RETRACTED: Platypnea–Orthodeoxia Syndrome: Multiple Pathophysiological Interpretations of a Clinical Picture Primarily Consisting of Orthostatic Dyspnea
Abstract
:1. Definition
2. Pathophysiological Issues
2.1. Cardiac POS
2.2. Ventilation/Perfusion Mismatch as Primary Cause of POS in Parenchimal Pulmonary Diseases and Pulmonary Arteriovenous Shunts
3. Epidemiology
4. Clinical Picture
5. Diagnostic Assessment
6. Treatment
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Cheng, T.O. Platypnea–orthodeoxia syndrome: Etiology, differential diagnosis, and management. Catheter. Cardiovasc. Interv. 1999, 47, 64–66. [Google Scholar] [CrossRef]
- Cheng, T.O. Mechanisms of platypnea–orthodeoxia: What causes water to flow uphill? Circulation 2002, 105, e47. [Google Scholar] [PubMed]
- Bellato, V.; Brusa, S.; Balazova, J.; Marescotti, S.; De Caria, D.; Bordone, G. Platypnea–orthodeoxia syndrome in interatrial right to left shunt postpneumonectomy. Minerva. Anestesiol. 2008, 74, 271–275. [Google Scholar] [PubMed]
- Rodrigues, P.; Palma, P.; Sousa-Pereira, L. Platypnea–orthodeoxia syndrome in review: Defining a new disease? Cardiol. 2012, 123, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hagen, P.T.; Scholz, D.G.; Edwards, W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo. Clin. Proc. 1984, 59, 17–20. [Google Scholar] [CrossRef]
- De Vecchis, R.; Baldi, C.; Cantatrione, S. Transcatheter closure of PFO as secondary prevention of cryptogenic stroke. Herz 2016. [Google Scholar] [CrossRef] [PubMed]
- De Vecchis, R.; Baldi, C. Unresolved or contradictory issues about management of patients with patent foramen ovale and previous cryptogenic stroke: Additional randomized controlled trials are eagerly awaited. J. Clin. Med. Res. 2016, 8, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Blanche, C.; Noble, S.; Roffi, M.; Testuz, A.; Müller, H.; Meyer, P.; Bonvini, J.M.; Bonvini, R.F. Platypnea orthodeoxia syndrome in the elderly treated by percutaneous patent foramen ovale closure: A case series and literature review. Eur. J. Intern. Med. 2013, 24, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Godart, F.; Rey, C.; Prat, A.; Vincentelli, A.; Chmaït, A.; Francart, C.; Porte, H. Atrial right-to-left shunting causing severe hypoxaemia despite normal right-sided pressures. Report of 11 consecutive cases corrected by percutaneous closure. Eur. Heart J. 2000, 21, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hirai, N.; Fukunaga, T.; Kawamo, H.; Honda, O.; Sakamoto, T.; Yoshimura, M.; Kugiyama, K.; Ogawa, H. Platypnea–orthodeoxia Syndrome with atrial septal defect. Circ. J. 2003, 67, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Bovelli, D.; Khoury, G.; Consalvi, G.; Casali, L.; Savino, K.; Carminati, M.; Rasetti, G.; Ambrosio, G.; Onorato, E. An unusual type of dyspnea. G. Ital. Cardiol. (Rome) 2008, 9, 367–371. [Google Scholar]
- Akin, E.; Krüger, U.; Braun, P.; Stroh, E.; Janicke, I.; Rezwanian, R.; Akin, I.; Schöls, W.H. The platypnea–orthodeoxia syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2599–2604. [Google Scholar] [PubMed]
- Takhar, R.; Biswas, R.; Arora, A.; Jain, V. Platypnoea–orthodeoxia syndrome: Novel cause for a known condition. BMJ Case Rep. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Tenholder, M.F.; Russell, M.D.; Knight, E.; Rajagopal, K.R. Orthodeoxia: A new finding in interstitial fibrosis. Am. Rev. Respir. Dis. 1987, 136, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, K.; Minasidis, I.; Vainas, A.; Bikas, C.; Kontakiotis, T.; Vakianis, P. Platypnea and orthodeoxia associated with Pneumocystis jiroveci and Cytomegalovirus pneumonia: A case report. J. Med. Case Rep. 2009, 5, 9319. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.N.; Wakefield, A.E.; Goldin, R.; Govan, J. Pneumocystis carinii pneumonia with pleurisy, platypnoea and orthodeoxia. Thorax 2003, 58, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Roisin, R.; Krowka, M.J. Hepatopulmonary syndrome—A liver-induced lung vascular disorder. N. Engl. J. Med. 2008, 358, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.T.; Fallon, M.B. The hepatopulmonary syndrome. J. Hepatol. 2006, 45, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Cremona, G.; Higenbottam, T.W.; Mayoral, V.; Alexander, G.; Demoncheaux, E.; Borland, C.; Roe, P.; Jones, G.J. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur. Respir. J. 1995, 8, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Godart, F.; Rey, C. Platypnea Orthodeoxia Syndrome: A probably underestimated syndrome? Chest 2001, 5, 1624–1625. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheng, S.T. Shortness of breath while sitting up: Hepatopulmonary syndrome. Candian Med. Assoc. J. 2011, 183, 80. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, A.; Matsumura, Y.; Tatsumi, H.; Sasaki, Y.; Hirai, H.; Hanatani, A.; Muro, T.; Yoshiyama, M.; Suehiro, S. Platypnea-orthodeoxia diagnosed by sitting transesophageal echocardiography. Ann. Thorac. Surg. 2010, 89, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Adolph, E.A.; Lacy, W.O.; Hermoni, Y.I.; Wexler, L.F.; Javaheri, S. Reversible orthodeoxia and platypnea due to right-to-left intracardiac shunting related to pericardial effusion. Ann. Intern. Med. 1992, 116, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Nourmand, H.; Fraiman, M.H.; Cooper, C.B.; Bellamy, P.E.; Farmer, D.G.; Vierling, J.M.; Ghobrial, R.M.; Busuttill, R.W. Retrospective analysis of the results of liver transplantation for adults with severe hepatopulmonary syndrome. Liver Transpl. 2002, 8, 925–931. [Google Scholar] [CrossRef] [PubMed]
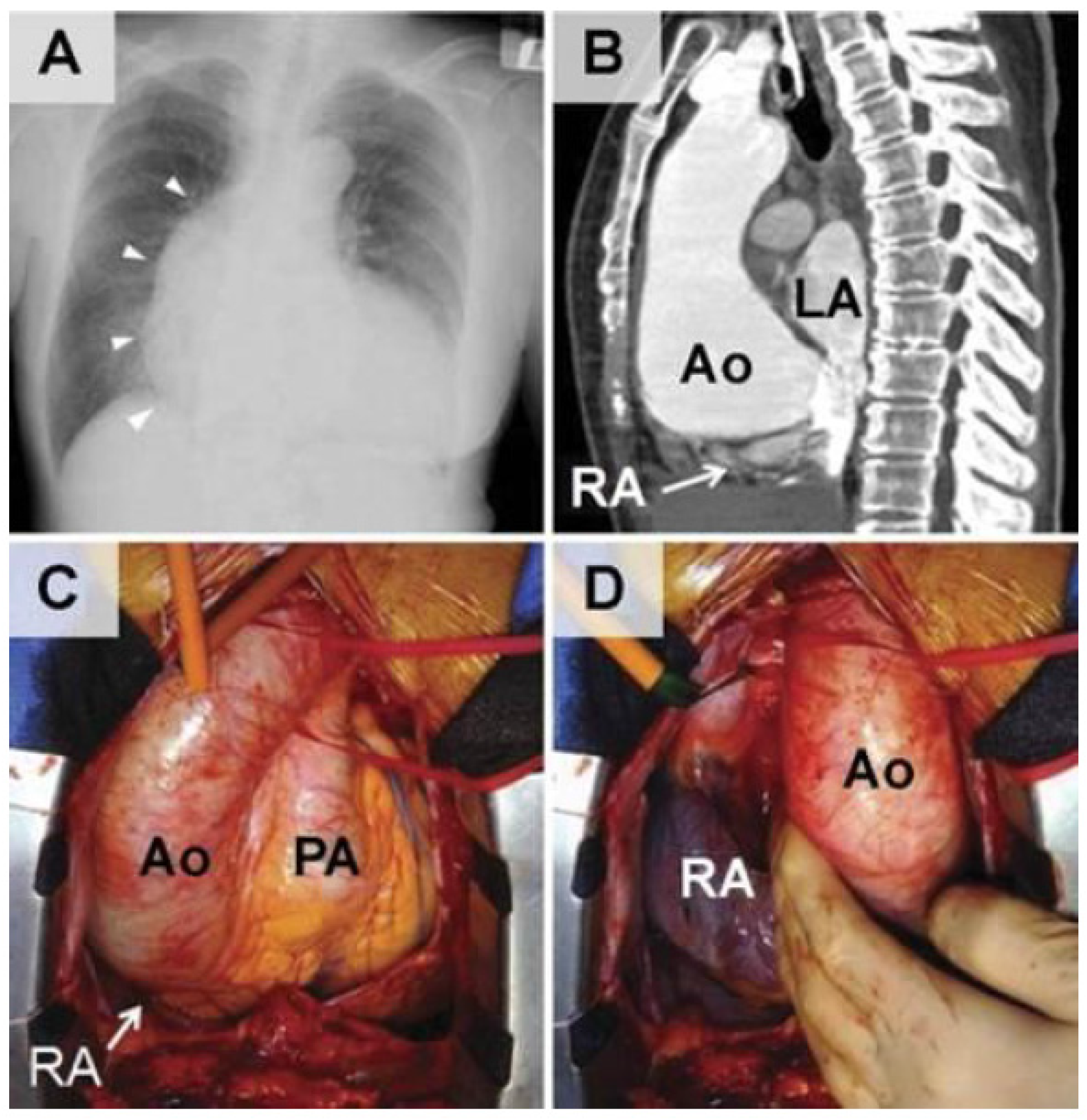
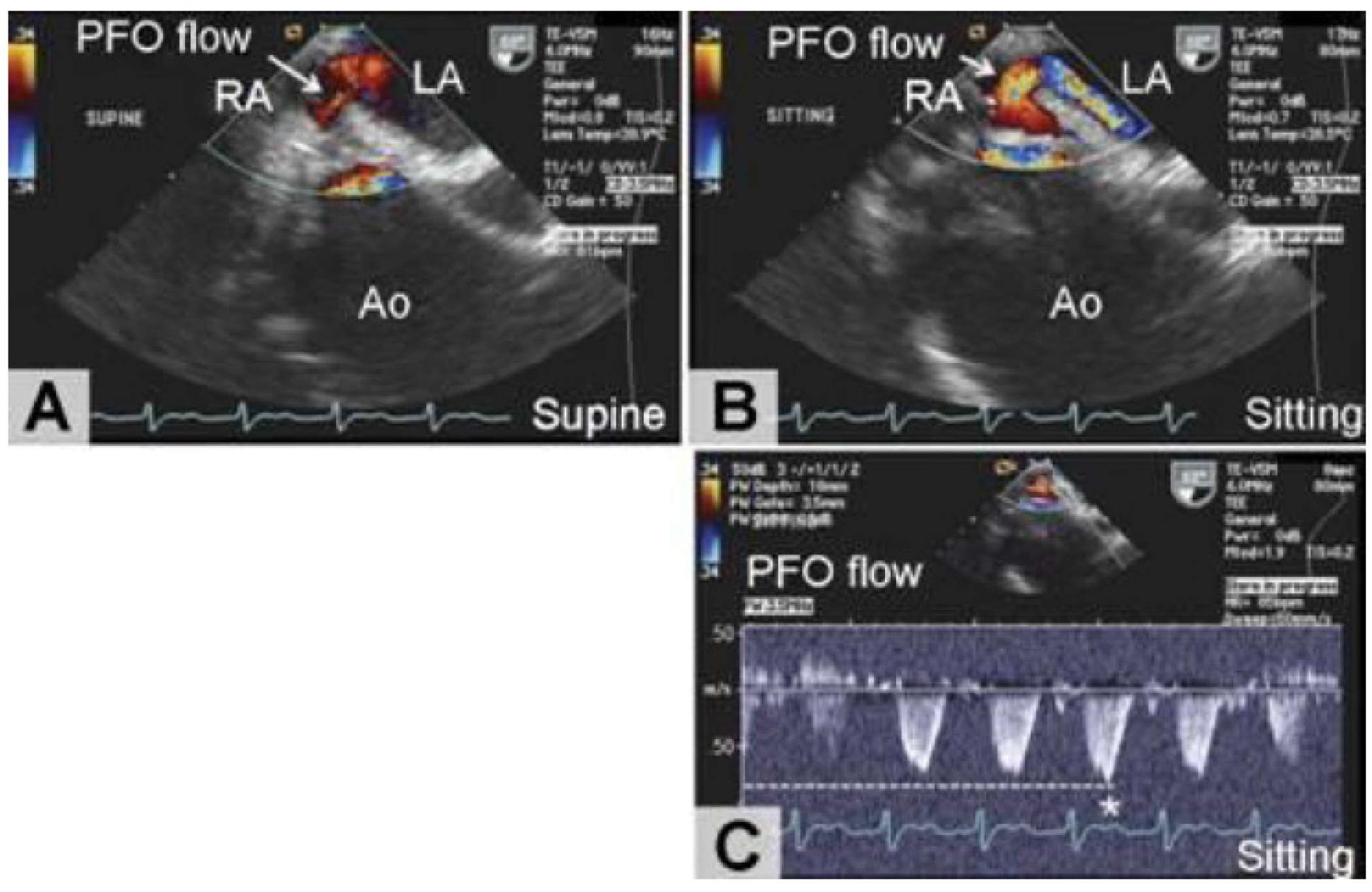
| Underlying Anatomical or Functional Alteration | Pathophysiologic Mechanism | Accompanying Pathologic Condition |
|---|---|---|
| Intracardiac shunt PFO ASD ASA with fenestration | Transient right–left shunt without elevated right–left pressure gradient | Compression of RA by aortic dilatation, elongation or aneurysm Pericardial effusion or constrictive pericarditis Postpneumectomy 1 Eosinophilic endomyocardial disease Abnormally lying Eustachian valve or Chiari network RA myxoma RA lipomatosis hypertrophy Kyphosis |
| Transient right–left shunt with elevated right–left pressure gradient | Pulmonary thromboembolism Idiopathic pulmonary hypertension Right hydrothorax Long duration lung disease causing pulmonary hypertension Postpneumectomy | |
| Pulmonary diseases with ventilation/perfusion mismatch | High V/Q ratio | Emphysema COPD Interstitial lung disease |
| Low V/Q ratio | Hepatopulmonary syndrome Pulmonary arteriovenous malformations or fistulae Rendu–Osler–Weber syndrome |
| Dyspnea elicited by upright position that disappears with lying position |
| Orthodeoxia (sPO2 < 90% or pO2 < 60 mmHg in upright position, normalization in lying position) |
| Ascertained interatrial communication |
| Right-to-left shunt |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vecchis, R.; Baldi, C.; Ariano, C. RETRACTED: Platypnea–Orthodeoxia Syndrome: Multiple Pathophysiological Interpretations of a Clinical Picture Primarily Consisting of Orthostatic Dyspnea. J. Clin. Med. 2016, 5, 85. https://doi.org/10.3390/jcm5100085
De Vecchis R, Baldi C, Ariano C. RETRACTED: Platypnea–Orthodeoxia Syndrome: Multiple Pathophysiological Interpretations of a Clinical Picture Primarily Consisting of Orthostatic Dyspnea. Journal of Clinical Medicine. 2016; 5(10):85. https://doi.org/10.3390/jcm5100085
Chicago/Turabian StyleDe Vecchis, Renato, Cesare Baldi, and Carmelina Ariano. 2016. "RETRACTED: Platypnea–Orthodeoxia Syndrome: Multiple Pathophysiological Interpretations of a Clinical Picture Primarily Consisting of Orthostatic Dyspnea" Journal of Clinical Medicine 5, no. 10: 85. https://doi.org/10.3390/jcm5100085
APA StyleDe Vecchis, R., Baldi, C., & Ariano, C. (2016). RETRACTED: Platypnea–Orthodeoxia Syndrome: Multiple Pathophysiological Interpretations of a Clinical Picture Primarily Consisting of Orthostatic Dyspnea. Journal of Clinical Medicine, 5(10), 85. https://doi.org/10.3390/jcm5100085




