Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery
Abstract
:1. Introduction
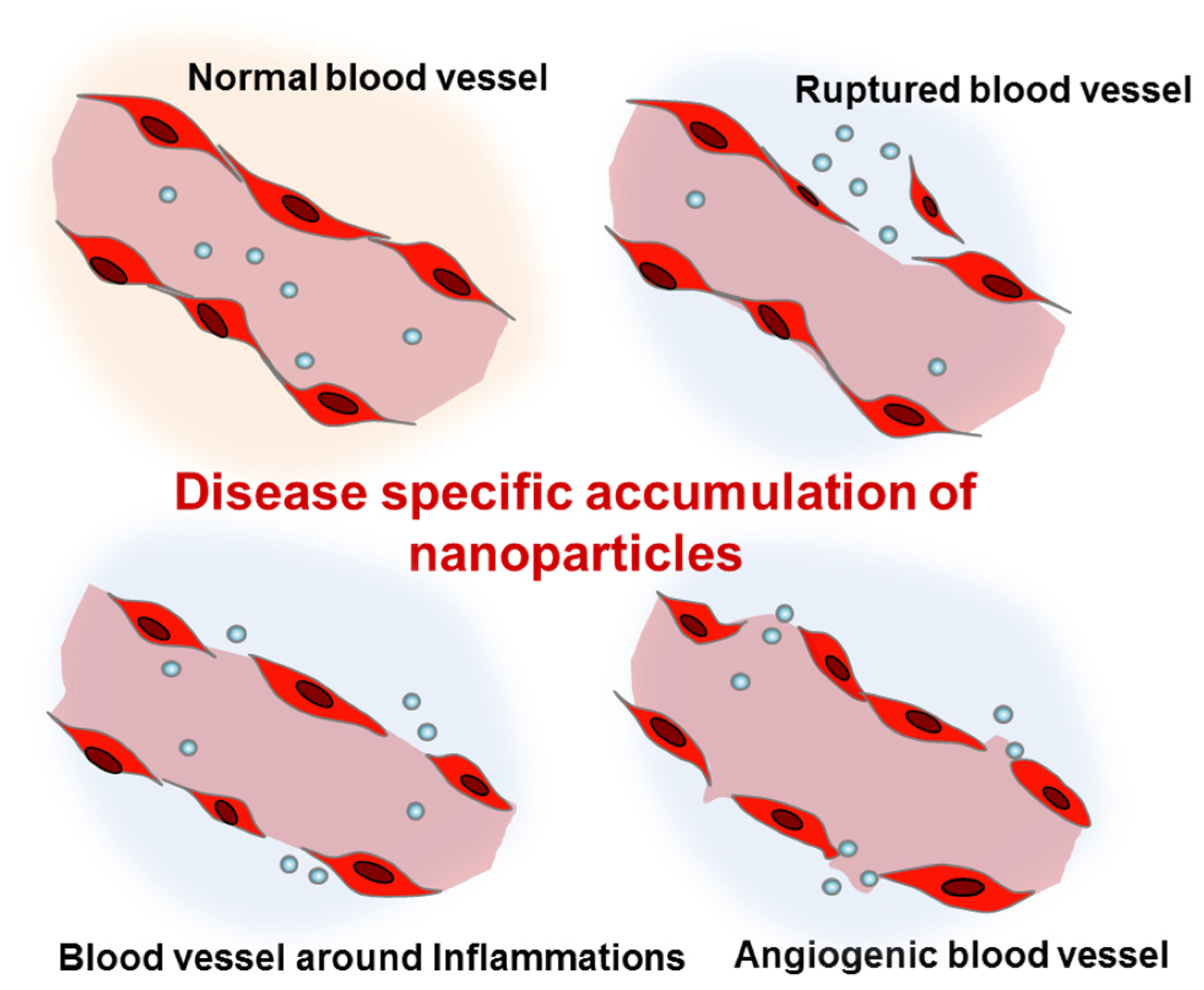
2. Concept of Targeted Delivery and in Vivo Behavior of Nanoparticles
2.1. Route of Delivery and in Vivo Behavior of Nanoparticles
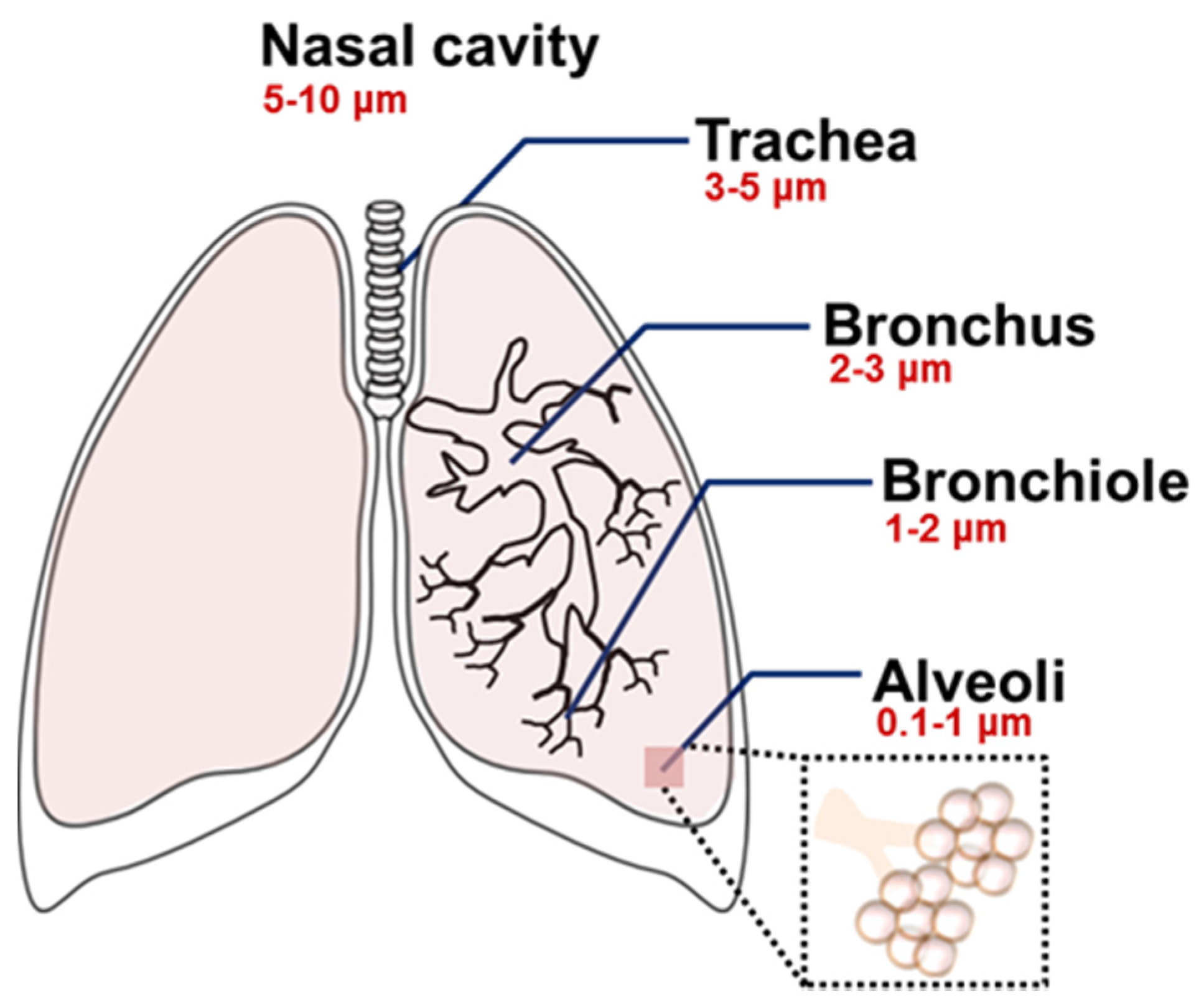
2.2. Determinants for the Pulmonary Delivery of Nanoparticles
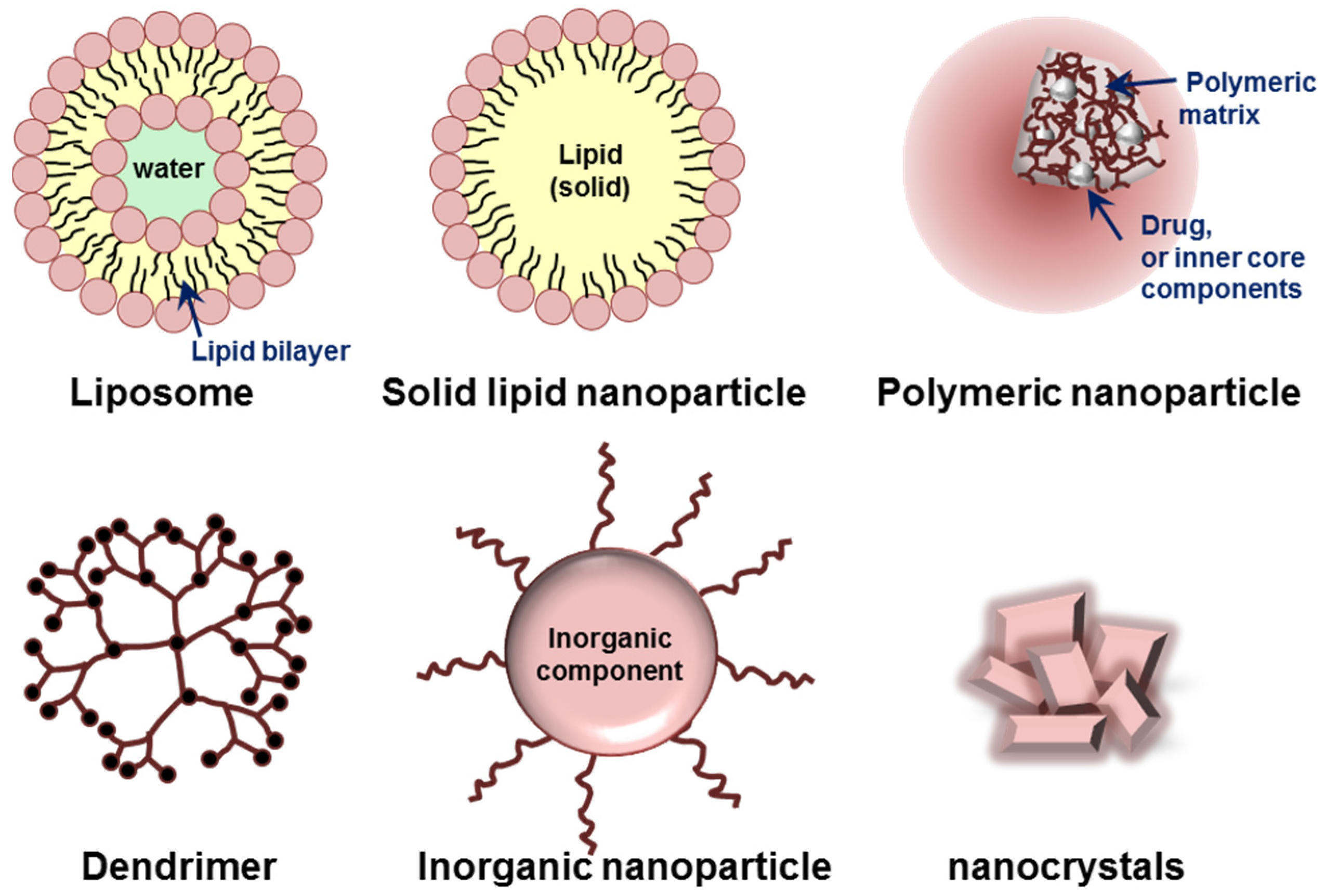
3. Various Nanoparticles for Chronic Lung Diseases
3.1. Liposomes and Solid Lipid Nanoparticles
3.2. Natural and Synthetic Polymer-Based Nanoparticles
3.3. Dendrimers
3.4. Inorganic Nanoparticles
4. Current Nanomedicine for Chronic Lung Diseases
5. Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2013; World Health Organization: Geneva, Switzerland, 2013; pp. 6–27. [Google Scholar]
- World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach; World Health Organization: Geneva, Switzerland, 2007; pp. 12–36. [Google Scholar]
- Halbert, R.J.; Natoli, J.L.; Gano, A.; Badamgarav, E.; Buist, A.S.; Mannino, D.M. Global burden of COPD: Systematic review and meta-analysis. Eur. Respir. J. 2006, 28, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R.; Global Initiative for Asthma P. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R. Epidemiology of idiopathic pulmonary fibrosis. Clin. Epidemiol. 2013, 5, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, C.; Schmidt, R.; Grimminger, F.; Suzuki, Y.; Seeger, W.; Lehr, C.M.; Gunther, A. Chemical coupling of a monoclonal antisurfactant protein-B antibody to human urokinase for targeting surfactant-incorporating alveolar fibrin. Bioconjug. Chem. 2002, 13, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Caramori, G.; Chung, K.F.; Adcock, I.M. Targeted anti-inflammatory therapeutics in asthma and chronic obstructive lung disease. Transl. Res. 2016, 167, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.E.; Bonvini, S.J.; Donovan, C.; Foong, R.E.; Han, B.; Jha, A.; Shaifta, Y.; Smit, M.; Johnson, J.R.; Moir, L.M. Novel drug targets for asthma and COPD: Lessons learned from in vitro and in vivo models. Pulm. Pharmacol. Ther. 2014, 29, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Takeshita, F.; Kuwano, K.; Ochiya, T. RNAi Therapeutic Platforms for Lung Diseases. Pharmaceuticals 2013, 6, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Diagnosis and management of interstitial lung disease. Transl. Respir. Med. 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Burhan, E.; Ruesen, C.; Ruslami, R.; Ginanjar, A.; Mangunnegoro, H.; Ascobat, P.; Donders, R.; van Crevel, R.; Aarnoutse, R. Isoniazid, rifampin, and pyrazinamide plasma concentrations in relation to treatment response in Indonesian pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 2013, 57, 3614–3619. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.; Mohammadi, F. Nanomedicine for respiratory diseases. Tanaffos 2012, 11, 18–22. [Google Scholar] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. Int. J. Nanomed. 2008, 3, 1–19. [Google Scholar]
- Di Gioia, S.; Trapani, A.; Castellani, S.; Carbone, A.; Belgiovine, G.; Craparo, E.F.; Puglisi, G.; Cavallaro, G.; Trapani, G.; Conese, M. Nanocomplexes for gene therapy of respiratory diseases: Targeting and overcoming the mucus barrier. Pulm. Pharmacol. Ther. 2015, 34, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Ratemi, E.; Sultana Shaik, A.; Al Faraj, A.; Halwani, R. Alternative approaches for the treatment of airway diseases: Focus on nanoparticle medicine. Clin. Exp. Allergy 2016, 46, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Serkova, N.J. Non-invasive imaging to monitor lupus nephritis and neuropsychiatric systemic lupus erythematosus. F1000Research 2015, 4, 153. [Google Scholar] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Son, S.; Kim, S.H.; Park, K.; Choi, K.; Kwon, I.C. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J. Control Release 2014, 193, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, J.T.; Kuo, Y.C. Polybutylcyanoacrylate nanoparticles for delivering hormone response element-conjugated neurotrophin-3 to the brain of intracerebral hemorrhagic rats. Biomaterials 2013, 34, 9717–9727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, A.; Hwang, S.R.; Park, J.S.; Jang, J.; Huh, M.S.; Jo, D.G.; Yoon, S.Y.; Byun, Y.; Kim, S.H.; Kwon, I.C.; Youn, I.; Kim, K. TNF-alpha gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol. Ther. 2014, 22, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Ali, S.F.; Dong, W.; Tian, Z.R.; Patnaik, R.; Patnaik, S.; Sharma, A.; Boman, A.; Lek, P.; Seifert, E.; Lundstedt, T. Drug delivery to the spinal cord tagged with nanowire enhances neuroprotective efficacy and functional recovery following trauma to the rat spinal cord. Ann. N. Y. Acad. Sci. 2007, 1122, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Putz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control Release 2016, 238, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Hatoum, H.; Dy, G.K. First line treatment of advanced non-small-cell lung cancer—Specific focus on albumin bound paclitaxel. Int. J. Nanomed. 2014, 9, 209–221. [Google Scholar]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Ait-Oudhia, S.; Mager, D.E.; Straubinger, R.M. Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology. Pharmaceutics 2014, 6, 137–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ipe, B.I.; Misra, P.; Lee, J.H.; Bawendi, M.G.; Frangioni, J.V. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009, 9, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Gipps, E.M.; Arshady, R.; Kreuter, J.; Groscurth, P.; Speiser, P.P. Distribution of polyhexyl cyanoacrylate nanoparticles in nude mice bearing human osteosarcoma. J. Pharm. Sci. 1986, 75, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.; Collet, B.; Le Verge, R.; Toujas, L. Blood clearance and organ distribution of intravenously administered polymethacrylic nanoparticles in mice. J. Pharm. Sci. 1989, 78, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Bazile, D.V.; Ropert, C.; Huve, P.; Verrecchia, T.; Marlard, M.; Frydman, A.; Veillard, M.; Spenlehauer, G. Body distribution of fully biodegradable [14C]-poly(lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials 1992, 13, 1093–1102. [Google Scholar] [CrossRef]
- Azarmi, S.; Roa, W.H.; Lobenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wang, L.; Ling, R.; Li, Y.; Wang, Z.; Yao, Q.; Ma, Z. Body distribution of nanoparticle-containing adriamycin injected into the hepatic artery of hepatoma-bearing rats. Dig. Dis. Sci. 2004, 49, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.K.; Lu, Z.; Wientjes, M.G.; Au, J.L. Formulating paclitaxel in nanoparticles alters its disposition. Pharm. Res. 2005, 22, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.P.; Cavalli, R.; Fundaro, A.; Bargoni, A.; Caputo, O.; Gasco, M.R. Pharmacokinetics of doxorubicin incorporated in solid lipid nanospheres (SLN). Pharmacol. Res. 1999, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Santhi, K.; Dhanaraj, S.A.; Koshy, M.; Ponnusankar, S.; Suresh, B. Study of biodistribution of methotrexate-loaded bovine serum albumin nanospheres in mice. Drug Dev. Ind. Pharm. 2000, 26, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Traini, D.; Young, P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J. Pharm. Sci. 2015, 10, 481–489. [Google Scholar] [CrossRef]
- Savla, R.; Minko, T. Nanotechnology approaches for inhalation treatment of fibrosis. J. Drug Target. 2013, 21, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Kuzmov, A.; Minko, T. Nanotechnology approaches for inhalation treatment of lung diseases. J. Control Release 2015, 219, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Lehofer, B.; Bloder, F.; Jain, P.P.; Marsh, L.M.; Leitinger, G.; Olschewski, H.; Leber, R.; Olschewski, A.; Prassl, R. Impact of atomization technique on the stability and transport efficiency of nebulized liposomes harboring different surface characteristics. Eur. J. Pharm. Biopharm. 2014, 88, 1076–1085. [Google Scholar] [CrossRef]
- Jiang, H.L.; Hong, S.H.; Kim, Y.K.; Islam, M.A.; Kim, H.J.; Choi, Y.J.; Nah, J.W.; Lee, K.H.; Han, K.W.; Chae, C.; et al. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int. J. Pharm. 2011, 420, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Serigano, T.; Hino, T.; Yamamoto, H.; Takeuchi, H. A new powder design method to improve inhalation efficiency of pranlukast hydrate dry powder aerosols by surface modification with hydroxypropylmethylcellulose phthalate nanospheres. Pharm. Res. 1998, 15, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Sham, J.O.; Zhang, Y.; Finlay, W.H.; Roa, W.H.; Lobenberg, R. Formulation and characterization of spray-dried powders containing nanoparticles for aerosol delivery to the lung. Int. J. Pharm. 2004, 269, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. 2016, 25, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, M.; Frederik, P.M.; Vallner, J.J.; Lasic, D.D. Stealth(R) liposomes: From theory to product. Adv. Drug Deliv. Rev. 1997, 24, 165–177. [Google Scholar]
- Allen, T.M. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs 1998, 56, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chen, F.; Mozhi, A.; Zhang, X.; Zhao, Y.; Xue, X.; Hao, Y.; Zhang, X.; Wang, P.C.; Liang, X.J. Innovative pharmaceutical development based on unique properties of nanoscale delivery formulation. Nanoscale 2013, 5, 8307–8325. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Bein, T.; Meiners, S. Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 2014, 44, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Malapit, M.; Mallory, E.; Hayes, D., Jr.; Mansour, H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine 2015, 11, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; McLeod, V.M.; Ryan, G.M.; Kelly, B.D.; Haynes, J.M.; Williamson, M.; Thienthong, N.; Owen, D.J.; Porter, C.J. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J. Control Release 2014, 183, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.M.; Kaminskas, L.M.; Kelly, B.D.; Owen, D.J.; McIntosh, M.P.; Porter, C.J. Pulmonary administration of PEGylated polylysine dendrimers: Absorption from the lung versus retention within the lung is highly size-dependent. Mol. Pharm. 2013, 10, 2986–2995. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, M.; Muller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.G.; Wong, J.; Zhou, Q.T.; Leung, S.S.; Chan, H.K. Advances in device and formulation technologies for pulmonary drug delivery. AAPS PharmSciTech 2014, 15, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Faiyazuddin, M.; Mujahid, M.; Hussain, T.; Siddiqui, H.H.; Bhatnagar, A.; Khar, R.K.; Ahmad, F.J. Aerodynamics and deposition effects of inhaled submicron drug aerosol in airway diseases. Recent Pat. Inflamm. Allergy Drug Discov. 2013, 7, 49–61. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, I.; Perfetto, B.; Costabile, G.; Ambrosini, V.; Caputo, P.; Miro, A.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Donnarumma, G.; Quaglia, F.; Ungaro, F. Large Porous Particles for Sustained Release of a Decoy Oligonucelotide and Poly(ethylenimine): Potential for Combined Therapy of Chronic Pseudomonas aeruginosa Lung Infections. Biomacromolecules 2016, 17, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Elhissi, A.M.A.; Faizi, M.; Naji, W.F.; Gill, H.S.; Taylor, K.M.G. Physical stability and aerosol properties of liposomes delivered using an air-jet nebulizer and a novel micropump device with large mesh apertures. Int. J. Pharm. 2007, 334, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Niven, R.W. Delivery of biotherapeutics by inhalation aerosol. Crit. Rev. Ther. Drug Carr. Syst. 1995, 12, 151–231. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Son, S.; Kim, N.; Choi, K.; Kwon, I.C. Theranostic applications of organic nanoparticles for cancer treatment. Mrs Bull. 2014, 39, 239–249. [Google Scholar] [CrossRef]
- Kim, T.; Hyeon, T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 2014, 25, 012001. [Google Scholar] [CrossRef] [PubMed]
- Estella-Hermoso de Mendoza, A.; Campanero, M.A.; Mollinedo, F.; Blanco-Prieto, M.J. Lipid nanomedicines for anticancer drug therapy. J. Biomed. Nanotechnol. 2009, 5, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Nassimi, M.; Schleh, C.; Lauenstein, H.D.; Hussein, R.; Hoymann, H.G.; Koch, W.; Pohlmann, G.; Krug, N.; Sewald, K.; Rittinghausen, S.; Braun, A.; Muller-Goymann, C. A toxicological evaluation of inhaled solid lipid nanoparticles used as a potential drug delivery system for the lung. Eur. J. Pharm. Biopharm. 2010, 75, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Nassimi, M.; Schleh, C.; Lauenstein, H.D.; Hussein, R.; Lubbers, K.; Pohlmann, G.; Switalla, S.; Sewald, K.; Muller, M.; Krug, N.; Muller-Goymann, C.C.; Braun, A. Low cytotoxicity of solid lipid nanoparticles in in vitro and ex vivo lung models. Inhal. Toxicol. 2009, 21, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Chougule, M.B.; Ian, T.; Patlolla, R.; Wang, G.; Singh, M. Efficacy of aerosolized celecoxib encapsulated nanostructured lipid carrier in non-small cell lung cancer in combination with docetaxel. Pharm. Res. 2013, 30, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Elhissi, A.M.A.; Islam, M.A.; Arafat, B.; Taylor, M.; Ahmed, W. Development and characterisation of freeze-dried liposomes containing two anti-asthma drugs. Micro Nano Lett. 2010, 5, 184–188. [Google Scholar] [CrossRef]
- Hoesel, L.M.; Flierl, M.A.; Niederbichler, A.D.; Rittirsch, D.; McClintock, S.D.; Reuben, J.S.; Pianko, M.J.; Stone, W.; Yang, H.; Smith, M.; et al. Ability of antioxidant liposomes to prevent acute and progressive pulmonary injury. Antioxid. Redox Signal. 2008, 10, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, J.; Dai, Q.; Yin, X.; Zhang, X.; Zheng, A. In vitro and in vivo evaluation of ciprofloxacin liposomes for pulmonary administration. Drug Dev. Ind. Pharm. 2015, 41, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Romeijn, S.; Junginger, H.E.; Borchard, G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 2004, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Gupta, K.C. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin. Drug Deliv. 2013, 10, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.H.; Lee, K.M.; Kim, S.T.; Jin, S.E.; Kim, C.K. Polyethylenimine-based antisense oligodeoxynucleotides of IL-4 suppress the production of IL-4 in a murine model of airway inflammation. J. Gene Med. 2006, 8, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Surti, N.; Naik, S.; Bagchi, T.; Dwarkanath, B.S.; Misra, A. Intracellular delivery of nanoparticles of an antiasthmatic drug. AAPS PharmSciTech 2008, 9, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly (dl-lactide-co-glycolide) nanoparticle-based inhalable sustained drug delivery system for experimental tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Egashira, K.; Chen, L.; Nakano, K.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Hara, K.; Morishita, R.; Sueishi, K.; Tominaga, R.; Sunagawa, K. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009, 53, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Bharatwaj, B.; Mohammad, A.K.; Dimovski, R.; Cassio, F.L.; Bazito, R.C.; Conti, D.; Fu, Q.; Reineke, J.; da Rocha, S.R. Dendrimer nanocarriers for transport modulation across models of the pulmonary epithelium. Mol. Pharm. 2015, 12, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.G.; Guimaraes, A.P.; Pacheco, M.A.; Dias, D.M.; Furtado, V.R.; de Alencastro, R.B.; Horta, B.A. Association of the anti-tuberculosis drug rifampicin with a PAMAM dendrimer. J. Mol. Graph. Model. 2015, 60, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Inapagolla, R.; Guru, B.R.; Kurtoglu, Y.E.; Gao, X.; Lieh-Lai, M.; Bassett, D.J.; Kannan, R.M. In vivo efficacy of dendrimer-methylprednisolone conjugate formulation for the treatment of lung inflammation. Int. J. Pharm. 2010, 399, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Glaus, C.; Chen, J.; Welch, M.J.; Xia, Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol. Med. 2010, 16, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Lee, S.; Chen, X. Inorganic nanoparticles for multimodal molecular imaging. Mol. Imaging 2011, 10, 3–16. [Google Scholar] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Capek, I.; Tiwari, A. DNA Engineered Noble Metal Nanoparticles: Fundamentals and State-of-the-Art of Nanobiotechnology; Wiley: New Jersey, NJ, USA, 2015; pp. 173–280. [Google Scholar]
- Geiser, M.; Quaile, O.; Wenk, A.; Wigge, C.; Eigeldinger-Berthou, S.; Hirn, S.; Schaffler, M.; Schleh, C.; Moller, W.; Mall, M.A.; Kreyling, W.G. Cellular uptake and localization of inhaled gold nanoparticles in lungs of mice with chronic obstructive pulmonary disease. Part. Fibre Toxicol. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Su, S.F.; Chien, C.T.; Lin, W.H.; Yu, S.L.; Chou, C.C.; Chen, J.J.; Yang, P.C. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. 2006, 20, 2393–2395. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Chandolu, V.; Dass, C.R. Treatment of lung cancer using nanoparticle drug delivery systems. Curr. Drug Discov. Technol. 2013, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- McLendon, J.M.; Joshi, S.R.; Sparks, J.; Matar, M.; Fewell, J.G.; Abe, K.; Oka, M.; McMurtry, I.F.; Gerthoffer, W.T. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control Release 2015, 210, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, R.; Xie, Q.; Li, A.; Xiao, Y.; Li, K.; Liu, H.; Cui, D.; Chen, Y.; Wang, S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Malerba, M.; Santini, G.; Miravitlles, M. Pharmacological treatment of chronic obstructive pulmonary disease: From evidence-based medicine to phenotyping. Drug Discov. Today 2014, 19, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.L.; Santos, R.S.; Xisto, D.G.; Alonso Sdel, V.; Morales, M.M.; Rocco, P.R. Nanoparticle-based therapy for respiratory diseases. Anais da Academia Brasileira de Ciencias 2013, 85, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Misra, A.N. Pulmonary disposition of budesonide from liposomal dry powder inhaler. Methods Find Exp. Clin. Pharmacol. 2001, 23, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Konduri, K.S.; Nandedkar, S.; Duzgunes, N.; Suzara, V.; Artwohl, J.; Bunte, R.; Gangadharam, P.R. Efficacy of liposomal budesonide in experimental asthma. J. Allergy Clin. Immunol. 2003, 111, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Ishihara, T.; Ishizaki, J.; Miyamoto, K.; Higaki, M.; Yamashita, N. Effect of betamethasone phosphate loaded polymeric nanoparticles on a murine asthma model. Cell Immunol. 2009, 260, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Redente, E.F.; Thakur, A.; Riches, D.W.; Kompella, U.B. Local delivery of biodegradable pirfenidone nanoparticles ameliorates bleomycin-induced pulmonary fibrosis in mice. Nanotechnology 2012, 23, 505101. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Martin, G.; Medina, M.; Ask, K.; Gauldie, J. Gene therapy for pulmonary diseases. Chest 2006, 130, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Hellermann, G.R.; Zhang, W.; Jena, P.; Kumar, M.; Behera, A.; Behera, S.; Lockey, R.; Mohapatra, S.S. Chitosan Interferon-gamma Nanogene Therapy for Lung Disease: Modulation of T-Cell and Dendritic Cell Immune Responses. Allergy Asthma Clin. Immunol. 2008, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Pizzichini, M.M.; Kjarsgaard, M.; Inman, M.D.; Efthimiadis, A.; Pizzichini, E.; Hargreave, F.E.; O’Byrne, P.M. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 2009, 360, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Boppana, N.B.; Devarajan, A.; Gopal, K.; Barathan, M.; Bakar, S.A.; Shankar, E.M.; Ebrahim, A.S.; Farooq, S.M. Blockade of CXCR2 signalling: A potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp. Biol. Med. 2014, 239, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Oiso, Y.; Sakai, H.; Motomura, T.; Yamashita, C. Pulmonary administration of phosphoinositide 3-kinase inhibitor is a curative treatment for chronic obstructive pulmonary disease by alveolar regeneration. J. Control Release 2015, 213, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Afzal, S.; Al Sayed, B.; Halwani, R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int. J. Nanomed. 2014, 9, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc. Am. Thorac. Soc. 2010, 7, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, B.M.; Park, S.; Han, B.; Yeo, Y. A strategy to deliver genes to cystic fibrosis lungs: A battle with environment. J. Control Release 2011, 155, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Lai, S.K.; Wang, Y.Y.; Ensign, L.M.; Zeitlin, P.L.; Boyle, M.P.; Hanes, J. The penetration of fresh undiluted sputum expectorated by cystic fibrosis patients by non-adhesive polymer nanoparticles. Biomaterials 2009, 30, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Broughton-Head, V.J.; Smith, J.R.; Shur, J.; Shute, J.K. Actin limits enhancement of nanoparticle diffusion through cystic fibrosis sputum by mucolytics. Pulm. Pharmacol. Ther. 2007, 20, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tsifansky, M.D.; Shin, S.; Lin, Q.; Yeo, Y. Mannitol-Guided Delivery of Ciprofloxacin in Artificial Cystic Fibrosis Mucus Model. Biotechnol. Bioeng. 2010, 108, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Duncan, G.A.; Hanes, J.; Suk, J.S. Barriers to inhaled gene therapy of obstructive lung diseases: A review. J. Control Release 2016, in press. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yhee, J.Y.; Im, J.; Nho, R.S. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82. https://doi.org/10.3390/jcm5090082
Yhee JY, Im J, Nho RS. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. Journal of Clinical Medicine. 2016; 5(9):82. https://doi.org/10.3390/jcm5090082
Chicago/Turabian StyleYhee, Ji Young, Jintaek Im, and Richard Seonghun Nho. 2016. "Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery" Journal of Clinical Medicine 5, no. 9: 82. https://doi.org/10.3390/jcm5090082
APA StyleYhee, J. Y., Im, J., & Nho, R. S. (2016). Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. Journal of Clinical Medicine, 5(9), 82. https://doi.org/10.3390/jcm5090082




