Hyponatraemia in Emergency Medical Admissions—Outcomes and Costs
Abstract
:1. Introduction
2. Methods
2.1. Background
2.2. Data Collection
2.3. Statistical Methods
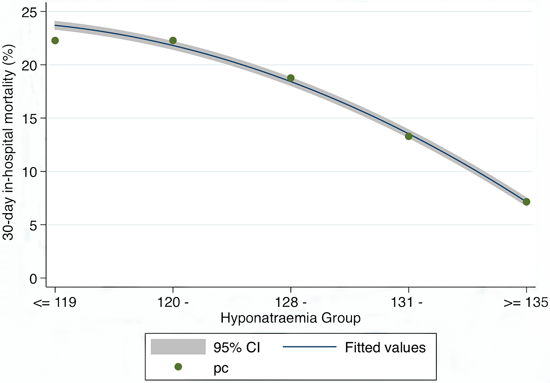
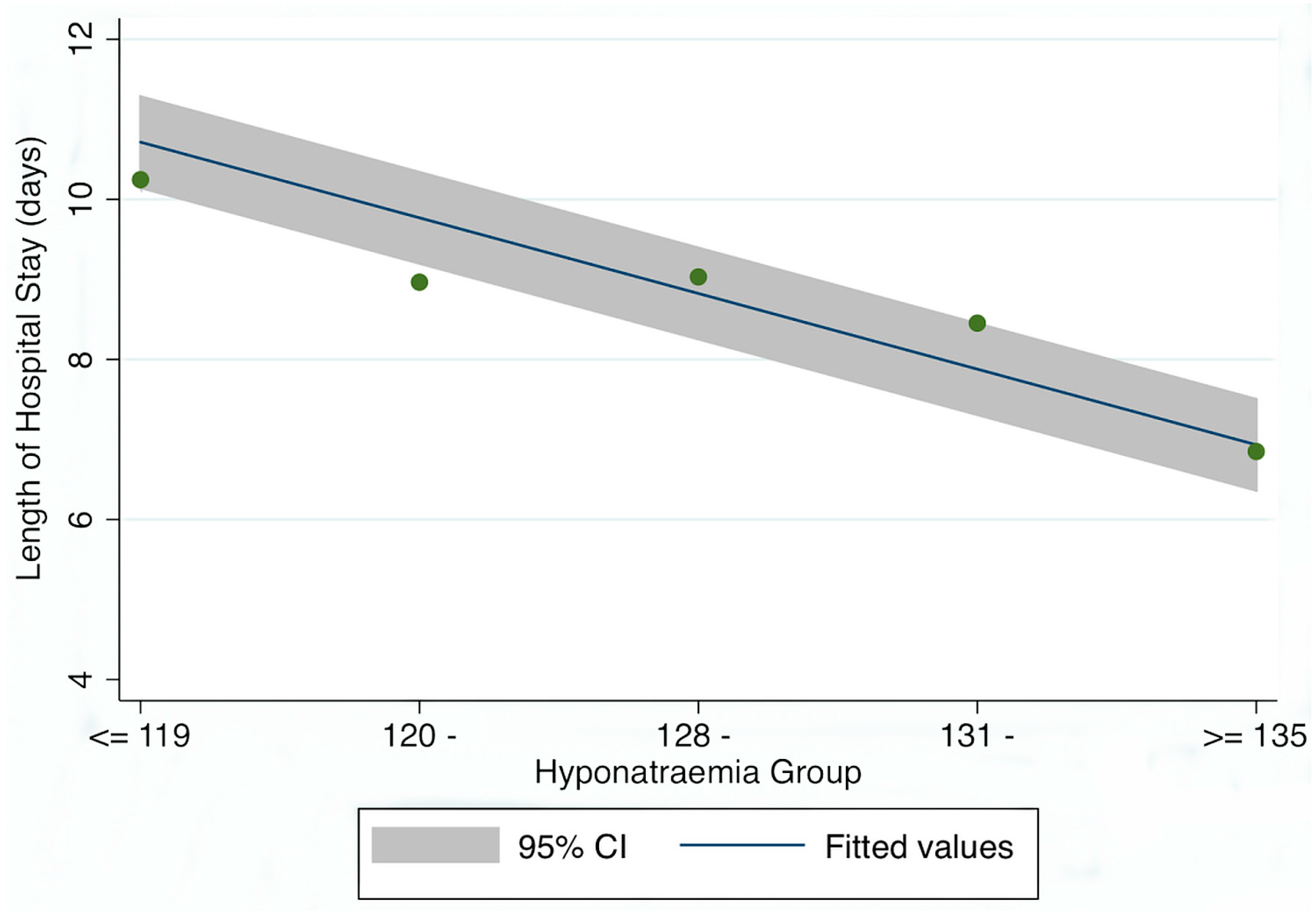
3. Results
Patient Demographics
| Variable | Level | >Na+ ≥ 135 mEq/L | Hyponatraemia | p-Value |
|---|---|---|---|---|
| N | 51,749 | 15,183 | ||
| Gender | Male | 24,924 (48.2%) | 7809 (51.4%) | <0.001 |
| Female | 26,825 (51.8%) | 7374 (48.6%) | ||
| Outcome | Alive | 49,762 (96.2%) | 14,012 (92.3%) | <0.001 |
| Died | 1987 (3.8%) | 1171 (7.7%) | ||
| Age (years: IQR) | 60.5 (40.2, 76.3) | 67.1 (49.0, 79.0) | <0.001 | |
| LOS (days: IQR) | 4.7 (1.9, 9.0) | 6.6 (3.1, 12.0) | <0.001 | |
| Manchester Triage | 3 | 30,661 (59.2%) | 8655 (57.0%) | <0.001 |
| 2 | 20,253 (39.1%) | 6316 (41.6%) | ||
| 1 | 835 (1.6%) | 212 (1.4%) | ||
| Charlson Index | 0 | 25,167 (48.6%) | 5420 (35.7%) | <0.001 |
| 1 | 13,853 (26.8%) | 4396 (29.0%) | ||
| 2 | 12,729 (24.6%) | 5367 (35.3%) |
| Variable | Level | >Na+ ≥ 135 mEq/L | Hyponatraemia | p-Value |
|---|---|---|---|---|
| Age Profile (years) | 10–39 | 12,856 (24.8%) | 2366 (15.6%) | <0.001 |
| 40–59 | 12,687 (24.5%) | 3550 (23.4%) | ||
| 60–74 | 11,927 (23.1%) | 3985 (26.2%) | ||
| 85+ | 9864 (19.1%) | 3572 (23.5%) | ||
| Disabling Score | 0 | 6622 (12.8%) | 937 (6.2%) | <0.001 |
| 1 | 13,446 (26.0%) | 3067 (20.2%) | ||
| 2 | 14,998 (29.0%) | 4574 (30.1%) | ||
| 3 | 10,234 (19.8%) | 3848 (25.3%) | ||
| 4 | 6449 (12.5%) | 2757 (18.2%) | ||
| Acute Illness Severity | 1 | 1876 (4.0%) | 109 (0.7%) | <0.001 |
| 2 | 4306 (9.3%) | 391 (2.7%) | ||
| 3 | 6844 (14.7%) | 990 (6.8%) | ||
| 4 | 8481 (18.2%) | 1984 (13.6%) | ||
| 5 | 9472 (20.4%) | 2949 (20.3%) | ||
| 6 | 15,504 (33.4%) | 8125 (55.8%) |

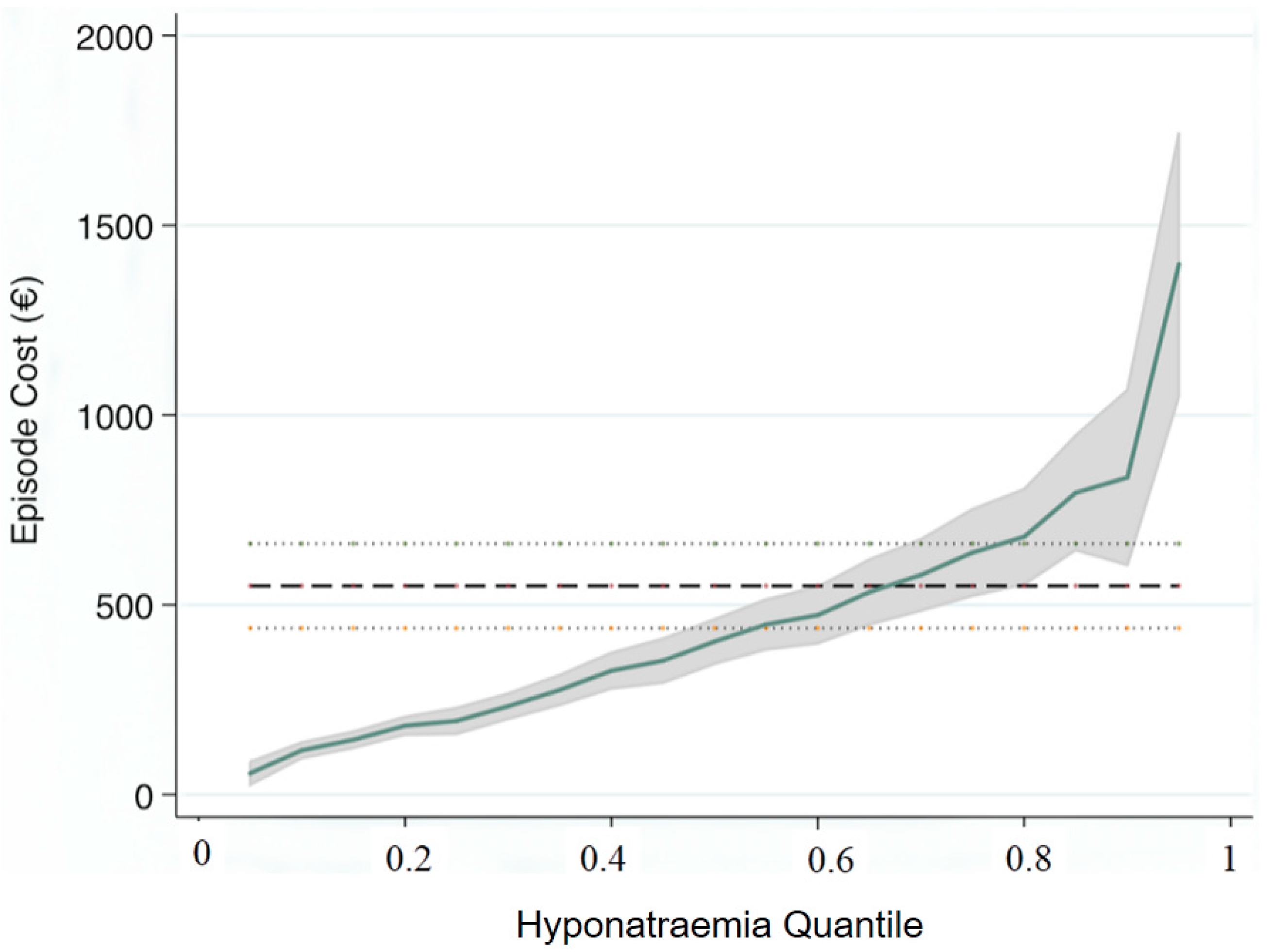
| Variables | Quantile | Parameter | 95% CI |
|---|---|---|---|
| Illness Severity | 0.25 | 43 | (17, 68) |
| Charlson Index | 131 | (79, 184) | |
| Disabling Disease | 528 | (490, 563) | |
| Triage Group | −24 | (−88, 41) | |
| Hyponatraemia | 194 | (146, 242) | |
| Illness Severity | 0.5 | 153 | (114, 192) |
| Charlson Index | 260 | (180, 340) | |
| Disabling Disease | 833 | (777, 889) | |
| Triage Group | −96 | (−194, 3) | |
| Hyponatraemia | 403 | (330, 477) | |
| Illness Severity | 0.75 | 434 | (362, 506) |
| Charlson Index | 559 | (412, 706) | |
| Disabling Disease | 1220 | (1117, 1323) | |
| Triage Group | −21 | (−202, 160) | |
| Hyponatraemia | 638 | (502, 774) |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kellett, J.; Deane, B. The Simple Clinical Score predicts mortality for 30 days after admission to an acute medical unit. QJM 2006, 99, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Whelan, B.; Bennett, K.; O’Riordan, D.; Silke, B. Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM 2009, 102, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, K.; Beeching, N.; Gill, G. Hyponatraemia as a risk factor for hospital mortality. QJM 2006, 99, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Stachon, A.; Segbers, E.; Hering, S.; Kempf, R.; Holland-Letz, T.; Krieg, M. A laboratory-based risk score for medical intensive care patients. Clin. Chem. Lab. Med. 2008, 46, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Mount, D.B.; Curhan, G.C. Mortality after Hospitalization with Mild, Moderate, and Severe Hyponatremia. Am. J. Med. 2009, 122, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.X.; Bridges, L.; Umpierrez, G.E.; Kuhl, D.; Kitabchi, A.E. Admission Hyperglycemia and Other Risk Factors as Predictors of Hospital Mortality in a Medical ICU Population. Chest 2005, 128, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Feldman, J. Association of serum albumin and mortality risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An Independent Marker of In-Hospital Mortality in Patients with Undiagnosed Diabetes. J. Clin. Endocrinol. MeTable 2002, 87, 978–982. [Google Scholar] [CrossRef]
- Suleiman, M.; Hammerman, H.; Boulos, M.; Kapeliovich, M.R.; Suleiman, A.; Agmon, Y.; Markiewicz, W.; Aronson, D. Fasting glucose is an important independent risk factor for 30 days mortality in patients with acute myocardial infarction: A prospective study. Circulation 2005, 111, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Stranders, I.; Diamant, M.; van Gelder, R.E.; Spruijt, H.J.; Twisk, J.W.; Heinem, R.J.; Visser, F.C. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch. Intern. Med. 2004, 164, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.S. Association between hyperglycemia and in-creased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin. Proc. 2003, 78, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; le Jeune, L.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS Guidelines for the Management of Community Acquired Pneumonia in Adults. Thorax 2009, 64. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Kellett, J.; Rooney, T.; Bennett, K.; O’Riordan, D. An improved medical admissions risk system using multivariable fractional polynomial logistic regression modelling. QJM 2010, 103, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, K.; Hastings, I.M.; Beeching, N.J.; Gill, G.V. Laboratory risk factors for hospital mortality in acutely admitted patients. QJM 2007, 100, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Froom, P.; Shimoni, Z. Prediction of hospital mortality rates by admission laboratory tests. Clin. Chem. 2006, 52, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified early warning score in medical admissions. QJM 2001, 94, 521–556. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.; Fisher, C.; Willitis, N. The Rapid Acute Physiology Score. Am. J. Emerg. Med. 1987, 5, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, S.; Turner, T.; Nicholl, J. Prediction of mortality among emergency medical admissions. Emerg. Med. J. 2006, 23, 371–375. [Google Scholar]
- Reynolds, R.M.; Padfield, P.L.; Seckl, J.R. Disorders of sodium balance. BMJ 2006, 332, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Holland-Bill, L.; Christiansen, C.F.; Ulrichsen, S.P.; Ring, T.; Jørgensen, J.O.; Sørensen, H.T. Validity of the International Classification of Diseases, 10th revision discharge diagnosis codes for hyponatraemia in the Danish National Registry of Patients. BMJ Open 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Nardi, R.; Fiorino, S.; Borioni, D.; Agostini, D.; D’Anastasio, C.; Marchetti, C.; Muratori, M. Comprehensive complexity assessment as a key tool for the prediction of in-hospital mortality in heart failure of aged patients admitted to internal medicine wards. Arch. Gerontol. Geriatr. 2007, 44, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, K.E.; Schmidt, J.M.; Claassen, J.; Temes, R.E.; Frontera, J.A.; Ostapkovich, N.; Parra, A.; Connolly, E.S.; Mayer, S.A. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006, 34, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.D.; Btaiche, I.F.; Sacks, G.S.; Kudsk, K.A. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am. J. Health Syst. Pharm. 2005, 62, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; Abraham, W.T.; Albert, N.M.; Gattis Stough, W.; Greenberg, B.H.; O’Connor, C.M.; She, L.; Yancy, C.W.; Young, J.; Fonarow, G.C.; et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur. Heart J. 2007, 28, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; van Kimmenade, R.R.; Richards, M.; Bayes-Genis, A.; Pinto, Y.; Moore, S.A.; Januzzi, J.L., Jr. Hyponatremia, natriuretic peptides, and outcomes in acutely decompensated heart failure: Results from the International Collaborative of NT-proBNP Study. Circ. Heart Fail. 2010, 3, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Forfia, P.R.; Mathai, S.C.; Fisher, M.R.; Housten-Harris, T.; Hemnes, A.R.; Champion, H.C.; Girgis, R.E.; Hassoun, P.M. Hyponatremia Predicts Right Heart Failure and Poor Survival in Pulmonary Arterial Hypertension. Am. J. Respir. Crit Care Med. 2008, 177, 1364–1349. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Sterns, R.H.; Nigwekar, S.U.; Cappuccio, J.D. Mortality and serum sodium: Do patients die from or with hyponatremia? Clin. J. Am. Soc. Nephrol. 2011, 6, 960–965. [Google Scholar] [CrossRef]
- Rooney, T.; Moloney, E.D.; Bennett, K.; O’Riordan, D.; Silke, B. Impact of an acute medical admission unit on hospital mortality: A 5 year prospective study. QJM 2008, 101, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; O’Riordan, D.; Silke, B. Long-term outcome of an AMAU—A decade’s experience. QJM 2014, 107, 43–49. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; Colgan, M.P.; McGuigan, C.; Smyth, F.; Haider, N.; O’Neill, S.; Moore, D.; Madhavan, P. A critical evaluation of HIPE data. Ir. Med. J. 2012, 105, 21–23. [Google Scholar] [PubMed]
- O’Sullivan, E.; Callely, E.; O’Riordan, D.; Bennett, K.; Silke, B. Predicting outcomes in emergency medical admissions—Role of laboratory data and co-morbidity. Acute Med. 2012, 2, 59–65. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Manchester Triage Group. Emergency Triage, 2nd ed.; Blackwell Publishing Ltd.: London, UK, 2006. [Google Scholar]
- Chotirmall, S.H.; Picardo, S.; Lyons, J.; D’Alton, M.; O’Riordan, D.; Silke, B. Disabling disease codes predict worse outcomes for acute medical admissions. Intern. Med. J. 2014, 44, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Mikulich, O.; Callaly, E.; Bennett, K.; O’Riordan, D.; Silke, B. The increased mortality associated with a weekend emergency admission is due to increased illness severity and altered case-mix. Acute Med. 2011, 10, 182–187. [Google Scholar] [PubMed]
- Armstrong, P. The Costs of Activity-Based Management. Account. Organ. Soc. 2002, 27, 99–120. [Google Scholar] [CrossRef]
- Arnaboldi, M.; Lapsley, I. Modern costing innovations and legitimation: A health care study. Abacus 2004, 40, 1–20. [Google Scholar] [CrossRef]
- Williams, R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012, 12, 308–331. [Google Scholar]
- Cameron, A.C.; Trivedi, P.K. Microeconometrics Using Stata; Stata Press: College Station, TX, USA, 2009. [Google Scholar]
- Stoltzfus, J.; Nishijima, D.; Melnikow, J. Why Quantile Regression Makes Good Sense for Analyzing Economic Outcomes in Medical Research. Acad. Emerg. Med. 2012, 19, 850–851. [Google Scholar] [CrossRef] [PubMed]
- Cody, C.S.; Clark, A.E.; Thomas, A.M.; Cook, L.J. Comparing least-squares and quantile regression approaches to analyzing median hospital charges. Acad. Emerg. Med. 2012, 19, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Koenker, R. Confidence intervals for regression quantiles. In Asymptotic Statistics, Proceedings of the Fifth Prague Symposium, Prague, 4–9 September 1993; Koenker, R., Ed.; Springer: Heidelberg, Gemany, 1993. [Google Scholar]
- Wald, R.; Jaber, B.L.; Price, L.L.; Upadhyay, A.; Madias, N.E. Impact of Hospital-Associated Hyponatremia on Selected Outcomes. Arch. Intern. Med. 2010, 170, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Assen, A.; Abouem, D.; Vandergheynst, F.; Nguyen, T.; Taccone, F.S.; Melot, C. Hyponatremia at the Emergency Department: A case-control study. Minerva Anestesiol. 2014, 80, 419–428. [Google Scholar] [PubMed]
- Tzoulis, P.; Bagkeris, E.; Bouloux, P.M. A case—Control study of hyponatraemia as an independent risk factor for inpatient mortality. Clin. Endocrinol. 2014, 81, 401–407. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conway, R.; Byrne, D.; O'Riordan, D.; Silke, B. Hyponatraemia in Emergency Medical Admissions—Outcomes and Costs. J. Clin. Med. 2014, 3, 1220-1233. https://doi.org/10.3390/jcm3041220
Conway R, Byrne D, O'Riordan D, Silke B. Hyponatraemia in Emergency Medical Admissions—Outcomes and Costs. Journal of Clinical Medicine. 2014; 3(4):1220-1233. https://doi.org/10.3390/jcm3041220
Chicago/Turabian StyleConway, Richard, Declan Byrne, Deirdre O'Riordan, and Bernard Silke. 2014. "Hyponatraemia in Emergency Medical Admissions—Outcomes and Costs" Journal of Clinical Medicine 3, no. 4: 1220-1233. https://doi.org/10.3390/jcm3041220
APA StyleConway, R., Byrne, D., O'Riordan, D., & Silke, B. (2014). Hyponatraemia in Emergency Medical Admissions—Outcomes and Costs. Journal of Clinical Medicine, 3(4), 1220-1233. https://doi.org/10.3390/jcm3041220