An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement
Abstract
:1. Introduction
2. Material and Methods
2.1. Zebra Fish Embryos
2.2. Rat Liver Mitochondria
2.3. Cell Culture
2.4. Mouse Astrocytes
2.5. Ethics Statement
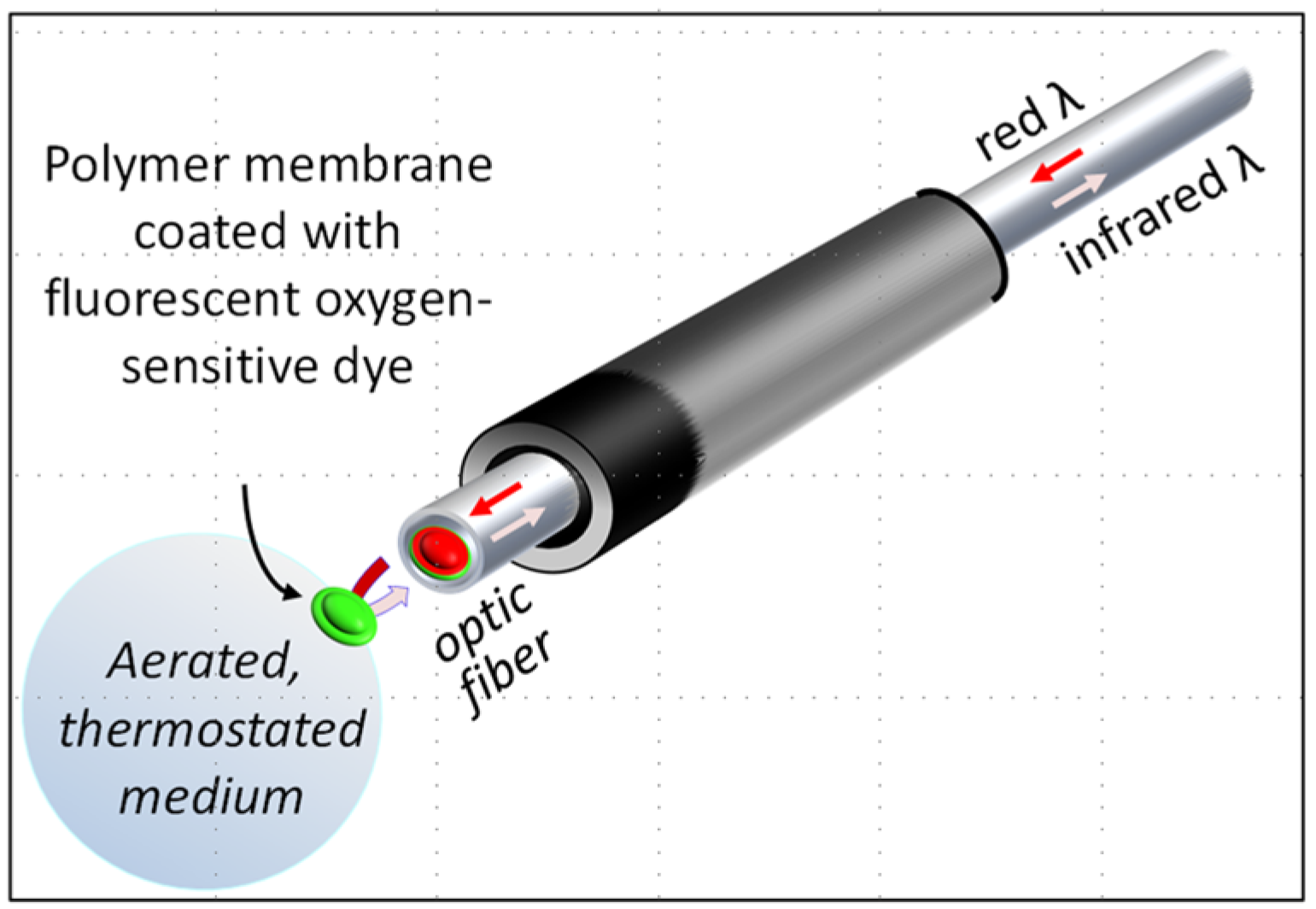
2.6. Organism, Organs, or Cells Respiration
2.7. Mitochondrial Substrate Oxidation and Membrane Potential
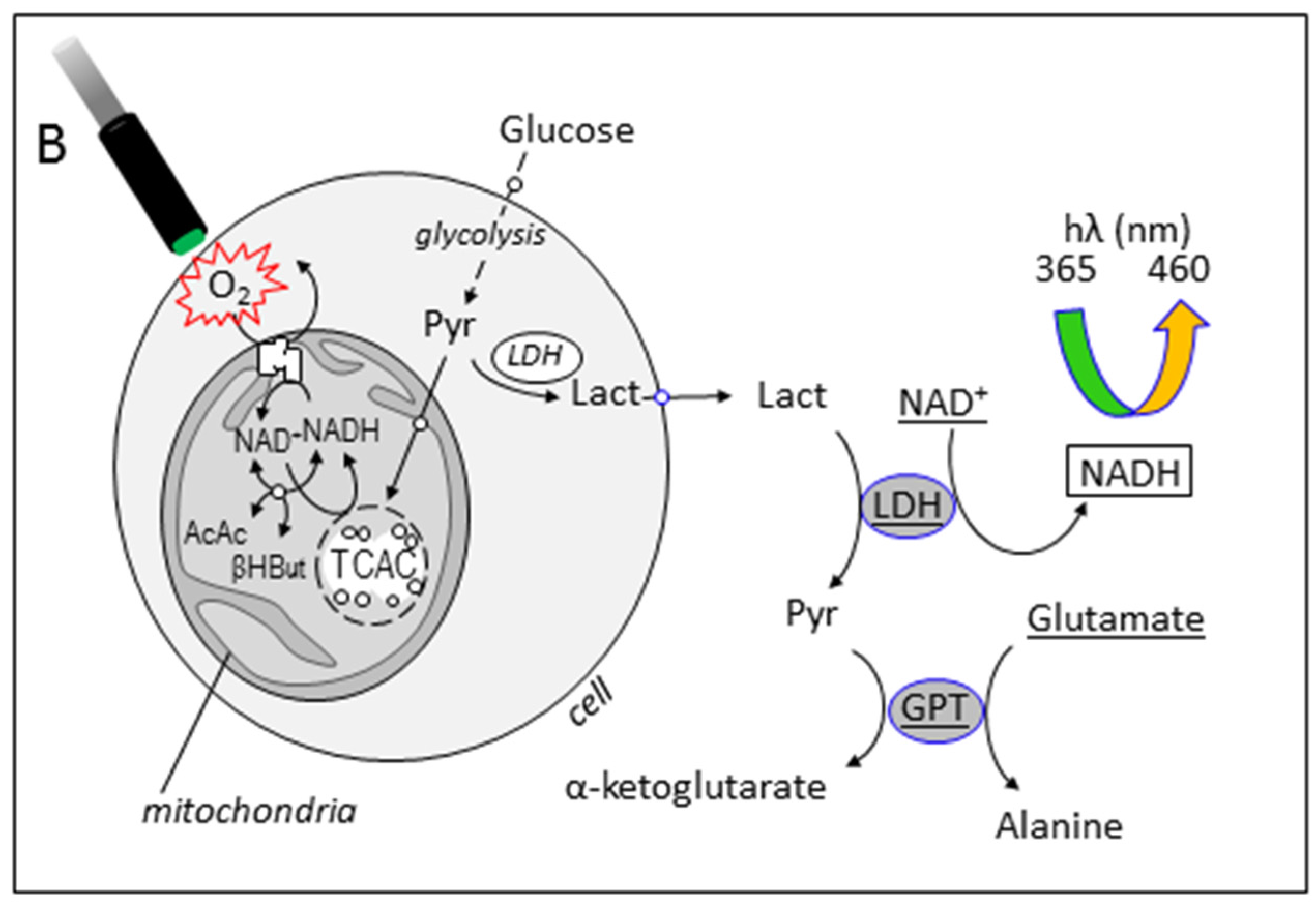
2.8. Respiration and Lactate Excretion
2.9. Protein Determination and Chemicals
2.10. Free 3D Printable Model Accessories
3. Results
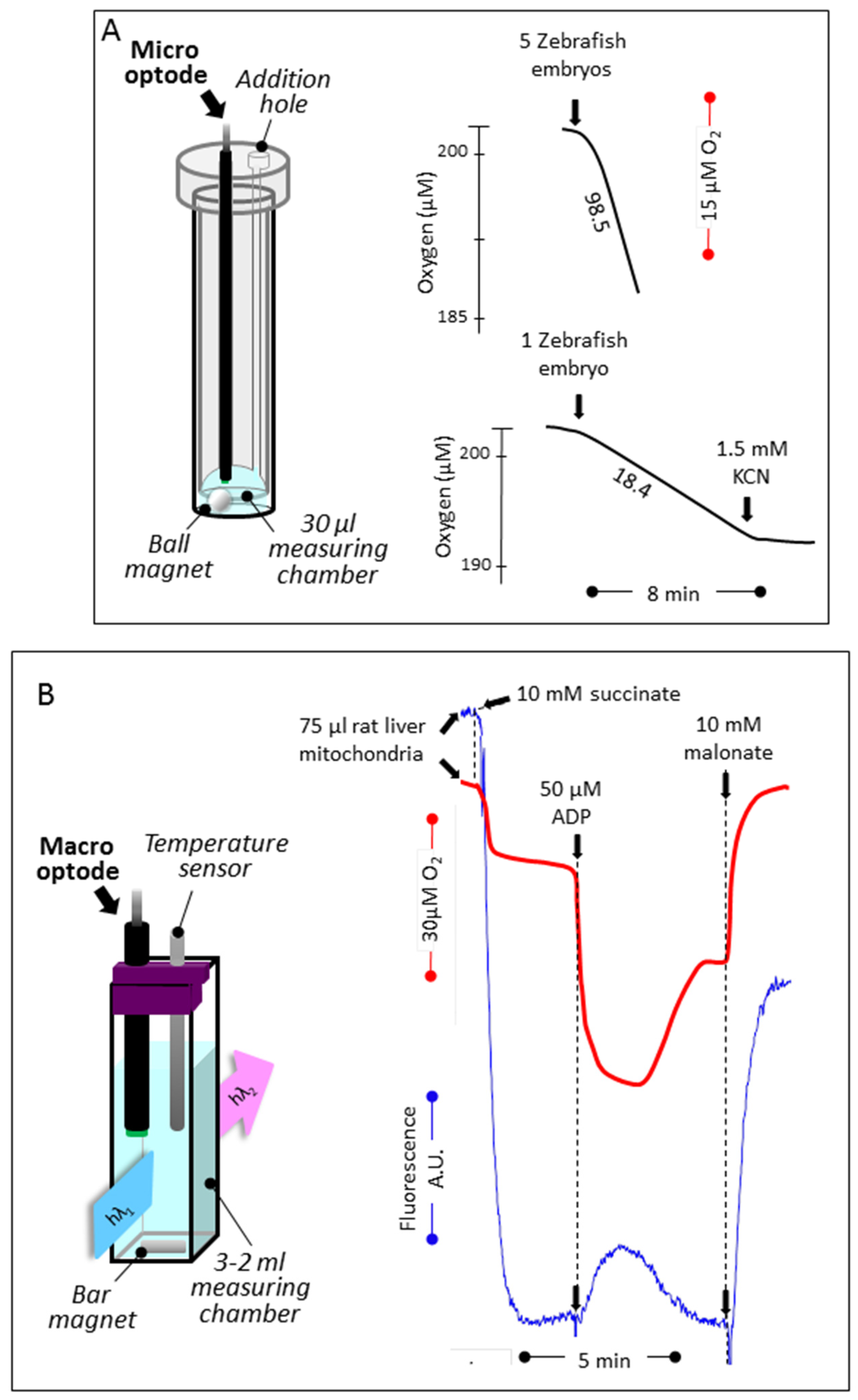
3.1. Reducing the Volume for Oxygen Consumption
3.2. Simultaneous Determination of Mitochondrial Oxygen Consumption and Membrane Potential
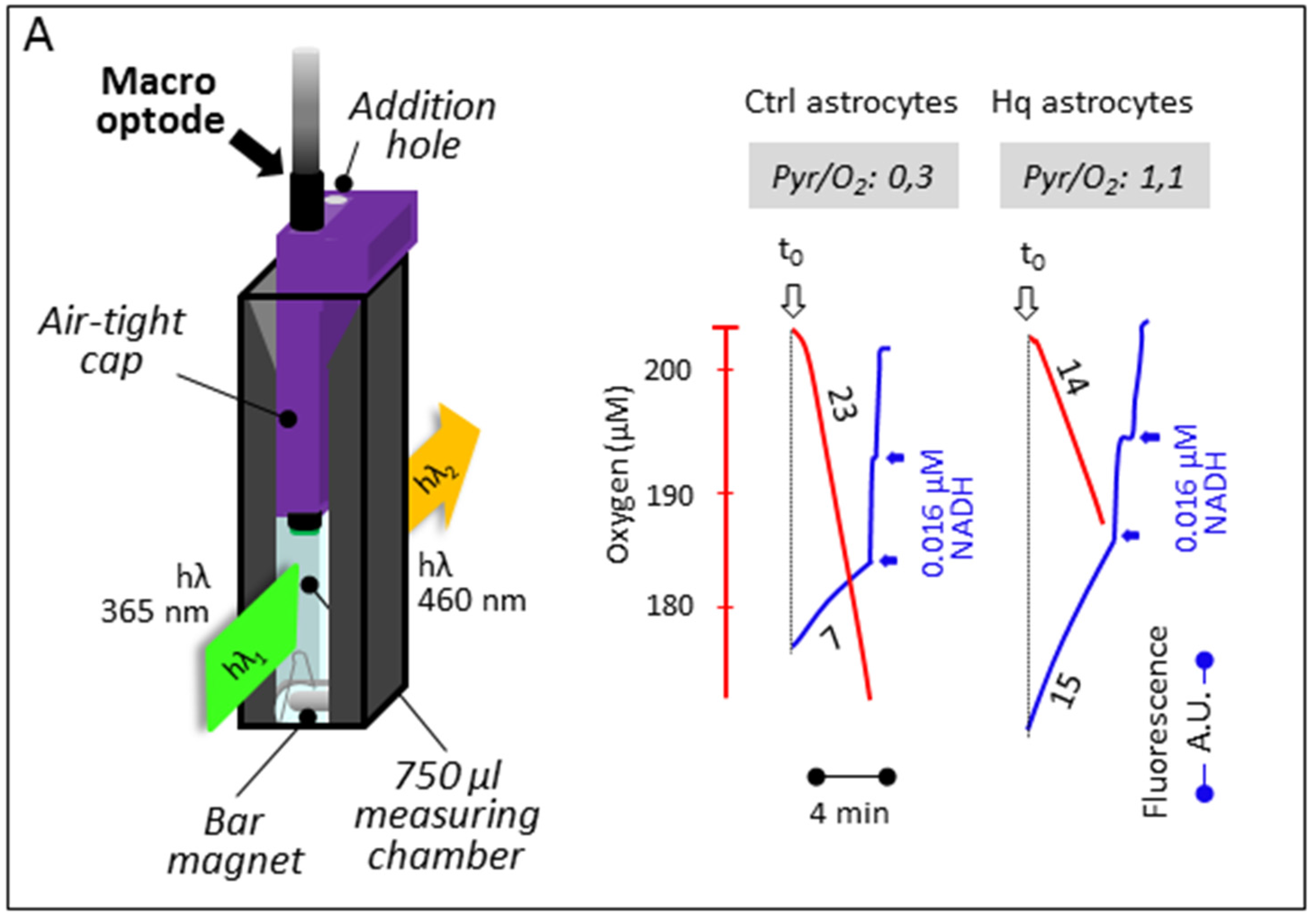
3.3. Simultaneous Determination of Cell Respiration and Lactate Excretion
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Rustin, P.; Chretien, D.; Bourgeron, T.; Gerard, B.; Rotig, A.; Saudubray, J.M.; Munnich, A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 1994, 228, 35–51. [Google Scholar] [CrossRef]
- Turnbull, D.M.; Rustin, P. Genetic and biochemical intricacy shapes mitochondrial cytopathies. Neurobiol. Dis. 2016, 92, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Benit, P.; Letouze, E.; Rak, M.; Aubry, L.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P.; Rustin, P. Unsuspected task for an old team: Succinate, fumarate and other krebs cycle acids in metabolic remodeling. Biochim. Biophys. Acta 2014, 1837, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Horobin, R.W.; Trapp, S.; Weissig, V. Mitochondriotropics: A review of their mode of action, and their applications for drug and DNA delivery to mammalian mitochondria. J. Control. Release 2007, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.L.; Ehninger, D. Environmental chemicals and aging. Curr. Environ. Health Rep. 2017, 4, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Munnich, A.; Rustin, P. Clinical spectrum and diagnosis of mitochondrial disorders. Am. J. Med. Genet. 2001, 106, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.; Newport, E.; Morten, K.J. The warburg effect: 80 years on. Biochem. Soc. Trans. 2016, 44, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Krippahl, G. Further development of manometric methods. J. Natl. Cancer Inst. 1960, 24, 51–55. [Google Scholar] [PubMed]
- Severinghaus, J.W. The invention and development of blood gas analysis apparatus. Anesthesiology 2002, 97, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, D.A.; Neilson, A.; Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 2008, 13, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Porceddu, M.; Buron, N.; Roussel, C.; Labbe, G.; Fromenty, B.; Borgne-Sanchez, A. Prediction of liver injury induced by chemicals in human with a multiparametric assay on isolated mouse liver mitochondria. Toxicol. Sci. 2012, 129, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Buron, N.; Porceddu, M.; Brabant, M.; Desgue, D.; Racoeur, C.; Lassalle, M.; Pechoux, C.; Rustin, P.; Jacotot, E.; Borgne-Sanchez, A. Use of human cancer cell lines mitochondria to explore the mechanisms of bh3 peptides and abt-737-induced mitochondrial membrane permeabilization. PLoS ONE 2010, 5, e9924. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, H.; Langonne, A.; Baux, L.; Rebouillat, D.; Rustin, P.; Prevost, M.C.; Brenner, C.; Edelman, L.; Jacotot, E. Real-time flow cytometry analysis of permeability transition in isolated mitochondria. Exp. Cell Res. 2004, 294, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Vahsen, N.; Cande, C.; Briere, J.J.; Benit, P.; Joza, N.; Larochette, N.; Mastroberardino, P.G.; Pequignot, M.O.; Casares, N.; Lazar, V.; et al. Aif deficiency compromises oxidative phosphorylation. EMBO J. 2004, 23, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar] [PubMed]
- Thorn, M.B. Inhibition by malonate of succinic dehydrogenase in heart-muscle preparations. Biochem. J. 1953, 54, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Benit, P.; Pelhaitre, A.; Saunier, E.; Bortoli, S.; Coulibaly, A.; Rak, M.; Schiff, M.; Kroemer, G.; Zeviani, M.; Rustin, P. Paradoxical inhibition of glycolysis by pioglitazone opposes the mitochondriopathy caused by aif deficiency. EBioMedicine 2017, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mosquera, L.; Diogo, C.V.; Yambire, K.F.; Santos, G.L.; Luna Sanchez, M.; Benit, P.; Rustin, P.; Lopez, L.C.; Milosevic, I.; Raimundo, N. Acute and chronic mitochondrial respiratory chain deficiency differentially regulate lysosomal biogenesis. Sci. Rep. 2017, 7, 45076. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Oellermann, M.; Portner, H.O.; Mark, F.C. Simultaneous high-resolution pH and spectrophotometric recordings of oxygen binding in blood microvolumes. J. Exp. Biol. 2014, 217, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, M.B.; Bushnell, P.G.; Steffensen, J.F. Design and setup of intermittent-flow respirometry system for aquatic organisms. J. Fish Biol. 2016, 88, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F. Some errors in respirometry of aquatic breathers: How to avoid and correct for them. Fish Physiol. Biochem. 1989, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; Li, Z.; Thomas, Z.; Stevens, C.W. Assessment of tissue oxygen tension: Comparison of dynamic fluorescence quenching and polarographic electrode technique. Crit. Care 2002, 6, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Sica, V.; Bravo-San Pedro, J.M.; Pietrocola, F.; Izzo, V.; Maiuri, M.C.; Kroemer, G.; Galluzzi, L. Assessment of glycolytic flux and mitochondrial respiration in the course of autophagic responses. Methods Enzymol. 2017, 588, 155–170. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bénit, P.; Chrétien, D.; Porceddu, M.; Yanicostas, C.; Rak, M.; Rustin, P. An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement. J. Clin. Med. 2017, 6, 58. https://doi.org/10.3390/jcm6060058
Bénit P, Chrétien D, Porceddu M, Yanicostas C, Rak M, Rustin P. An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement. Journal of Clinical Medicine. 2017; 6(6):58. https://doi.org/10.3390/jcm6060058
Chicago/Turabian StyleBénit, Paule, Dominique Chrétien, Mathieu Porceddu, Constantin Yanicostas, Malgorzata Rak, and Pierre Rustin. 2017. "An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement" Journal of Clinical Medicine 6, no. 6: 58. https://doi.org/10.3390/jcm6060058
APA StyleBénit, P., Chrétien, D., Porceddu, M., Yanicostas, C., Rak, M., & Rustin, P. (2017). An Effective, Versatile, and Inexpensive Device for Oxygen Uptake Measurement. Journal of Clinical Medicine, 6(6), 58. https://doi.org/10.3390/jcm6060058




