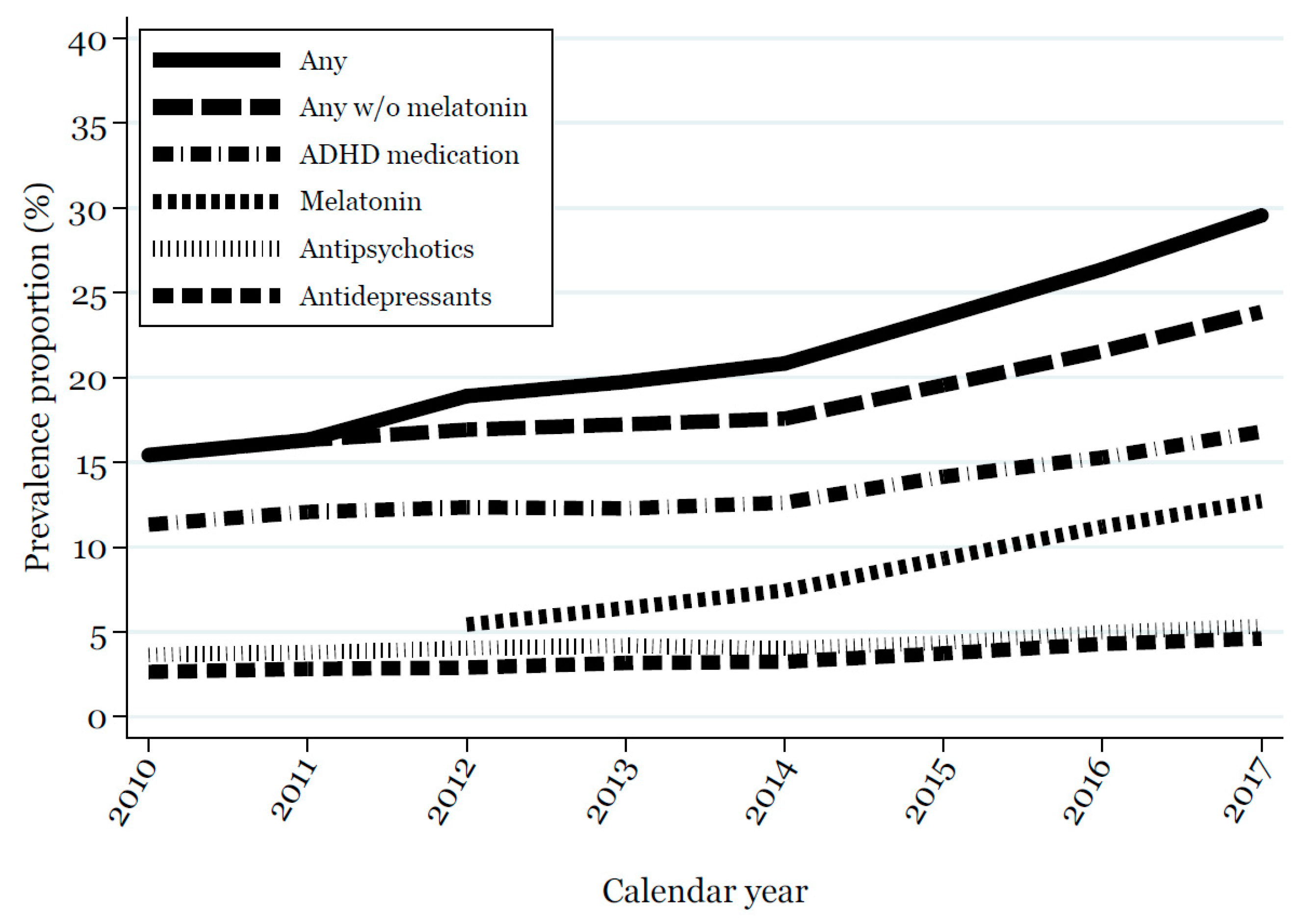
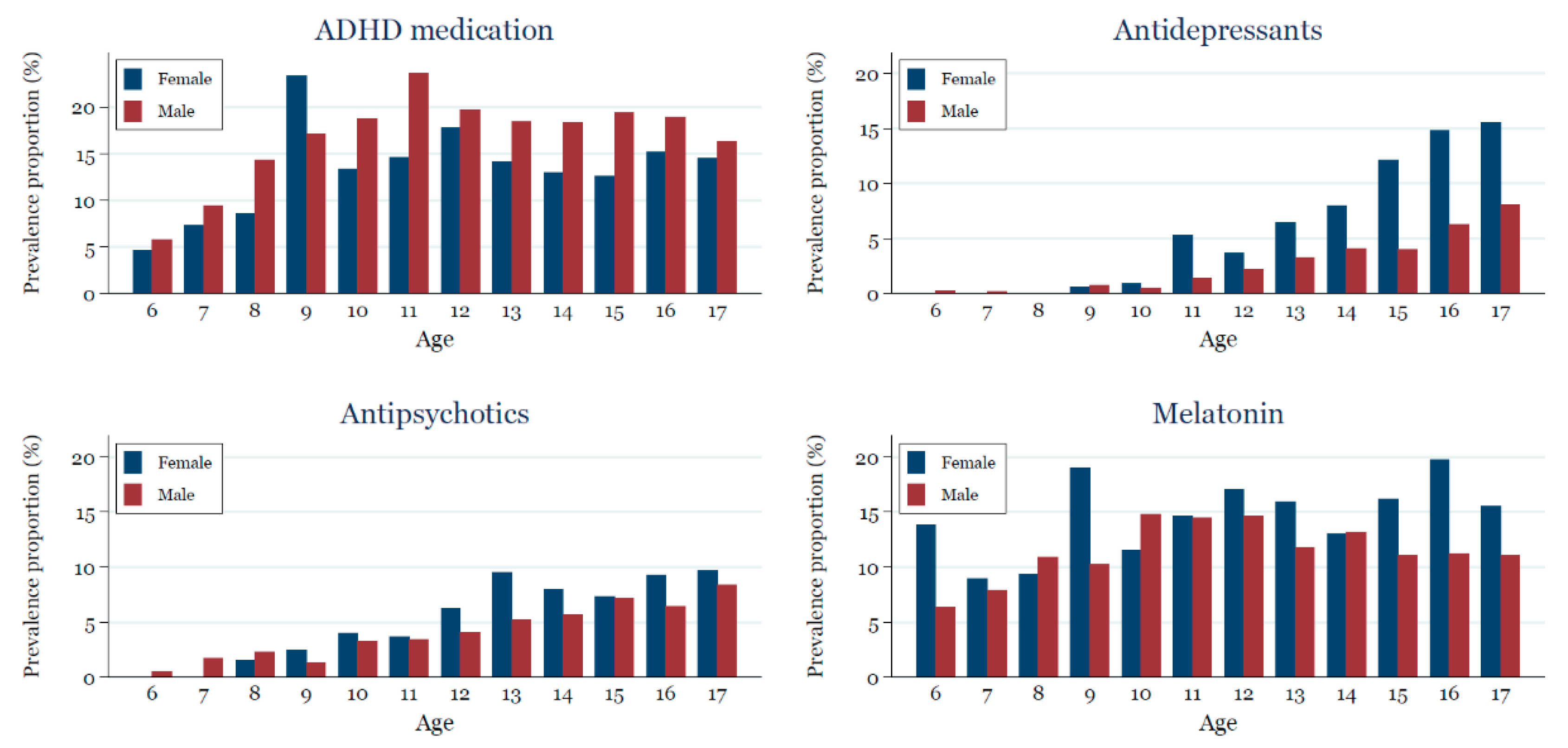
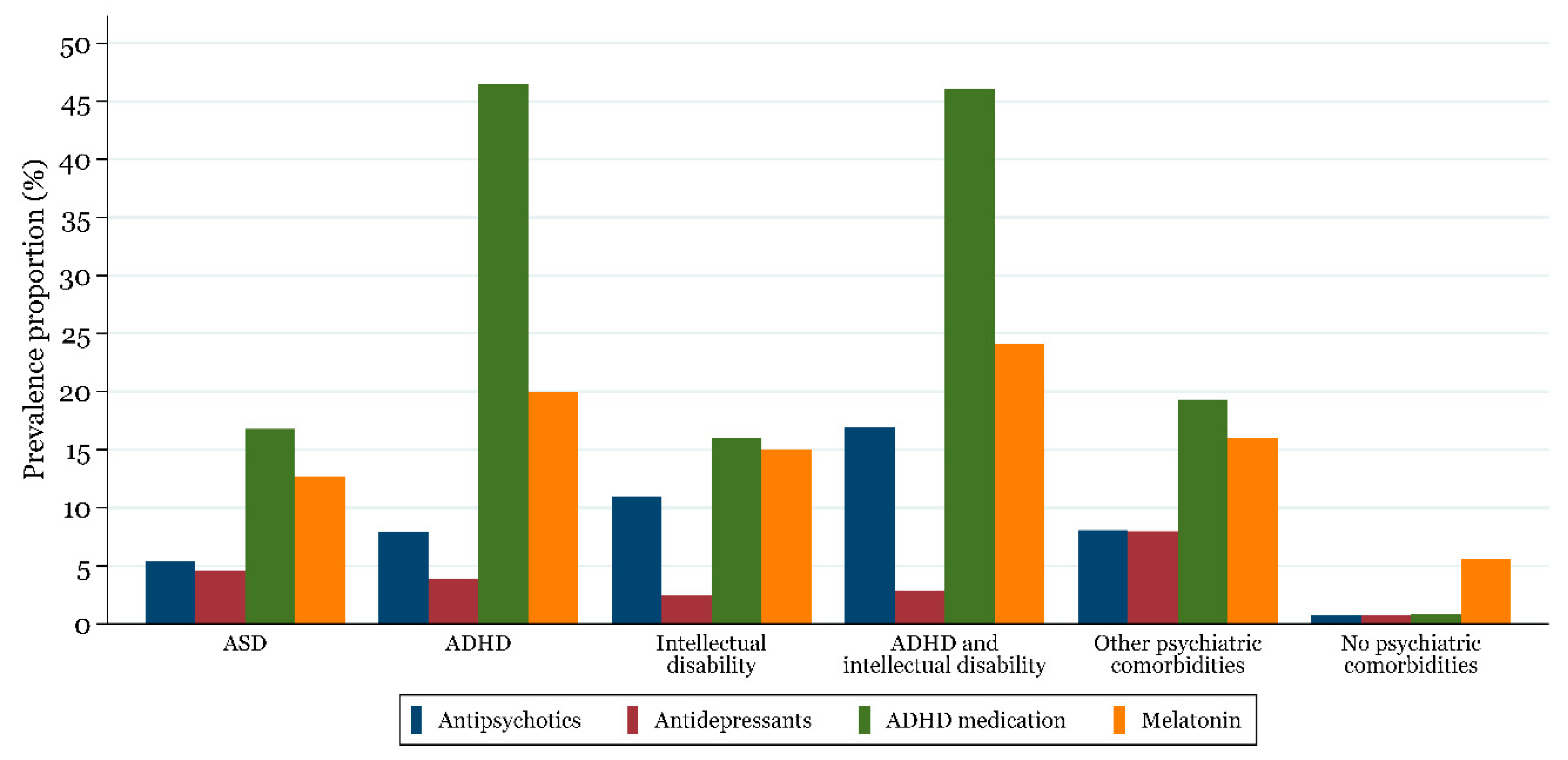
| Overall (N = 23,935) | 2017 Cohort (N = 14,210) | |
|---|---|---|
| ASD subtype | ||
| Childhood autism | 9303 (38.9%) | 6365 (44.8%) |
| Atypical autism | 4314 (18.0%) | 2682 (18.9%) |
| Asperger’s syndrome | 6950 (29.0%) | 3368 (23.7%) |
| Other pervasive developmental disorders | 3368 (14.1%) | 1795 (12.6%) |
| Median age at first diagnosis, years (IQR) | ||
| ASD | 9 (6–13) | 8 (5–11) |
| Childhood autism | 7 (4–11) | 6 (4–9) |
| Atypical autism | 11 (7–14) | 9 (6–12) |
| Asperger’s syndrome | 11 (8–14) | 10 (8–13) |
| Other pervasive developmental disorders | 10 (7–13) | 9 (6–11) |
| Sex (%) | ||
| Male | 17,966 (75.1%) | 10,731 (75.5%) |
| Female | 5969 (24.9%) | 3479 (24.5%) |
| Ten most common ICD-10 psychiatric comorbidities (%) | ||
| Hyperkinetic disorders | 6922 (28.9%) | 4570 (32.2%) |
| Reaction to severe stress and adjustment disorders | 3340 (14.0%) | 1905 (13.4%) |
| Other behavioural and emotional disorders with onset usually occurring in childhood and adolescence | 3201 (13.4%) | 1883 (13.3%) |
| Mild mental retardation | 2052 (8.6%) | 1147 (8.1%) |
| Tic disorders | 1763 (7.4%) | 1172 (8.2%) |
| Depressive episode | 1656 (6.9%) | 706 (5.0%) |
| Mixed specific developmental disorders | 1553 (6.5%) | 881 (6.2%) |
| Specific developmental disorders of speech and language | 1384 (5.8%) | 842 (5.9%) |
| Other anxiety disorders | 1288 (5.4%) | 701 (4.9%) |
| Unspecified mental retardation | 1190 (5.0%) | 675 (4.8%) |
| Selected psychiatric comorbidities (%) | ||
| ADHD | 7293 (30.5%) | 4851 (34.1%) |
| Intellectual disability | 3587 (15.0%) | 2007 (14.1%) |
| ADHD and intellectual disability | 1092 (4.6%) | 656 (4.6%) |
| Other | 13,284 (55.5%) | 7566 (53.2%) |
| None | 6484 (27.1%) | 3890 (27.4%) |
| Total Number of Patients Initiating Treatment | Fills a Second Prescription within the First 180 Days | Day 180 | Day 365 | Day 730 | |
|---|---|---|---|---|---|
| ADHD medication | |||||
| 3–5 | 179 | 93.30% | 82.12% | 76.54% | 72.63% |
| 6–11 | 1687 | 94.01% | 81.42% | 73.81% | 67.10% |
| 12–17 | 771 | 94.09% | 76.21% | 62.00% | 55.05% |
| Antidepressants | |||||
| 3–5 | 5 | - | - | - | - |
| 6–11 | 315 | 90.16% | 70.16% | 61.90% | 48.57% |
| 12–17 | 1336 | 89.76% | 72.22% | 62.01% | 46.54% |
| Antipsychotics | |||||
| 3–5 | 30 | - | - | - | - |
| 6–11 | 599 | 80.97% | 64.77% | 55.43% | 48.08% |
| 12–17 | 1087 | 76.85% | 60.44% | 52.25% | 44.87% |
| Melatonin | |||||
| 3–5 | 210 | 71.90% | 61.90% | 55.50% | 46.41% |
| 6–11 | 1520 | 74.52% | 62.80% | 58.10% | 51.52% |
| 12–17 | 1892 | 63.98% | 50.34% | 43.31% | 37.85% |
| ADHD Medication | Antidepressants | Antipsychotics | ||||
|---|---|---|---|---|---|---|
| 6–11 years | Total number of prescriptions (n = 6813) | Total number of prescriptions (n = 180) | Total number of prescriptions (n = 622) | |||
| Methylphenidate | 4641 (68.1%) | Sertraline | 137 (76.1%) | Risperidone | 471 (75.7%) | |
| Atomoxetine | 1217 (17.9%) | Fluoxetine | 15 (8.3%) | Aripiprazole | 126 (20.3%) | |
| Lisdexamfetamine | 848 12.4%) | Imipramine | 9 (5.0%) | Levomepromazine | 11 (1.8%) | |
| Dexamfetamine | 107 (1.6%) | Venlafaxine | 8 (4.4%) | Pimozide | 5 (0.8%) | |
| - | Escitalopram | 5 (2.8%) | Chlorprothixene | 5 (0.8%) | ||
| 12–17 years | Total number of prescriptions (n = 12,832) | Total number of prescriptions (n = 2903) | Total number of prescriptions (n = 4203) | |||
| Methylphenidate | 7817 (60.9%) | Sertraline | 2156 (74.3%) | Risperidone | 1808 (43.0%) | |
| Atomoxetine | 3353 (26.1%) | Fluoxetine | 587 (20.2%) | Aripiprazole | 1328 (31.6%) | |
| Lisdexamfetamine | 1560 (12.2%) | Citalopram | 71 (2.4%) | Quetiapine | 665 (15.8%) | |
| Dexamfetamine | 92 (0.7%) | Mirtazapine | 17 (0.6%) | Olanzapine | 157 (3.7%) | |
| Modafinil | 10 (0.1%) | Escitalopram | 16 (0.6%) | Chlorprothixene | 134 (3.2%) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, L.; Bilenberg, N.; Thomsen Ernst, M.; Abitz Boysen, S.; Pottegård, A. Use of Psychotropic Drugs among Children and Adolescents with Autism Spectrum Disorders in Denmark: A Nationwide Drug Utilization Study. J. Clin. Med. 2018, 7, 339. https://doi.org/10.3390/jcm7100339
Rasmussen L, Bilenberg N, Thomsen Ernst M, Abitz Boysen S, Pottegård A. Use of Psychotropic Drugs among Children and Adolescents with Autism Spectrum Disorders in Denmark: A Nationwide Drug Utilization Study. Journal of Clinical Medicine. 2018; 7(10):339. https://doi.org/10.3390/jcm7100339
Chicago/Turabian StyleRasmussen, Lotte, Niels Bilenberg, Martin Thomsen Ernst, Sidsel Abitz Boysen, and Anton Pottegård. 2018. "Use of Psychotropic Drugs among Children and Adolescents with Autism Spectrum Disorders in Denmark: A Nationwide Drug Utilization Study" Journal of Clinical Medicine 7, no. 10: 339. https://doi.org/10.3390/jcm7100339
APA StyleRasmussen, L., Bilenberg, N., Thomsen Ernst, M., Abitz Boysen, S., & Pottegård, A. (2018). Use of Psychotropic Drugs among Children and Adolescents with Autism Spectrum Disorders in Denmark: A Nationwide Drug Utilization Study. Journal of Clinical Medicine, 7(10), 339. https://doi.org/10.3390/jcm7100339





