Plasma Vascular Endothelial Growth Factor Concentration and Alveolar Nitric Oxide as Potential Predictors of Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Study Design
2.3. Exhaled Nitric Oxide Measurement
2.4. Vascular Endothelial Growth Factor (VEGF)
2.5. Measurement of Progression
2.6. Statistical Analysis
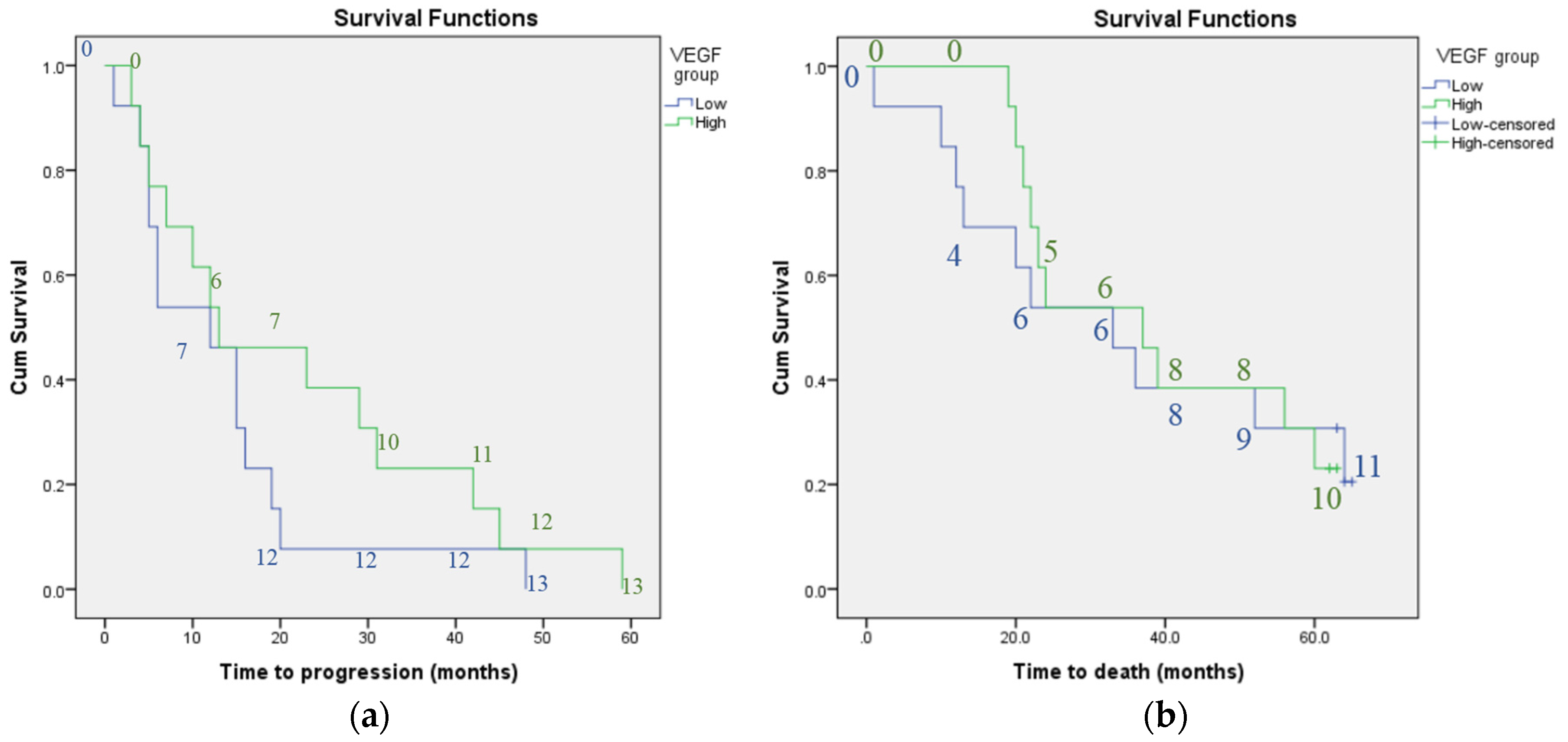
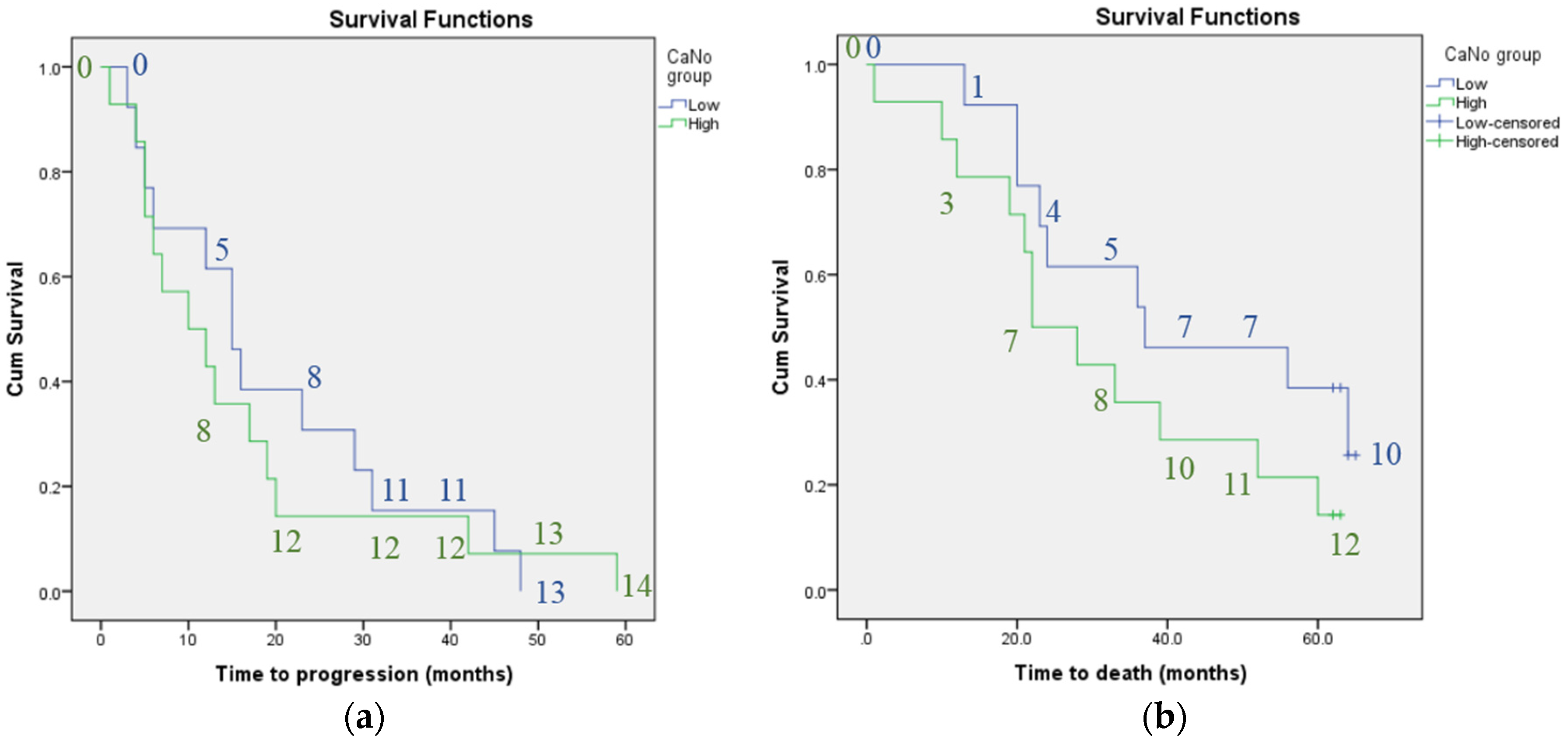
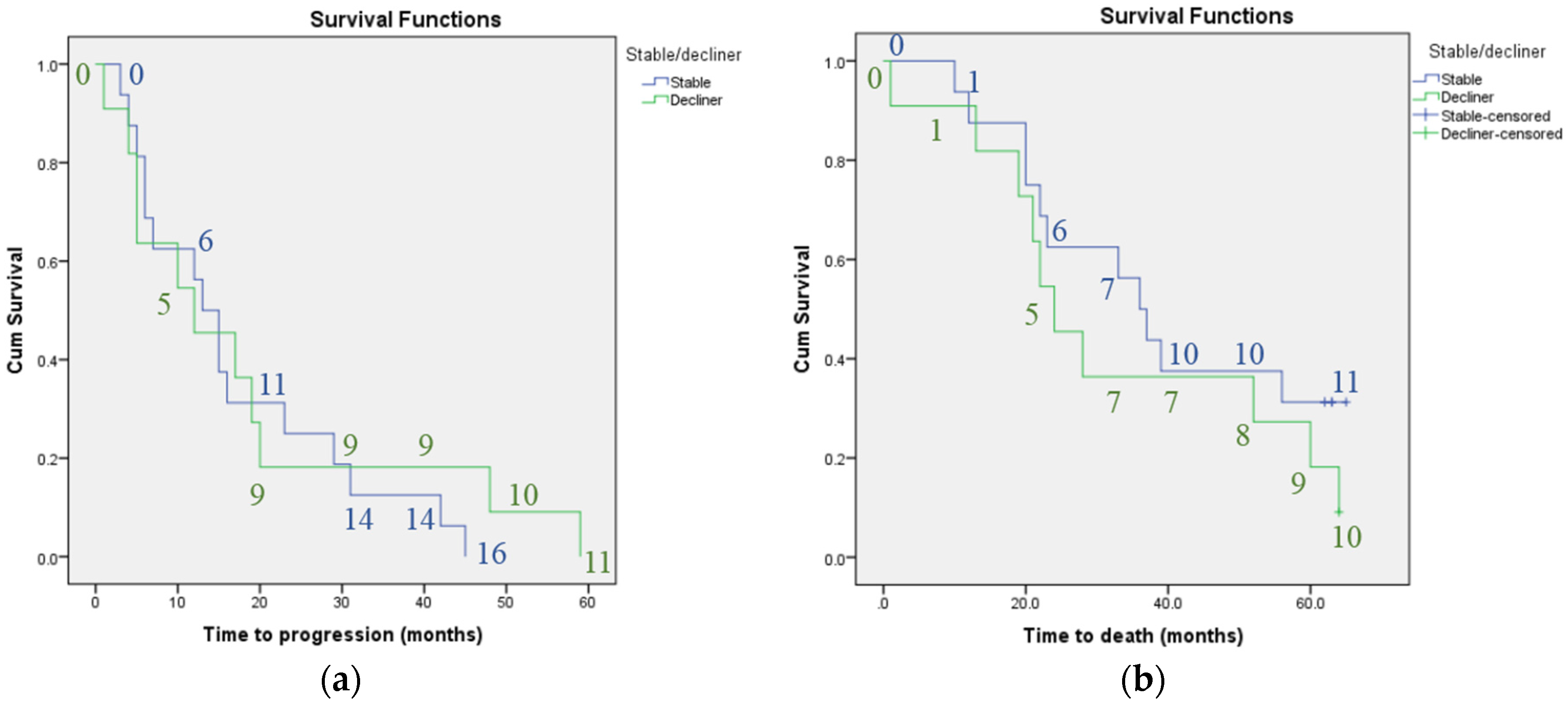
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ando, M.; Miyazaki, E.; Ito, T.; Hiroshige, S.; Nureki, S.I.; Ueno, T.; Takenaka, R.; Fukami, T.; Kumamoto, T. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung 2010, 188, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Borensztajn, K.; Crestani, B.; Kolb, M. Idiopathic pulmonary fibrosis: From epithelial injury to biomarkers-insights from the bench side. Respiration 2014, 86, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Simler, N.R.; Brenchley, P.E.; Horrocks, A.W.; Greaves, S.M.; Hasleton, P.S.; Egan, J.J. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax 2004, 59, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Bjoraker, J.A.; Ryu, J.H.; Edwin, M.K.; Myers, J.L.; Tazelaar, H.D.; Schroeder, D.R.; Offord, K.P. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1998, 157, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.; Porretta, M.A.; Bargagli, E.; Sergiacomi, G.; Zompatori, M.; Sverzellati, N.; Taglieri, A.; Mezzasalma, F.; Rottoli, P.; Saltini, C.; et al. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: A 3-year prospective study. Eur. Respir. J. 2012, 40, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Shlobin, O.A.; Weir, N.; Ahmad, S.; Kaldjob, J.M.; Battle, E.; Sheridan, M.J.; du Bois, R.M. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011, 140, 221–219. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Lancaster, L.; Noble, P.W.; Raghu, G.; Sahn, S.A.; et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Collard, H.R.; King, T.E.; Bartelson, B.B.; Vourlekis, J.S.; Schwarz, M.I.; Brown, K.K. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Zappala, C.J.; Latsi, P.I.; Nicholson, A.G.; Colby, T.V.; Cramer, D.; Renzoni, E.A.; Hansell, D.M.; du Bois, R.M.; Wells, A.U. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010, 35, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.R.; Mumford, J.A.; Murray, S.; Kazerooni, E.A.; Gross, B.H.; Colby, T.V.; Travis, W.D.; Flint, A.; Toews, G.B.; Lynch, J.P., III; et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2003, 168, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Ryerson, C.J.; Lee, J.S.; Wolters, P.J.; Koth, L.L.; Ley, B.; Elicker, B.M.; Jones, K.D.; King, T.E., Jr.; Ryu, J.H.; et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax 2012, 67, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Keane, M.P.; Arenberg, D.A.; Lynch, J.P.; Whyte, R.I.; Iannettoni, M.D.; Burdick, M.D.; Wilke, C.A.; Morris, S.B.; Glass, M.C.; DiGiovine, B.; et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J. Immunol. 1997, 159, 1437–1443. [Google Scholar] [PubMed]
- Wells, A.U.; Hansell, D.M.; Rubens, M.B.; Cailes, J.B.; Black, C.M.; du Bois, R.M. Functional impairment in lone cryptogenic fibrosing alveolitis and fibrosing alveolitis associated with systemic sclerosis: A comparison. Am. J. Respir. Crit. Care Med. 1997, 155, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Turner-Warwick, M. Precapillary systemic-pulmonary anastomoses. Thorax 1963, 18, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L.; Farkas, D.; Ask, K.; Möller, A.; Gauldie, J.; Margetts, P.; Inman, M.; Kolb, M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Investig. 2009, 119, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L.; Gauldie, J.; Voelkel, N.F.; Kolb, M. Pulmonary hypertension and idiopathic pulmonary fibrosis: A tale of angiogenesis, apoptosis, and growth factors. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, G.P.; Brown, K.K.; Schiemann, W.P.; Serls, A.E.; Parr, J.E.; Geraci, M.W.; Schwarz, M.I.; Cool, C.D.; Worthen, G.S. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: A role in aberrant angiogenesis. Am. J. Respir. Crit. Care Med. 2004, 170, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Ebina, M.; Shimizukawa, M.; Shibata, N.; Kimura, Y.; Suzuki, T.; Endo, M.; Sasano, H.; Kondo, T.; Nukiwa, T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2004, 169, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, E.A. Neovascularization in idiopathic pulmonary fibrosis: Too much or too little? Am. J. Respir. Crit. Care Med. 2004, 169, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Veikkola, T.; Alitalo, K. VEGFs, receptors and angiogenesis. Semin. Cancer Biol. 1999, 9, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Kuwano, K.; Yamada, M.; Hagimoto, N.; Hiasa, K.; Egashira, K.; Nakashima, N.; Maeyama, T.; Yoshimi, M.; Nakanishi, Y. Anti-Vascular Endothelial Growth Factor Gene Therapy Attenuates Lung Injury and Fibrosis in Mice 1. J. Immunol. 2005, 175, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, K.E.; Zhong, J.; Papakonstantinou, E.; Karakiulakis, G.; Tamm, M.; Seidel, P.; Sun, Q.; Mandal, J.; Lardinois, D.; Lambers, C.; et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ventetuolo, C.E.; Kawut, S.M.; Lederer, D.J. Plasma endothelin-1 and vascular endothelial growth factor levels and their relationship to hemodynamics in idiopathic pulmonary fibrosis. Respiration 2012, 84, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Schildge, J. Stickstoffmonoxyd in der Atemluft von Patienten mit interstitiellen Lungenkrankheiten Nitric Oxide in Exhaled Breath of Patients with Interstitial Lung Diseases. Pneumologie 2011, 65, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Vyas-Read, S.; Shaul, P.W.; Yuhanna, I.S.; Willis, B.C. Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L212. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Bargagli, E.; Rottoli, P. Exhaled nitric oxide is not increased in pulmonary sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2016, 33, 39–40. [Google Scholar] [PubMed]
- Tiev, K.P.; Cabane, J.; Aubourg, F.; Kettaneh, A.; Ziani, M.; Mouthon, L.; Duong-Quy, S.; Fajac, I.; Guillevin, L.; Dinh-Xuan, A.T. Severity of scleroderma lung disease is related to alveolar concentration of nitric oxide. Eur. Respir. J. 2007, 30, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.E.; Gugnani, M.K.; Abrams, J.; Mayes, M.D. Partitioning of alveolar and conducting airway nitric oxide in scleroderma lung disease. Am. J. Respir. Crit. Care Med. 2002, 165, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Tiev, K.P.; Coste, J.; Ziani, M.; Aubourg, F.; Cabane, J.; Dinh-Xuan, A.T. Diagnostic value of exhaled nitric oxide to detect interstitial lung disease in systemic sclerosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2009, 26, 32–38. [Google Scholar] [PubMed]
- Tiev, K.P.; Hua-Huy, T.; Kettaneh, A.; Allanore, Y.; Le-Dong, N.-N.; Duong-Quy, S.; Cabane, J.; Dinh-Xuan, A.T. Alveolar concentration of nitric oxide predicts pulmonary function deterioration in scleroderma. Thorax 2012, 67, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Hogman, M.; Permutt, S.; Silkoff, P.E. Modeling pulmonary nitric oxide exchange. J. Appl. Physiol. 2004, 96, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Tsoukias, N.M.; George, S.C. A two-compartment model of pulmonary nitric oxide exchange dynamics. J. Appl. Physiol. 1998, 85, 653–666. [Google Scholar] [PubMed]
- Tsoukias, N.M.; Shin, H.W.; Wilson, A.F.; George, S.C. A single-breath technique with variable flow rate to characterize nitric oxide exchange dynamics in the lungs. J. Appl. Physiol. 2001, 91, 477–487. [Google Scholar] [PubMed]
- Chaudhary, N.I.; Roth, G.J.; Hilberg, F.; Müller-Quernheim, J.; Prasse, A.; Zissel, G.; Schnapp, A.; Park, J.E. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 2007, 29, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.-M.; Li, W.-C.; Liu, D.-S.; Li, Y.-P.; Wen, F.-Q.; Feng, Y.-L.; Zhang, S.F.; Huang, X.Y.; Wang, T.; Wang, K.; et al. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int. Immunopharmacol. 2009, 9, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, T.T.; Barber, C.L.; Jordan, M.C.; Murdock, J.; Desai, S.; Ferrara, N.; Nagy, A.; Roos, K.P.; Iruela-Arispe, M.L. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007, 130, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, C.; Kerdiles, Y.; Nomaksteinsky, M.; Weidemann, A.; Takeda, N.; Doedens, A.; Torres-Collado, A.X.; Iruela-Arispe, L.; Nizet, V.; Johnson, R.S. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Banks, R.E.; Forbes, M.A.; Kinsey, S.E.; Stanley, A.; Ingham, E.; Walters, C.; Selby, P.J. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: Significance for VEGF measurements and cancer biology. Br. J. Cancer 1998, 77, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Hormbrey, E.; Gillespie, P.; Turner, K.; Han, C.; Roberts, A.; McGrouther, D.; Harris, A.L. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: Is the current literature meaningful? Clin. Exp. Metastasis 2002, 19, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Hong, Y.J.; Han, C.J.; Hwang, D.Y.; Hong, S.I. Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: Which is the optimal specimen? Int. J. Oncol. 2000, 17, 149–201. [Google Scholar] [CrossRef] [PubMed]
- Crooks, M.G.; Fahim, A.; Naseem, K.M.; Morice, A.H.; Hart, S.P. Increased Platelet Reactivity in Idiopathic Pulmonary Fibrosis Is Mediated by a Plasma Factor. PLoS ONE 2014, 9, e111347. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Albera, C.; Bradford, W.Z.; Costabel, U.; du Bois, R.M.; Fagan, E.A.; Fishman, R.S.; Glaspole, I.; Glassberg, M.K.; Glasscock, K.F.; et al. Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: Analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax 2016, 71, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.L.; Tayob, N.; Han, M.K.; Zappala, C.; Kervitsky, D.; Murray, S.; Wells, A.U.; Brown, K.K.; Martinez, F.J.; Flaherty, K.R. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest 2014, 145, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Network TIPFCR. Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar]
| Demographic | Number of Patients or Mean Parameter Values |
|---|---|
| Male sex (n (%)) | 23 (85%) |
| Ex-smokers * (n (%)) | 19 (70%) |
| Receiving LTOT at recruitment (n (%)) | 2 (7%) |
| Receiving immunosuppressants at recruitment (n (%)) | 5 (19%) |
| Age (years) (mean (S.D.)) | 72.8 (9.5) |
| Disease duration (months) (mean (S.D.)) | 35.0 (27.0) |
| Baseline FVC % predicted (mean (S.D.)) | 71.8 (18.1) |
| Baseline TLCO-SB % predicted (mean (S.D.)) | 43.3 (16.0) |
| Parameter | Subgroup | Median | Range |
|---|---|---|---|
| Plasma VEGF concentration (pg/mL) | Combined | 133.0 | 286.4 |
| High | 169.2 | 202.6 | |
| Low | 82.5 | 71.1 | |
| CANO (ppb) | Combined | 4.4 | 10.5 |
| High | 6.4 | 6.6 | |
| Low | 3.0 | 3.9 |
| Parameter | Time to Death (Months) | Time to Progression (Months) | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI Lower Bound | 95% CI Upper Bound | Hazard Ratio | 95% CI Lower Bound | 95% CI Upper Bound | |
| Plasma VEGF concentration | 0.93 | 0.38 | 2.28 | 0.62 | 0.28 | 1.40 |
| CANO | 1.88 | 0.77 | 4.62 | 0.96 | 0.84 | 1.10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotecha, J.; Shulgina, L.; Sexton, D.W.; Atkins, C.P.; Wilson, A.M. Plasma Vascular Endothelial Growth Factor Concentration and Alveolar Nitric Oxide as Potential Predictors of Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2016, 5, 80. https://doi.org/10.3390/jcm5090080
Kotecha J, Shulgina L, Sexton DW, Atkins CP, Wilson AM. Plasma Vascular Endothelial Growth Factor Concentration and Alveolar Nitric Oxide as Potential Predictors of Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine. 2016; 5(9):80. https://doi.org/10.3390/jcm5090080
Chicago/Turabian StyleKotecha, Jalpa, Ludmila Shulgina, Darren W. Sexton, Christopher P. Atkins, and Andrew M. Wilson. 2016. "Plasma Vascular Endothelial Growth Factor Concentration and Alveolar Nitric Oxide as Potential Predictors of Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis" Journal of Clinical Medicine 5, no. 9: 80. https://doi.org/10.3390/jcm5090080
APA StyleKotecha, J., Shulgina, L., Sexton, D. W., Atkins, C. P., & Wilson, A. M. (2016). Plasma Vascular Endothelial Growth Factor Concentration and Alveolar Nitric Oxide as Potential Predictors of Disease Progression and Mortality in Idiopathic Pulmonary Fibrosis. Journal of Clinical Medicine, 5(9), 80. https://doi.org/10.3390/jcm5090080






