Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia
Abstract
:1. Introduction
2. Epidemiological Aspects
| Disciplines | Subjects | Prevalence of EAH | References |
|---|---|---|---|
| Marathoners | up to 22% | [13,15,37,38] | |
| Ultra-marathoners | Asymptomatic | 30%–51% | [7,10,11] |
| Athletes seeking medical care | 38% | [17] | |
| Mountain bikers | 7.1% | [32] | |
| Ultra-mountain bikers | 3.7% | [33] | |
| Ironman triathletes | 1.8%–28% | [16,28,39] | |
| Hikers | 16% | [5,6,9,20,21,22] | |
| Military | Indreased trend; one case of death | [23,24,25] | |
| Swimmers | Males | 8% | [36] |
| Females | 36% |
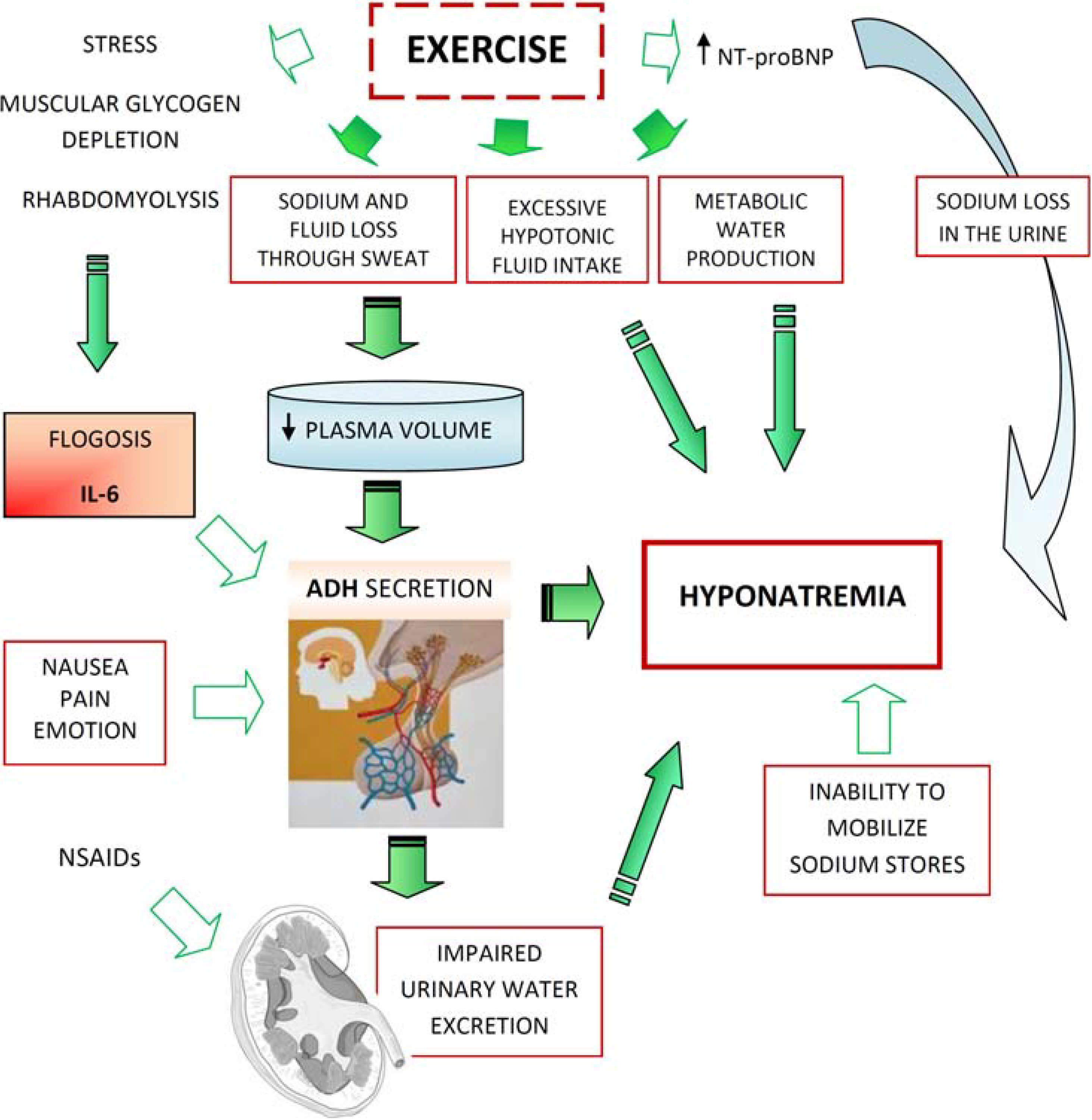
3. Physiopathology
- -
- Excessive fluid intake
- -
- Impaired urinary water excretion, largely as a result of persistent secretion of antidiuretic hormone (ADH) (Figure 1).
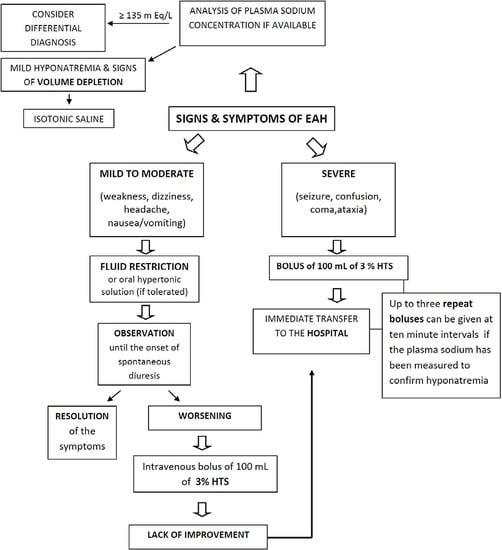
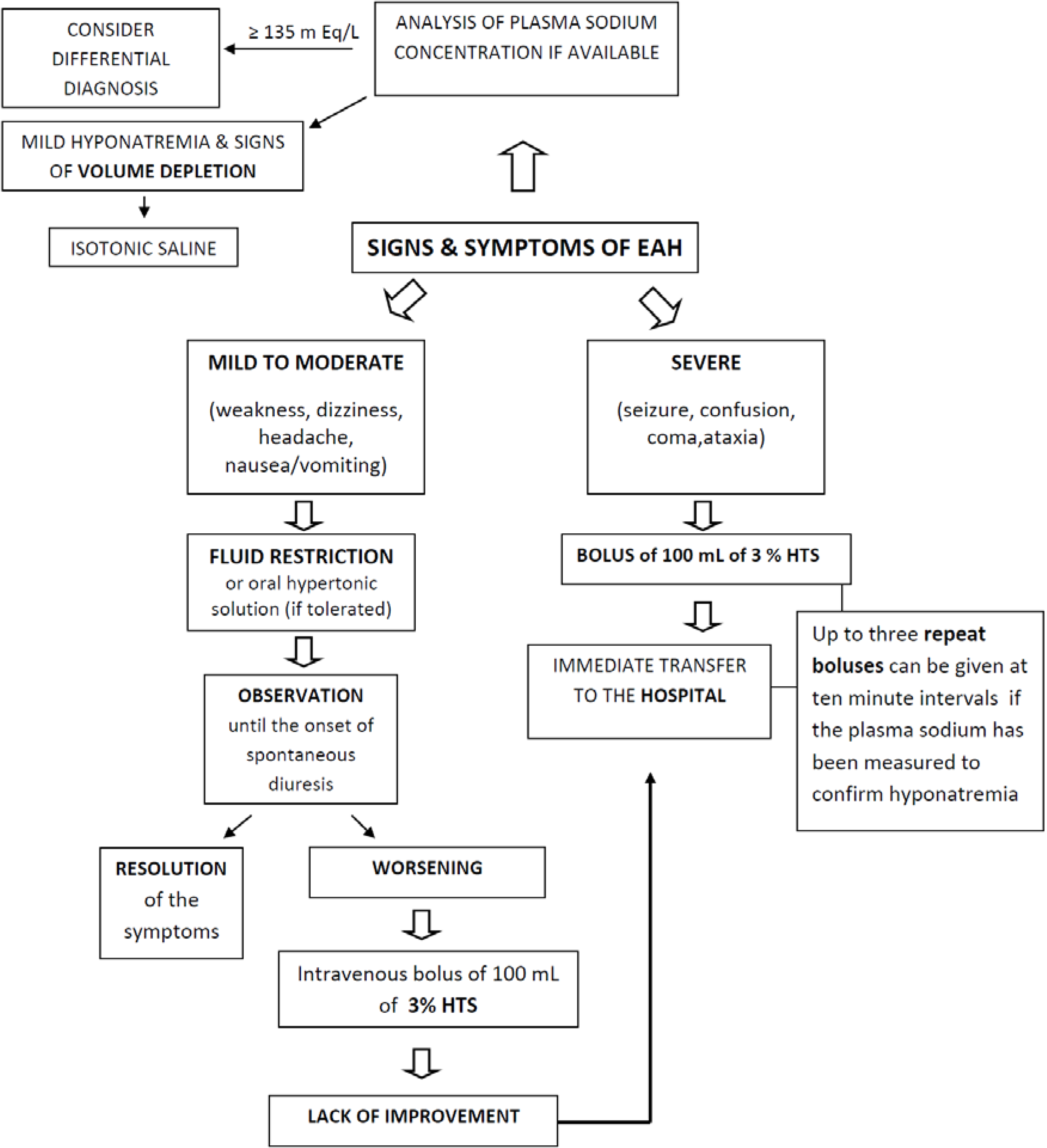
4. Clinical Aspects
5. Prevention and Treatment
| Signs and symptoms | EAH | Heat illness | AMS | HACE or HAPE |
|---|---|---|---|---|
| Fatigue/weakness | +/− | +/− | + | + |
| Increased thirst | +/− | + | +/− | +/− |
| Temperature elevated | +/− | +++ | − | − |
| Tachycardia | +/− | + | +/− | +/− |
| Nausea/vomiting | +/− | +/− | +/− | +/− |
| Headache/dizzines | +/− | +/− | +++ | +++ |
| Blurred vision | +/− | +/− | +/− | +/− |
| Confusion/disorientation | +/− | +/− | +/− | +/− |
| Obtundation | +/− | +/− | +/− | +/− |
| Seizure | +/− | +/− | +/− | +/− |
| Coma | +/− | +/− | +/− | +/− |
| Respiratory distress | +/− | − | +/− | +/− |
| Oliguria | +/− | + | +/− | +/− |
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Hew-Butler, T.; Ayus, J.C.; Kipps, C. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin. J. Sport Med. 2008, 18, 111–121. [Google Scholar] [PubMed]
- Bennet, B.L.; Hew-Butler, T.; Hoffman, M. Wilderness Medical Society Practise Guidelines for treatment of exercise-associated hyponatremia. Wilderness Environ. Med. 2013, 24, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.R.; Hook, G.; Stuempfle, K.J.; Hoffman, M.D.; Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: A preliminary randomized trial. Clin. J. Sport Med. 2011, 21, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Goodwin, N.; Rayner, B.L.; Branken, T.; Taylor, R.K. Water intoxication: A possible complication during endurance exercise. Med. Sci. Sports Exerc. 1985, 17, 370–375. [Google Scholar] [CrossRef]
- Backer, H.D.; Shopes, E.; Collins, S.L. Hyponatremia in recreational hikers in Grand Canyon National Park. J. Wilderness Med. 1993, 4, 391–406. [Google Scholar] [CrossRef]
- Basnyat, B.; Sleggs, J.; Spinger, M. Seizures and delirium in a trekker: The consequences of excessive water drinking? Wilderness Environ. Med. 2000, 11, 69–70. [Google Scholar]
- Hoffman, M.D.; Hew-Butler, T.; Stuempfle, K.J. Exercise associated hyponatremia and hydration status in 161-km ultramarathoners. Med. Sci. Sports Exerc. 2013, 45, 784–791. [Google Scholar] [PubMed]
- Kormann, F.; Philippart, S.; Bruel, C. Marathon Runner with Acute Hyponatremia: A Neurological Disorder. Case Rep. Emerg. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Severac, M.; Orban, J.C. A near-fatal case of exercise-associated hyponatremia. Am. J. Emerg. Med. 2014, 32. [Google Scholar] [CrossRef]
- Stuempfle, K.J.; Lehmann, D.R.; Case, H.S. Hyponatremia in a cold weather ultraendurance race. Alaska Med. 2002, 44, 51–55. [Google Scholar] [PubMed]
- Lebus, D.K.; Casazza, G.A.; Hoffman, M.D.; van Loan, M.D. Can changes in body mass and total body water accurately predict hyponatremia after a 161-km running race? Clin. J. Sport Med. 2010, 20, 193–199. [Google Scholar]
- Wellershoff, G. Hyponatremic encephalopathy with non-cardiogenic pulmonary edema. Development following marathon run. Med. Klin. Intensiv. Notfmed. 2013, 108, 234–237. [Google Scholar] [CrossRef]
- Kipps, C.; Sharma, S.; Tunstall Pedoe, D. The incidence of exercise-associated hyponatraemia in the London marathon. Br. J. Sports Med. 2011, 45, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ayus, J.C.; Varon, J.; Arieff, A.I. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann. Intern. Med. 2000, 132, 711–714. [Google Scholar] [CrossRef]
- Hew, T.D.; Chorley, J.N.; Cianca, J.C.; Divine, J.G. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin. J. Sport Med. 2003, 13, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Speedy, D.B.; Noakes, T.D.; Rogers, I.R. Hyponatremia in ultradistance triathletes. Med. Sci. Sports Exerc. 1999, 31, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Nio, A.Q.; Ang, W.H. First reported cases of exercise-associated hyponatremia in Asia. Int. J. Sports Med. 2011, 32, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Glace, B.; Murphy, C. Severe hyponatremia develops in a runner following a half-marathon. JAAPA 2008, 21, 27–29. [Google Scholar] [PubMed]
- Shapiro, S.A.; Ejaz, A.A.; Osborne, M.D.; Taylor, W.C. Moderate exercise-induced hyponatremia. Clin. J. Sport Med. 2006, 16, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Zafren, K. Hyponatremia in a cold environment. Wilderness Environ. Med. 1998, 9, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.P.; Rosengren, D. Severe exercise-associated hyponatremia on the Kokoda Trail, Papua, New Guinea. Wilderness Environ. Med. 2008, 19, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Spano, M.D.; Zacharia Reagle, D.O. Timothy Evans Symptomatic Hypotonic Hyponatremia Presenting at High Altitude. Wilderness Environ. Med. 2014, 25, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, J.W. Death by water intoxication. Mil. Med. 2002, 167, 432–434. [Google Scholar] [PubMed]
- Garigan, T.P.; Ristedt, D.E. Death from hyponatremia as a result of acute water intoxication in an Army basic trainee. Mil. Med. 1999, 164, 234–238. [Google Scholar] [PubMed]
- O’Donnell, F.L. Army Medical Surveillance Activity. Update: Exertional hyponatremia, active component, U.S. Armed Forces, 1999–2011. Med. Surveill. Mon. Rep. 2012, 19, 20–23. [Google Scholar]
- Knechtle, B.; Knechtle, P.; Rosemann, T. No exercise-associated hyponatremia found in an observational field study of male ultra-marathoners participating in a 24-hour ultra-run. Phys. Sportsmed 2010, 38, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Sharwood, K.A.; Collins, M.; Goedecke, J.H.; Wilson, G.; Noakes, T.D. Weight changes, medical complications and performance during an Ironman triathlon. Brit. J. Sports Med. 2004, 38, 718–724. [Google Scholar] [CrossRef]
- Speedy, D.B.; Faris, J.G.; Hamlin, M.; Gallagher, P.G.; Campbell, R.G. Hyponatremia and weight changes in an ultradistance triathlon. Clin. J. Sport Med. 1997, 7, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Gnädinger, M.; Knechtle, P.; Imoberdorf, R.; Kohler, G.; Ballmer, P.; Rosemann, T.; Senn, O. Prevalence of exercise-associated hyponatremia in male ultra endurance athletes. Clin. J. Sport Med. 2011, 21, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chlíbková, D.; Knechtle, B.; Rosemann, T.; Zakovska, A. The prevalence of exercise-associated hyponatremia in 24-hour ultra-mountain bikers, 24-hour ultra-runners and multi-stage ultra-mountain bikers in the Czech Republic. J. Int. Soc. Sports Nutr. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Reid, S.A.; Speedy, D.B.; Mulligan, G.P.; Thompson, J. Exercise-associated hyponatremia, renal function, and nonsteroidal anti-inflammatory drug use in an ultra endurance mountain run. Clin. J. Sport Med. 2007, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.C.; Peters-Futre, E.M. Ad libitum adjustments to fluid intake during cool environmental conditions maintain hydration status during a 3-day mountain bike race. Brit. J. Sports Med. 2010, 4, 430–436. [Google Scholar] [CrossRef]
- Schenk, K.; Gatterer, H.; Ferrari, M.; Ferrari, P.; Cascio, V.L.; Burtscher, M. Bike Transalp 2008: Liquid intake and its effect on the body’s fluid homeostasis in the course of a multistage, cross country, MTB marathon race in the central Alps. Clin. J. Sport Med. 2010, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Rust, C.A.; Knechtle, B.; Rosemann, T. No case of exercise-associated hyponatraemia in top male ultra-endurance cyclists: The “swiss cycling marathon”. Eur. J. Appl. Physiol. 2012, 112, 689–697. [Google Scholar] [CrossRef]
- Knechtle, B.; Knechtle, P.; Rosemann, T. No case of exercise associated hyponatremia in male ultra-endurance mountain bikers in the “Swiss bike masters”. Chin. J. Physiol. 2011, 54, 379–384. [Google Scholar] [PubMed]
- Wagner, S.; Knechtle, B. Higher prevalence of exercise-associated hyponatremia in female than in male open-water ultra-endurance swimmers: The “Marathon-Swimm” in Lake Zurich. Eur. J. Appl. Physiol. 2012, 112, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Mettler, S.; Rusch, C.; Frey, W.O.; Bestmann, L.; Wenk, C.; Colombani, P.C. Hyponatremia among runners in the Zurich Marathon. Clin. J. Sport Med. 2008, 18, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Almond, C.S.; Shin, A.Y.; Fortescue, E.B. Hyponatremia among runners in the Boston Marathon. N. Engl. J. Med. 2005, 352, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Wharam, P.C.; Speedy, D.B.; Noakes, T.D.; Thompson, J.M.; Reid, S.A.; Holtzhausen, L.M. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med. Sci. Sports Exerc. 2006, 38, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Kargotich, S.; Goodman, C.; Keast, D.; Morton, A.R. The influence of exercise-induced plasma volume changes on the interpretation of biochemical parameters used for monitoring exercise, training and sport. Sports Med. 1998, 26, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Ball, K.D.; Kolkhorst, F.W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 2007, 103, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Aragon-Vargas, L.M.; Chamorro, M.; Maughan, R.J.; Serratosa, L.; Zachwieja, J.J. The sweating response of elite professional soccer players to training in the heat. Int. J. Sports Med. 2005, 26, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.I.; Carroll, D.R.; Lowe, S.L.; Peterson, E.W.; Cooper, K.H. Serum electrolyte changes after marathon running. J. Appl. Physiol. 1970, 29, 449–451. [Google Scholar] [PubMed]
- Coyle, E.F.; Montain, S.J. Benefits of fluid replacement with carbohydrate during exercise. Med. Sci. Sports Exerc. 1992, 24, S324–S330. [Google Scholar] [PubMed]
- Davis, D.P.; Videen, J.S.; Marino, A.; Vilke, G.M.; Dunford, J.V.; van Camp, S.P.; Maharam, L.G. Exercise-associated hyponatremia in marathon runners: A two-year experience. J. Emerg. Med. 2001, 21, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Jordaan, E.; Stuempfle, K.J. Osmotic and nonosmotic regulation of arginine vasopressin during prolonged endurance exercise. J. Clin. Endocrinol. Metab. 2008, 93, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.D.; Post, T.W. Regulation of water and electrolyte balance. In Clinical Physiology of Acid-Base and Electrolyte Disorders, 5th ed.; McGraw Hill: New York, NY, USA, 2001; pp. 286–288. [Google Scholar]
- Siegel, A.J.; Verbalis, J.G.; Clement, S. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am. J. Med. 2007, 120, 461–467. [Google Scholar] [PubMed]
- Noakes, T.D.; Sharwood, K.; Speedy, D. Three independent biological mechanisms cause exercise-associated hyponatremia: Evidence from 2135 weighed competitive athletic performances. Proc. Natl. Acad. Sci. USA 2005, 102, 18550–18555. [Google Scholar] [CrossRef] [PubMed]
- Verbalis, J.G. Disorders of body water homeostasis. Best Pract. Res. 2003, 17, 471–503. [Google Scholar] [CrossRef]
- Rosner, M.H.; Kirven, J. Exercise-associated hyponatremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 151–161. [Google Scholar] [CrossRef]
- Siegel, A.J. Exercise-associated hyponatremia: Role of cytokines. Am. J. Med. 2006, 119 (Suppl. 1), 74–78. [Google Scholar] [CrossRef]
- Bennermo, M.; Held, C.; Stemme, S. Genetic predisposition of the interleukin-6 reswponse to inflammation: Implications for a variety of major disease? Clin. Chem. 2004, 50, 2136–2140. [Google Scholar]
- Hillman, A.R.; Vince, R.V.; Taylor, L.; Mc Naughton, L.; Mitchell, N.; Siegler, J. Exercise-induced dehydration with and without environmental heat stress results in increased oxidative stress. Appl. Physiol. Nutr. Metab. 2011, 36, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, Z.; Klapcinka, B.; Krepa, E.S. Acute metabolic responses to a 24 hours ultra marathon race in male amateur runners. Eur. J. Appl. Physiol. 2012, 112, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Reid, S.; Sikaris, K.; McCrory, P. Hyponatremia is associated with higher NT-proBNP than normonatremia after prolonged exercise. Clin. J. Sport Med. 2012, 22, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Edelman, I.S.; James, A.H.; Brooks, L.; Moore, F.D. Body sodium and potassium. IV. The normal total exchangeable sodium; its measurement and magnitude. Metabolism 1954, 3, 530–538. [Google Scholar] [PubMed]
- Hew-Butler, T.; Stuempfle, K.J.; Hoffman, M.D. Bone: An acute buffer of plasma sodium during exhaustive exercise? Horm. Metab. Res. 2013, 45, 697–700. [Google Scholar] [CrossRef]
- Halperin, M.L.; Kamel, K.S.; Sterns, R. Hyponatremia in marathon runners. N. Engl. J. Med. 2005, 353, 427–428. [Google Scholar] [PubMed]
- Lindinger, M.I.; Heigenhauser, G.J.; McKelvie, R.S.; Jones, N.L. Blood ion regulation during repeated maximal exercise and recovery in humans. Am. J. Physiol. 1992, 262, 126–136. [Google Scholar]
- Rüst, C.A.; Knechtle, B.; Patrizia Knechtle, P.; Rosemann, T. Higher Prevalence of Exercise-Associated Hyponatremia in Triple Iron Ultra-Triathletes than Reported for Ironman Triathletes. Chin. J. Physiol. 2012, 55, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Skjønnemand, M. A case of severe exercise-associated hyponatremia. Ugeskr. Laeger. 2013, 175, 1349–1350. [Google Scholar] [PubMed]
- Hew-Butler, T.D.; Boulter, J.; Bhorat, R.; Noakes, T.D. Avoid adding insult to injury—Correct management of sick female endurance athletes. S. Afr. Med. J. 2012, 102, 927–930. [Google Scholar] [PubMed]
- Stricker, E.M.; Thiels, E.; Verbalis, J.G. Sodium appetite in rats after prolonged dietary sodium deprivation: A sexually dimorphic phenomenon. Am. J. Physiol. 1991, 260, 1082–1088. [Google Scholar]
- Barron, W.M.; Schreiber, J.; Lindheimer, M.D. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am. J. Physiol. 1986, 250, 352–361. [Google Scholar]
- Fraser, C.L.; Kucharczyk, J.; Arieff, A.I.; Rollin, C.; Sarnacki, P.; Norman, D. Sex differences result in increased morbidity from hyponatremia in female rats. Am. J. Physiol. 1989, 256, 880–885. [Google Scholar]
- Stachenfeld, N.S.; DePietro, L.; Palter, S.F.; Nadel, E.R. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am. J. Physiol. 1998, 43, 187–195. [Google Scholar]
- Baker, J.; Cotter, J.D.; Gerrard, D.F.; Bell, M.L.; Walker, R.J. Effects of indomethacin and celecoxib on renal function in athletes. Med. Sci. Sports Exerc. 2005, 37, 712–717. [Google Scholar] [PubMed]
- Raymond, K.H.; Lifschitz, M.D. Effects of prostaglandins on renal salt and water excretion. Am. J. Med. 1986, 80, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Dibona, G.F. Prostaglandins and nonsteroidal anti-inflammatory drugs: Effects on renal hemodynamics. Am. J. Med. 1986, 80, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Putterman, C.; Levy, L.; Rubinger, D. Transient exercise-induced water intoxication and rhabdomyolysis. Am. J. Kidney Dis. 1993, 21, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Cuthill, J.; Hew-Butler, T.; George, S.M.; Rosner, M.H. Case report: Exercise-associated hyponatremia with rhabdomyolysis during endurance exercise. Phys. Sportsmed. 2009, 37, 126–132. [Google Scholar] [CrossRef]
- Kaskavage, J.; Sklansky, D. Hyponatremia-Associated Rhabdomyolysis Following Exercise in an Adolescent with Cystic Fibrosis. Pediatrics 2012, 130, 220. [Google Scholar] [CrossRef]
- Lewis, D.P.; Hoffman, M.D.; Stuempfle, K.J.; Owen, B.E.; Rogers, I.R.; Verbalis, J.G.; Hew-Butler, T.D. The need for salt: Does a relationship exist between cystic fibrosis and exercise-associated hyponatremia? J. Strength. Cond. Res. 2014, 28, 807–813. [Google Scholar]
- Hew-Butler, T.D.; Sharwood, K.; Collins, M.; Speedy, D.; Noakes, T. Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon. Br. J. Sports Med. 2006, 40, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Noakes, T.D.; Dennis, S.C. Sodium replacement and fluid shifts during prolonged exercise in humans. Eur. J. Appl. Physiol. 2001, 84, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, D.; Rehrer, N. Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J. Appl. Physiol. 1999, 86, 1847–1851. [Google Scholar] [PubMed]
- Anastasiou, C.A.; Kavouras, S.A.; Arnaoutis, G.; Gioxari, A.; Kollia, M.; Botoula, E.; Sidossis, L.S. Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J. Athl. Train 2009, 44, 117–123. [Google Scholar]
- Twerenbold, R.; Knechtle, B.; Kakebeeke, T.; Eser, P.; Miller, G.; Von Arx, P.; Knecht, P. Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br. J. Sports Med. 2003, 37, 300–303. [Google Scholar]
- Barr, S.; Costill, D.; Fink, W. Fluid replacement during prolonged exercise: Effects of water, saline, or no fluid. Med. Sci. Sports Exerc. 1991, 23, 811–817. [Google Scholar] [PubMed]
- Speedy, D.B.; Thompson, J.; Rodgers, I.; Collins, M.; Sharwood, K. Oral salt supplementation during ultradistance exercise. Clin. J. Sport Med. 2002, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.D.; Black, K.E. Sodium supplementation has no effect on endurance performance during a cyclin time-trial in cool conditions: A randomized cross-over trial. J. Int. Soc. Sports Nutr. 2013, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Asplund, C.A.; O’Connor, F.G.; Noakes, T.D. Exerciseassociated collapse: An evidence-based review and primer for clinicians. Br. J. Sports Med. 2011, 45, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J. Hypertonic (3%) sodium chloride for emergent treatment of exercise-associated hypotonic encephalopathy. Sport Med. 2007, 37, 459–462. [Google Scholar] [CrossRef]
- Surgenor, S.; Uphold, R.E. Acute hyponatremia in ultra-endurance athletes. Am. J. Emerg. Med. 1995, 13, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, T.; Peter, S.; Pang, M.D.; Sanjeev Malik, M.D.; Chiampas, T. Large-volume hypertonic saline therapy in endurance athlete with exercise-associated hyponatremic encephalopathy. J. Emerg. Med. 2013, 44, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, R.T.; Lang, G.H.; Lowance, D.C.; Lathan, S.R. Hyponatremia and ultramarathon running. JAMA 1986, 255, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J.; D’Hemecourt, P.; Adner, M.M. Exertional dysnatremia in collapsedmarathon runners: A critical role for point-of-care testing to guide appropriate therapy. Am. J. Clin. Pathol. 2009, 132, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.E.; Rogers, I.R.; Hoffman, M.D.; Stuempfle, K.; Lewis, D.; Fogard, K.; Verbalis, J.C.; Hew-Butler, T. Efficacy of oral versus intravenous hypertonic saline in runners with hyponatremia. J. Sci. Med. Sport 2014, 17, 457–462. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urso, C.; Brucculeri, S.; Caimi, G. Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia. J. Clin. Med. 2014, 3, 1258-1275. https://doi.org/10.3390/jcm3041258
Urso C, Brucculeri S, Caimi G. Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia. Journal of Clinical Medicine. 2014; 3(4):1258-1275. https://doi.org/10.3390/jcm3041258
Chicago/Turabian StyleUrso, Caterina, Salvatore Brucculeri, and Gregorio Caimi. 2014. "Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia" Journal of Clinical Medicine 3, no. 4: 1258-1275. https://doi.org/10.3390/jcm3041258
APA StyleUrso, C., Brucculeri, S., & Caimi, G. (2014). Physiopathological, Epidemiological, Clinical and Therapeutic Aspects of Exercise-Associated Hyponatremia. Journal of Clinical Medicine, 3(4), 1258-1275. https://doi.org/10.3390/jcm3041258