The Evolving Treatment of Peripheral Arterial Disease through Guideline-Directed Recommendations
Abstract
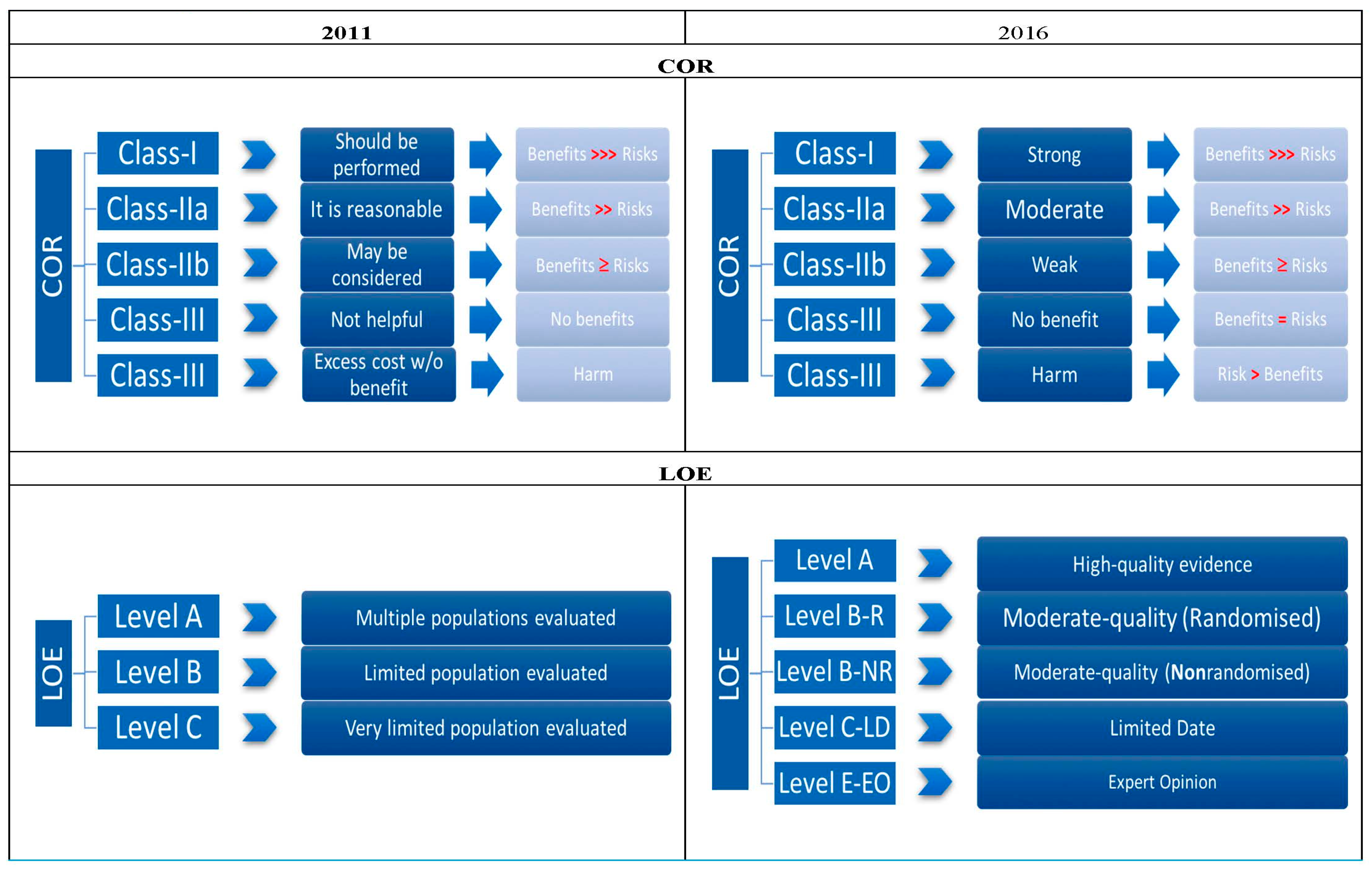
:1. Introduction
2. Guidelines
2.1. History and Physical Exam
2.2. Patients at Risk
2.3. Diagnostic Testing
2.4. Screening
2.5. Medical Management
2.6. Supervised Exercise
2.7. Foot Care and Minimizing Tissue Loss
2.8. Revascularization for Claudication
2.9. Critical Limb Ischemia
2.10. Acute Limb Ischemia
2.11. Longitudinal Follow-Up
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Olin, J.W.; Sealove, B.A. Peripheral artery disease: Current insight into the disease and its diagnosis and management. Mayo Clin. Proc. 2010, 85, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.A. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull. World Health Organ. 1962, 27, 645–658. [Google Scholar] [PubMed]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.A.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Hiatt, W.R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Sutton-Tyrrell, K.; Kuller, L.H. Lower-extremity arterial disease in older hypertensive adults. Arterioscler. Thromb. 1993, 13, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation 2006, 113, e463–e654. [Google Scholar] [PubMed]
- Margolis, J.; Barron, J.J.; Grochulski, W.D. Health care resources and costs for treating peripheral artery disease in a managed care population: Results from analysis of administrative claims data. J. Manag. Care Pharm. 2005, 11, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Pang, W.; Zhao, M.; Luo, Y.; Sun, Y.; Hu, D. Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ. J. 2008, 72, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Rahim, S.A.; Anand, S.S.; Simel, D.L.; Panju, A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA 2006, 295, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.J.; Tobin, C.; Matangi, M.F. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can. J. Cardiol. 2010, 26, e346–e350. [Google Scholar] [CrossRef]
- Bavry, A.A.; Anderson, R.D.; Gong, Y.; Denardo, S.J.; Cooper-DeHoff, R.M.; Bandberg, E.M.; Pepine, C.J. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease. Hypertension 2010, 55, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Rac-Albu, M.; Iliuta, L.; Guberna, S.M.; Sinescu, C. The role of ankle-brachial index for predicting peripheral arterial disease. Maedica 2014, 9, 295–302. [Google Scholar] [PubMed]
- Nicolai, S.P.; Viechtbauer, W.; Kruidenier, L.M.; Candel, M.J.; Prins, M.H.; Teijink, J.A. Reliability of treadmill testing in peripheral arterial disease: A meta-regression analysis. J. Vasc. Surg. 2009, 50, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Burbelko, M.; Augsten, M.; Kalinowski, M.O.; Heverhagen, J.T. Comparison of contrast-enhanced multi-station MR angiography and digital subtraction angiography of the lower extremity arterial disease. J. Magn. Reson. Imaging 2013, 37, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. Biomed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, G.; Laurenzano, E.; Rengo, C.; de Roza, G.; Brevetti, L.; Sannino, A.; Perrino, C.; Chiariotti, L.; Schiattarella, G.G. Abdominal aortic aneurysm in patients affected by intermittent claudication: Prevalence and clinical predictors. BMC Surg. 2012, 12, S17. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Estallo, L.; Rodríguez, L.; Baquer, M.; de Ceniga, V. Detection of abdominal aortic aneurysm in patients with peripheral artery disease. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Scaife, M.; Giannakopoulos, T.; Al-Khoury, G.E.; Chaer, R.A.; Avgerinos, E.D. Contemporary applications of ultrasound in abdominal aortic aneurysm management. Front. Surg. 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Anderson, D.R.; Yeo, K.K.; Singh, G.D.; Bang, H.; Amsterdam, E.A.; Freischlag, J.A.; Laird, J.R. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. J. Vasc. Surg. 2015, 62, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mackay, D.F.; Pell, J.P. Association between level of exposure to secondhand smoke and peripheral arterial disease: Cross-sectional study of 5686 never smokers. Atherosclerosis 2013, 229, 273–276. [Google Scholar] [CrossRef]
- Mannino, S.; Villa, M.; Apolone, G.; Weiss, N.S.; Groth, N.; Aqiono, I.; Boldori, L.; Caramaschi, F.; Gattinoni, A.; Malchiodi, G.; et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in Northern Italy. Am. J. Epidermiol. 2012, 176, 527–533. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Balady, G.J. Exercise rehabilitation in peripheral artery disease: Functional impact and mechanisms of benefits. Circulation 2011, 123, 87–97. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Domanchuk, K.; Liu, K.; Guralnik, J.M.; Tian, L.; Criqui, M.H.; Ferrucci, L.; Kibbe, M.; Jones, D.L.; Pearce, W.H.; et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemp. Clin. Trials 2012, 33, 1311–1320. [Google Scholar] [CrossRef]
- Pickwell, K.; Siersma, V.; Kars, M.; Apelqvist, J.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovská, A.; Jude, E.; Mauricio, D.; et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015, 38, 852–857. [Google Scholar] [CrossRef]
- Ouriel, K.; Veith, F.J.; Sasahara, A.A. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N. Engl. J. Med. 1998, 338, 1105–1111. [Google Scholar] [CrossRef]
- Rooke, T.W.; Hirsch, A.T.; Misra, S.; Sidawy, A.N.; Beckman, J.A.; Findeiss, L.K.; Golzarian, J.; Gornik, H.L.; Halperin, J.L.; Jaff, M.R.; et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 58, 2020–2045. [Google Scholar]
- Adam, D.J.; Bradbury, A.W. TASC II document on the management of peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 1–2. [Google Scholar] [CrossRef]
| Disease Aspects | 2005 | 2011 | 2016 | Comments |
|---|---|---|---|---|
| History + Examination | Required: Walking impairment, ischemic rest pain, and non-healing wounds. | Focused updates remained same. | Additions: Vascular examination for the patients with increased risk of PAD. Non-invasive BP measurements in both arms of the patients with PAD. | LOE changed from (IC) in 2005 to (IB-NR) in 2016. Two new recommendations with LOE of (IB-NR) were added. |
| Risks | Age (<50, 50–69, >70 years), leg symptoms with exertion, abnormal lower extremity pulse exam & K/C of atherosclerosis. | Focused updates remained same. | Modifications: Age (<50, 50–64, ≥65 years), K/C of atherosclerosis in another vascular bed or AAA. | The age categories were modified in terms of risk for the patients. |
| Screening | ABI can be used for PAD screening. | Focused updates remained same. | Screening DUS for symptomatic AAA. | A new recommendation was added. |
| Test | 2005 | 2011 | 2016 | Comments |
|---|---|---|---|---|
| ABI | ABI with segmental pressures for PAD in both legs, without categorization; LOE (C). | ABI for exertional leg symptoms, non-healing wounds and risk factors for atherosclerosis. ABI results categorized. Other focused updates remained same. | ABI & segmental leg pressure for patients with presentation suggestive of PAD. Resting ABI recommended for patients with increased risk of PAD. | LOE changed from (IB) for ABI and segmental leg pressure, and ABI categories in 2011 to (IB-NR) and (IC-LD), respectively, in 2016. A new recommendation was added with LOE (II 1B-NR). |
| Physiological testing | Pulse volume recordings (IIa B), leg segmental pressure (IB), continuous-wave DUS (IB) for location and severity, and TBI for patients in whom ABI is not reliable. Exercise treadmill test for claudication and response to therapy. Pre- and post-exercise ABI to differentiate between arterial & non-arterial claudication. | Focused updates remained same. | TBI for suspected PAD with ABI > 1.40. TBI with wave-form, TcPO2 for normal, borderline ABI with non-healing wounds or gangrene to diagnose CLI. TBI with wave-form, TcPO2 or SPP for abnormal ABI, ABI > 1.40 and TBI ≤ 0.70 with non-healing wounds or gangrene. Exercise treadmill test for patients with walking impairment and normal or abnormal ABI. ABI before and after treadmill test for patients with PAD and abnormal resting ABI. | LOE changed from (IB) in 2005 to (IB-NR) in 2016 for TBI. Two new recommendations for ABI with wave-form added (IIa B-NR). Old recommendation for exercise treadmill test modified in 2016. A new recommendation added for exercise treadmill test. |
| Imaging | Non-Invasive: DUS for extremities for location and degree of stenosis (IA), endovascular intervention (IIB), and surveillance of femoral-popliteal bypass. CTA for anatomical location and the degree of stenosis in lower extremities in PAD patients (IIb B) and as a substitute for MRA (IIb B). MRA for anatomical location and degree of stenosis in PAD patients (IA) with gadolinium enhancement (IB) as well as for the selection of endovascular intervention (IA) and post-vascularization surveillance (IIb B). | Focused updates remained the same | DUS, CTA, and MRA of lower extremities for location and severity of stenosis in symptomatic patients with PAD. | A new recommendation added for imaging (IB-NR). |
| Invasive: DSA for contrast angiographic studies (IA), and decisions for invasive therapeutic interventions (IB). Hydration for patients undergoing contrast angiography (IB). Follow-up for contrast angiography within two weeks (IC). | Angiography for patients with CLI in whom revascularization is considered (I C-EO). Angiography for patients with lifestyle-limiting symptoms with sub-optimal response to medical therapy (IIa C-EO). | New guidelines added (IC-EO & IIa C-EO). |
| Disease Aspects | New Additions |
|---|---|
| Structured exercise |
|
| Minimizing tissue loss in patients with PAD |
|
| Acute limb ischemia |
|
| Longitudinal follow-up |
|
| Influenza vaccine |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcos, R.; Louka, B.; Tseng, A.; Misra, S.; McBane, R.; Esser, H.; Shamoun, F. The Evolving Treatment of Peripheral Arterial Disease through Guideline-Directed Recommendations. J. Clin. Med. 2018, 7, 9. https://doi.org/10.3390/jcm7010009
Morcos R, Louka B, Tseng A, Misra S, McBane R, Esser H, Shamoun F. The Evolving Treatment of Peripheral Arterial Disease through Guideline-Directed Recommendations. Journal of Clinical Medicine. 2018; 7(1):9. https://doi.org/10.3390/jcm7010009
Chicago/Turabian StyleMorcos, Ramez, Boshra Louka, Andrew Tseng, Sanjay Misra, Robert McBane, Heidi Esser, and Fadi Shamoun. 2018. "The Evolving Treatment of Peripheral Arterial Disease through Guideline-Directed Recommendations" Journal of Clinical Medicine 7, no. 1: 9. https://doi.org/10.3390/jcm7010009
APA StyleMorcos, R., Louka, B., Tseng, A., Misra, S., McBane, R., Esser, H., & Shamoun, F. (2018). The Evolving Treatment of Peripheral Arterial Disease through Guideline-Directed Recommendations. Journal of Clinical Medicine, 7(1), 9. https://doi.org/10.3390/jcm7010009




