Immature Platelet Fraction as a Potential Biomarker of Dysregulated Thrombopoiesis in Philadelphia-Negative Myeloproliferative Neoplasms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistics
3. Results
3.1. Patients Characteristics
3.2. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Liu, Y.; Zhang, M.; Zhang, J. New advances in the role of JAK2 V617F mutation in myeloproliferative neoplasms. Cancer 2024, 130, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Bjorkholm, M.; Dickman, P.W.; Landgren, O.; Derolf, A.R.; Kristinsson, S.Y.; Andersson, T.M.L. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: A population-based cohort study. Ann. Intern. Med. 2018, 168, 317–325. [Google Scholar] [CrossRef]
- Kaifie, A.; Kirschner, M.; Wolf, D.; Maintz, C.; Hänel, M.; Gattermann, N.; Gökkurt, E.; Platzbecker, U.; Hollburg, W.; Göthert, J.R.; et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): Analysis from the German SAL-MPN-registry. J. Hematol. Oncol. 2016, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Smalberg, J.H.; Arends, L.R.; Valla, D.C.; Kiladjian, J.J.; Janssen, H.L.; Leebeek, F.W. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: A meta-analysis. Blood 2012, 120, 4921–4928. [Google Scholar] [CrossRef] [PubMed]
- Horvat, I.; Boban, A.; Zadro, R.; Antolic, M.R.; Serventi-Seiwerth, R.; Roncevic, P.; Radman, I.; Sertic, D.; Vodanovic, M.; Pulanic, D.; et al. Influence of Blood Count, Cardiovascular Risks, Inherited Thrombophilia, and JAK2 V617F Burden Allele on Type of Thrombosis in Patients with Philadelphia Chromosome Negative Myeloproliferative Neoplasms. Clin. Lymphoma Myeloma Leuk. 2019, 19, 53–63. [Google Scholar] [CrossRef]
- Kelliher, S.; Falanga, A. Thrombosis in myeloproliferative neoplasms: A clinical and pathophysiological perspective. Thromb. Update 2021, 5, 100081. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Marin Oyarzún, C.P.; Carestia, A.; Lev, P.R.; Glembotsky, A.C.; Ríos, M.A.C.; Moiraghi, B.; Molinas, F.C.; Marta, R.F.; Schattner, M.; Heller, P.G. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci. Rep. 2016, 6, 38738. [Google Scholar] [CrossRef]
- Guy, A.; Favre, S.; Labrouche-Colomer, S.; Garcia, G.; Gourdou-Latyszenok, V.; Wolff-Trombini, L.; Josserand, L.; Kimmerlin, Q.; Kilani, B.; Marty, C.; et al. Platelets and neutrophils cooperate to induce increased NETosis in JAK2-V617F MPN. J. Thromb. Haemost. 2024, 22, 172–187. [Google Scholar] [CrossRef]
- Matsuura, S.; Thompson, C.R.; Belghasem, M.E.; Bekendam, R.H.; Piasecki, A.; Leiva, O.; Ray, A.; Italiano, J.; Yang, M.; Merill-Skoloff, G.; et al. Platelet dysfunction and thrombosis in JAK2(V617F)-mutated primary myelofibrotic mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e262–e272. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Marchetti, M.; Russo, L.; Tartari, C.J.; Leuzzi, A.; Finazzi, G.; Rambaldi, A.; Cate, H.T.; Falanga, A. ADP-induced platelet aggregation and thrombin generation are increased in Essential Thrombocythemia and Polycythemia Vera. Thromb. Res. 2013, 132, 88–93. [Google Scholar] [CrossRef]
- Campbell, P.J.; MacLean, C.; Beer, P.A.; Buck, G.; Wheatley, K.; Kiladjian, J.-J.; Forsyth, C.; Harrison, C.N.; Green, A.R. Correlation of blood counts with vascular complications in essential thrombocythemia: Analysis of the prospective PT1 cohort. Blood 2012, 120, 1409–1411. [Google Scholar] [CrossRef]
- Barbui, T.; Vannucchi, A.; Buxhofer-Ausch, V.; De Stefano, V.; Betti, S.; Rambaldi, A.; Rumi, E.; Ruggeri, M.; Rodeghiero, F.; Randi, M.L.; et al. Practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined essential thrombocythemia. Blood Cancer J. 2015, 5, e369. [Google Scholar] [CrossRef]
- Lev, E.I. Immature Platelets: Clinical Relevance and Research Perspectives. Circulation 2016, 134, 987–988. [Google Scholar] [CrossRef]
- Hille, L.; Lenz, M.; Vlachos, A.; Grüning, B.; Hein, L.; Neumann, F.J.; Nührenberg, T.G.; Trenk, D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020, 18, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Grove, E.L.; Hvas, A.M.; Kristensen, S.D. Immature platelets in patients with acute coronary syndromes. Thromb. Haemost. 2009, 101, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Uchiyama, S.; Yamazaki, M.; Okubo, K.; Takakuwa, Y.; Iwata, M. Flow cytometric analysis of reticulated platelets in patients with ischemic stroke. Thromb. Res. 2002, 106, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ryningen, A.; Apelseth, T.; Hausken, T.; Bruserud, Ø. Reticulated platelets are increased in chronic myeloproliferative disorders, pure erythrocytosis, reactive thrombocytosis and prior to hematopoietic reconstitution after intensive chemotheapy. Platelets 2006, 17, 296–302. [Google Scholar] [CrossRef]
- Abe, Y.; Wada, H.; Tomatsu, H.; Sakaguchi, A.; Nishioka, J.; Yabu, Y.; Onishi, K.; Nakatani, K.; Morishita, Y.; Oguni, S.; et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb. Res. 2006, 118, 463–469. [Google Scholar] [CrossRef]
- Harrison, P.; Robinson, M.S.; Mackie, I.J.; Machin, S.J. Reticulated platelets. Platelets 1997, 8, 379–383. [Google Scholar] [CrossRef]
- Pons, I.; Monteagudo, M.; Lucchetti, G.; Muñoz, L.; Perea, G.; Colomina, I.; Guiu, J.; Obiols, J. Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur. J. Haematol. 2010, 85, 158–163. [Google Scholar] [CrossRef]
- Briere, J.; Kiladjian, J.J.; Peynaud-Debayle, E. Megakaryocytes and platelets in myeloproliferative disorders. Baillieres Clin. Haematol. 1997, 10, 65–88. [Google Scholar] [CrossRef]
- Cao, Z.; Soleimani Samarkhazan, H. Immature platelet fraction in cardiology. Clin. Chim. Acta 2026, 579, 120600. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2023, 98, 801–821. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.; Barbui, T. Essential thrombocythemia: 2024 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2024, 99, 697–718. [Google Scholar] [CrossRef] [PubMed]
- Panova-Noeva, M.; Marchetti, M.; Buoro, S.; Russo, L.; Leuzzi, A.; Finazzi, G.; Rambaldi, A.; Ottomano, C.; Ten Cate, H.; Falanga, A. JAK2V617F mutation and hydroxyurea treatment as determinants of immature platelet parameters in essential thrombocythemia and polycythemia vera patients. Blood 2011, 118, 2599–2601. [Google Scholar] [CrossRef]
- Lucijanic, M.; Cicic, D.; Stoos-Veic, T.; Pejsa, V.; Lucijanic, J.; Fazlic Dzankic, A.; Vlasac Glasnovic, J.; Soric, E.; Skelin, M.; Kusec, R. Elevated Neutrophil-to-Lymphocyte-ratio and Platelet-to-Lymphocyte Ratio in Myelofibrosis: Inflammatory Biomarkers or Representatives of Myeloproliferation Itself? Anticancer Res. 2018, 38, 3157–3163. [Google Scholar] [PubMed]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 26, 3636. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Thrombotic disease in the myeloproliferative neoplasms. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 571–581. [Google Scholar] [CrossRef]
| Variable | Ph− MPN (n = 45) | Control Group (n = 27) |
|---|---|---|
| ET | 24 (53%) | |
| PV | 13 (28%) | |
| MPN unclassified | 5 (11%) | |
| PMF | 3 (6%) | |
| Age | 65 (24–87) | 50 (21–76) |
| Sex, female | 19 (42%) | 8 (29%) |
| JAK2 | 33 (73%) |
| Variable | Ph− MPN (n = 45) | Control Group (n = 27) | p Value * |
|---|---|---|---|
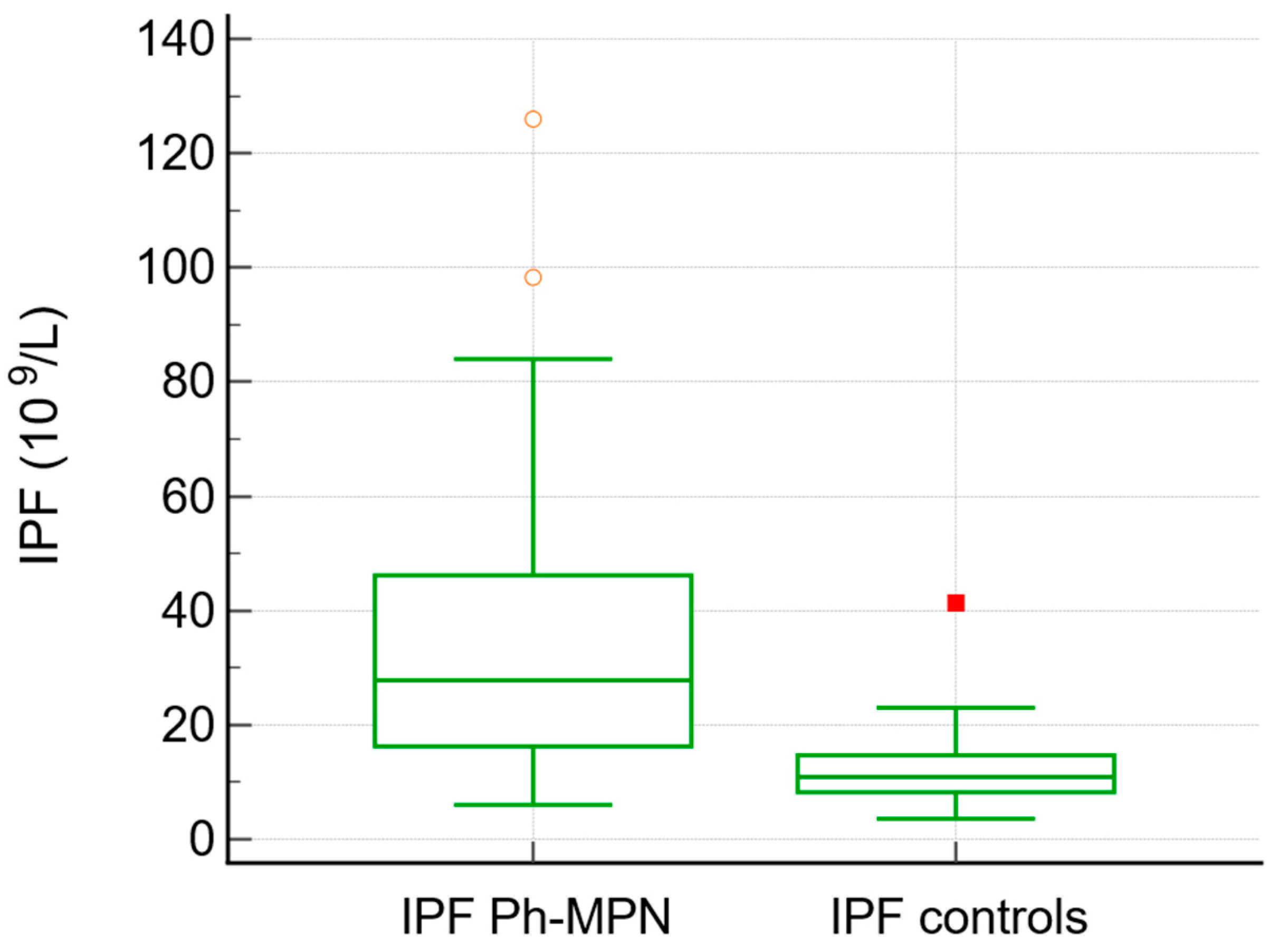
| IPF × 109/L (median, range) | 27 (6–126) | 10.9 (3.6–41) | <0.0001 |
| Platelets × 109/L (median, range) | 572 (141–1292) | 238 (133–690) | <0.0001 |
| Erythrocytes × 1012/L (median, range) | 5.23 (2.8–10.1) | 5.64 (3.57–6.98) | 0.913 |
| Leukocytes × 109/L (median, range) | 9.4 (4.93–24.9) | 9.35 (5.70–21.50) | 0.521 |
| Hematocrit % (median, range) | 0.46 (0.26–0.73) | 0.49 (0.32–0.57) | 0.332 |
| Granulocytes × 109/L (median, range) | 6.20 (2.32–38.59) | 5.64 (2.70–18.04) | 0.220 |
| Lymphocytes × 109/L (median, range) | 2.02 (0.79–5.49) | 2.28 (1.35–3.75) | 0.037 |
| Neutrophil to lymphocyte ratio (NLR) | 3.68 (0.85–16.28) | 2. 68 (1.18–5.18) | 0.013 |
| Creatinine μmol/L (median, range) | 78 (44–125) | 80 (50–206) | 0.443 |
| LDH U/L (median, range) | 226 (86–509) | 178 (81–592) | <0.0001 |
| D-dimer mg/mL (median, range) | 0.5 (0.19–6.21) | 0.33 (0.18–2.46) | 0.048 |
| Variable | Ph− MPN JAK2 Positive (n = 33) | Ph− MPN JAK2 Negative (n = 12) | p Value * |
|---|---|---|---|
| IPF × 109/L (median, range) | 26 (6–126) | 40.7 (8.5–98.3) | 0.254 |
| Platelets × 109/L (median, range) | 557 (141–1134) | 652 (296–1292) | 0.501 |
| Erythrocytes × 1012/L (median, range) | 5.4 (3.7–8.6) | 4.6 (2.88–10.15) | 0.056 |
| Leukocytes × 109/L (median, range) | 9.45 (4.93–24.90) | 10.20 (5.7–17.7) | 0.877 |
| Hematocrit % (median, range) | 0.47 (0.34–0.63) | 0.41 (0.26–0.73) | 0.032 |
| Granulocytes × 109/L (median, range) | 6.38 (3.75–38.5) | 5.78 (2.32–12.29) | 0.449 |
| Neutrophil-to-lymphocyte ratio (NLR) | 3.68 (1.58–16.28) | 2.48 (0.85–7.68) | 0.161 |
| D-dimer mg/mL (median, range) | 0.58 (0.10–6.21) | 0.47 (0.28–0.8) | 0.694 |
| Creatinine μmol/L (median, range) | 79 (54–125) | 69 (44–101) | 0.369 |
| LDH U/L (median, range) | 225 (86–509) | 240 (122–439) | 0.635 |
| Variable | Ph− MPN > 60 Years (n = 29) | Ph− MPN < 60 Years (n = 16) | p Value * |
|---|---|---|---|
| IPF × 109/L (median, range) | 38.8 (10.2–126) | 23.5 (6–50) | 0.0218 |
| Platelets × 109/L (median, range) | 572 (141–1264) | 604 (296–1292) | 0.849 |
| Erythrocytes × 1012/L (median, range) | 5.23 (2.88–10.15) | 5.21 (4.43–8.81) | 0.766 |
| Leukocytes, × 109/L (median, range) | 10 (4.93–23) | 8.6 (6.2–24.9) | 0.399 |
| Hematocrit % (median, range) | 0.46 (0.26–0.73) | 0.46 (0.36–0.66) | 0.803 |
| Granulocytes × 109/L (median, range) | 6.5 (2.32–38.59) | 5.34 (3.96–20.94) | 0.367 |
| Neutrophil-to-lymphocyte ratio (NLR) | 3.68 (0.85–16.2) | 2.92 (1.67–8.1) | 0.420 |
| D-dimer mg/mL (median, range) | 0.68 (0.2–6.21) | 0.31 (0.19–0.7) | 0.004 |
| Creatinine μmol/L (median, range) | 85 (44–125) | 72.5 (54–118) | 0.114 |
| LDH U/L (median, range) | 225 (86–509) | 233 (122–455) | 0.872 |
| Variable | ET (n = 24) | PV (n = 13) | PMF (n = 3) | MPN-U (n = 5) | p Value * |
|---|---|---|---|---|---|
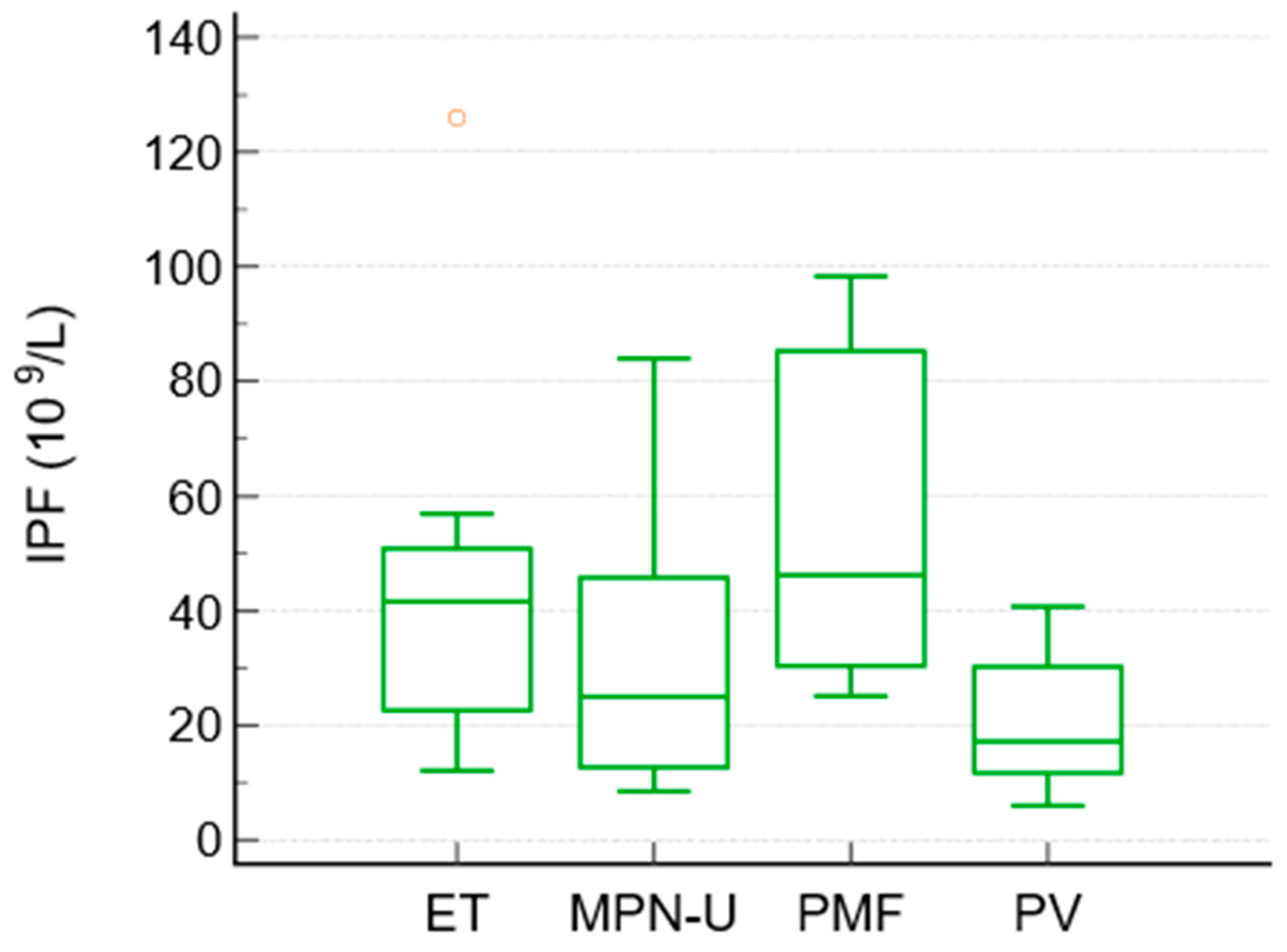
| IPF × 109/L (median, range) | 41.6 (12–126) | 17.2 (6–40.7) | 46.2 (25–98) | 25 (8.5–84) | 0.027 |
| Platelets × 109/L (median, range) | 675 (320–1292) | 422 (277–848) | 496 (301–638) | 436 (141–544) | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zekanovic, I.; Marketin, T.; Moric Peric, M.; Zekanovic, D.; Vulic, A.; Milos, M.; Bogic, A.; Marcinkovic, M.; Grbic Pavlovic, P.; Radic Antolic, M.; et al. Immature Platelet Fraction as a Potential Biomarker of Dysregulated Thrombopoiesis in Philadelphia-Negative Myeloproliferative Neoplasms. J. Clin. Med. 2026, 15, 1140. https://doi.org/10.3390/jcm15031140
Zekanovic I, Marketin T, Moric Peric M, Zekanovic D, Vulic A, Milos M, Bogic A, Marcinkovic M, Grbic Pavlovic P, Radic Antolic M, et al. Immature Platelet Fraction as a Potential Biomarker of Dysregulated Thrombopoiesis in Philadelphia-Negative Myeloproliferative Neoplasms. Journal of Clinical Medicine. 2026; 15(3):1140. https://doi.org/10.3390/jcm15031140
Chicago/Turabian StyleZekanovic, Ivan, Tina Marketin, Martina Moric Peric, Drazen Zekanovic, Ante Vulic, Marija Milos, Anamarija Bogic, Marta Marcinkovic, Petra Grbic Pavlovic, Margareta Radic Antolic, and et al. 2026. "Immature Platelet Fraction as a Potential Biomarker of Dysregulated Thrombopoiesis in Philadelphia-Negative Myeloproliferative Neoplasms" Journal of Clinical Medicine 15, no. 3: 1140. https://doi.org/10.3390/jcm15031140
APA StyleZekanovic, I., Marketin, T., Moric Peric, M., Zekanovic, D., Vulic, A., Milos, M., Bogic, A., Marcinkovic, M., Grbic Pavlovic, P., Radic Antolic, M., Knezevic, J., Jurlina, L., & Boban, A. (2026). Immature Platelet Fraction as a Potential Biomarker of Dysregulated Thrombopoiesis in Philadelphia-Negative Myeloproliferative Neoplasms. Journal of Clinical Medicine, 15(3), 1140. https://doi.org/10.3390/jcm15031140





