Characteristics and Outcomes of New-Onset Cardiomyopathy in Hospitalized COVID-19 Patients
Abstract
1. Introduction
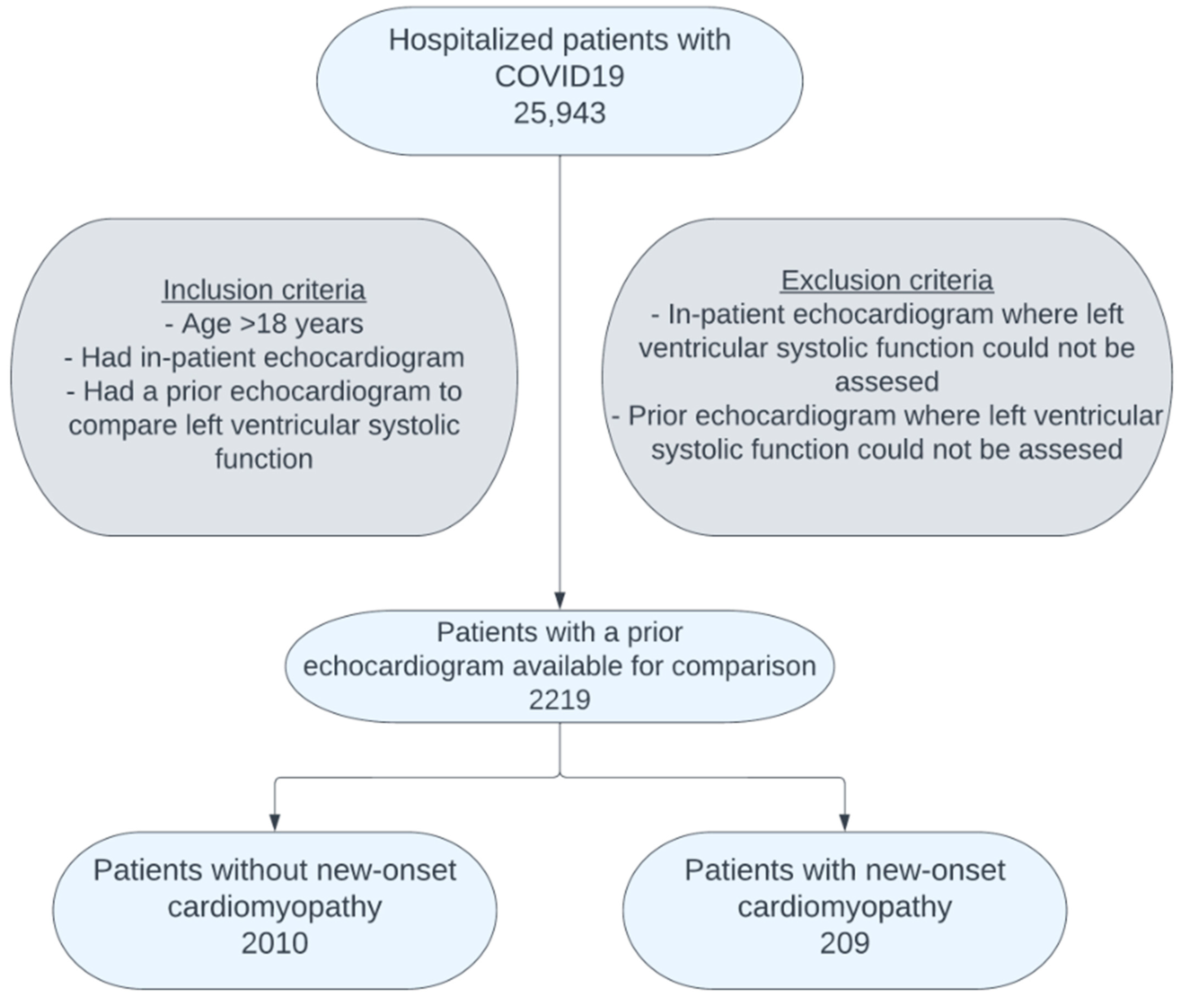
2. Materials and Method
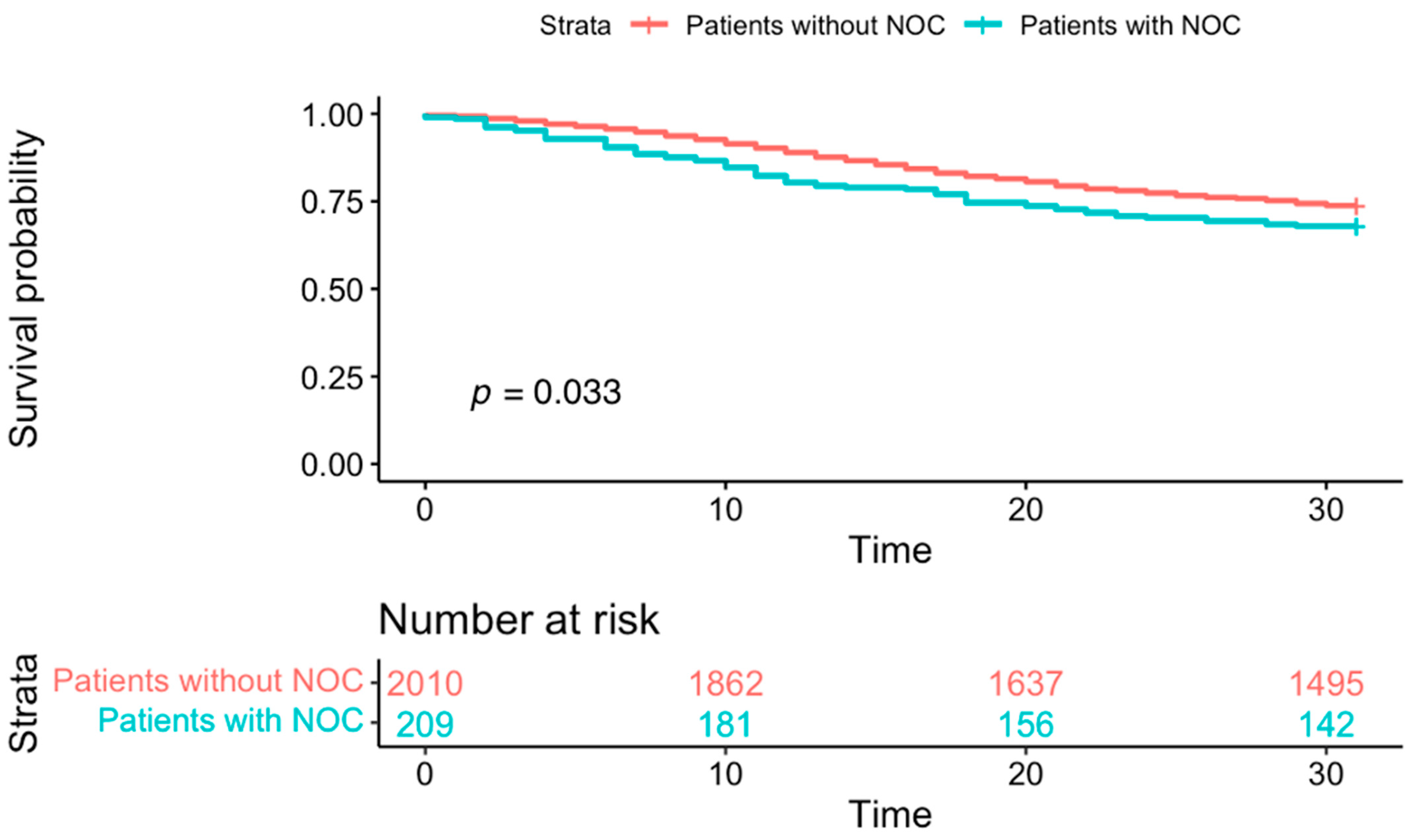
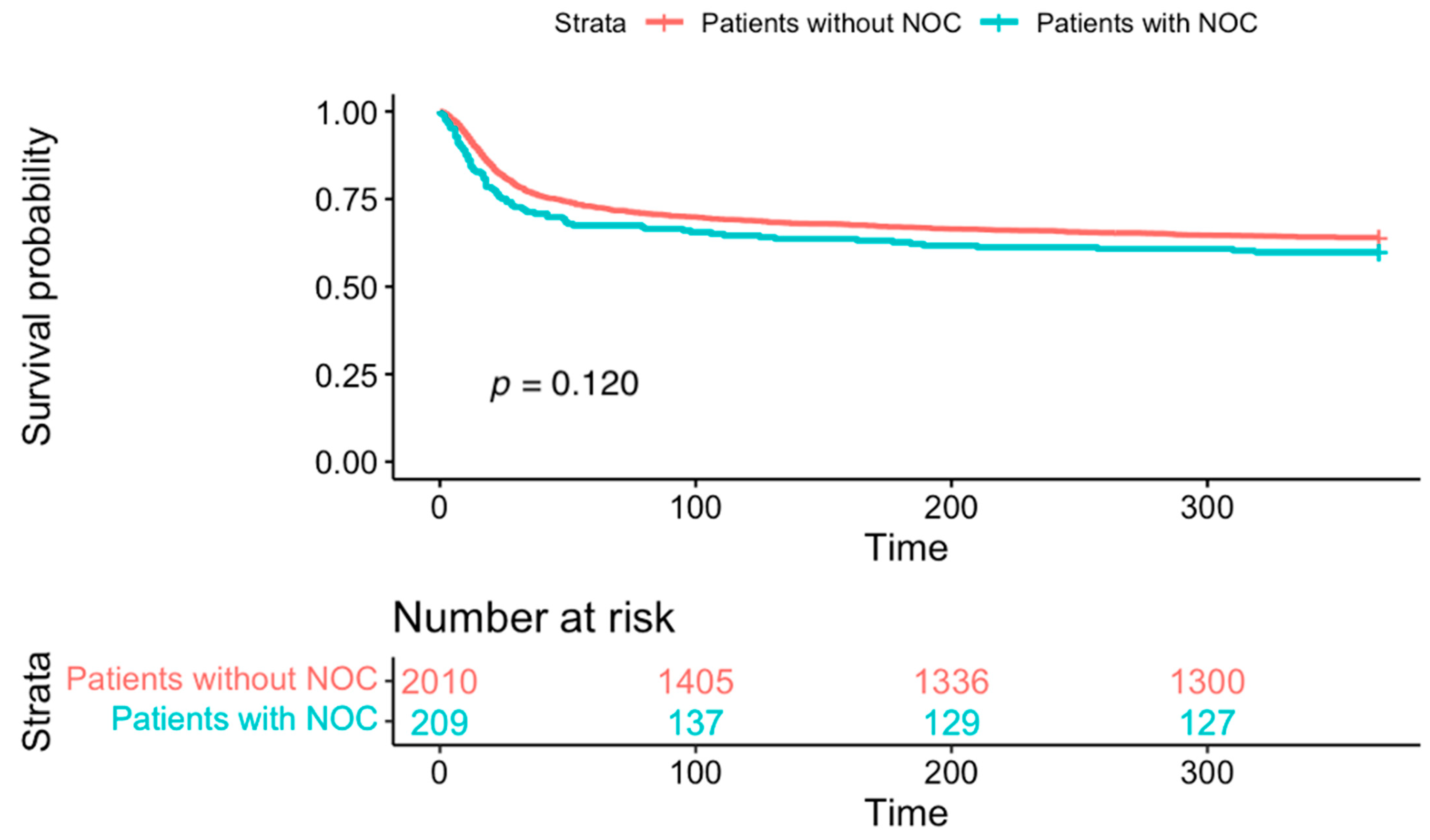
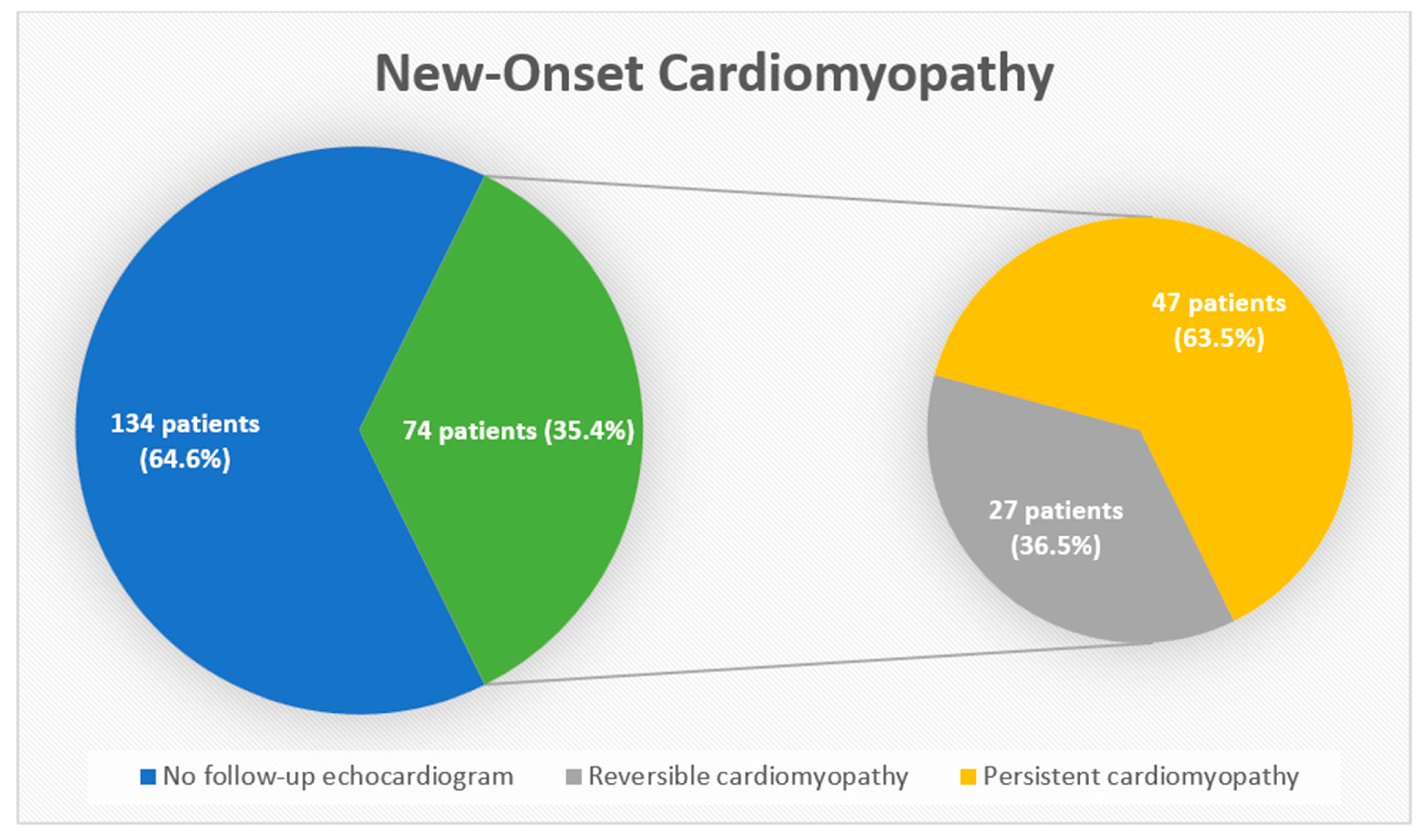
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 March 2025).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chawla, S.; Karimi, H.; Ahmed, T.; Shah, G. Cardiac Thromboembolism in COVID-19: A Case Series. Cureus 2022, 14, e25193. [Google Scholar] [CrossRef] [PubMed]
- Dy, L.F.; Lintao, R.C.V.; Cordero, C.P.; Cabaluna, I.T.G.; Dans, L.F. Prevalence and prognostic associations of cardiac abnormalities among hospitalized patients with COVID-19: A systematic review and meta-analysis. Sci Rep. 2021, 11, 8449. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Li, Y.; An, N.; Xing, Y.; Yang, F.; Tian, L.; Sun, J.; Gao, Y.; Shang, H.; et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: A meta-analysis and systematic review. Crit. Care 2020, 24, 468. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Bearse, M.; Hung, Y.P.; Krauson, A.J.; Bonanno, L.; Boyraz, B.; Harris, C.K.; Helland, T.L.; Hilburn, C.F.; Hutchison, B.; Jobbagy, S.; et al. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod. Pathol. 2021, 34, 1345–1357. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Madjiguène Ka, M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Di Salvo, G.; Di Salvo, L.E.; Pearce, P.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef]
- Karagodin, I.; Carvalho Singulane, C.; Woodward, G.M.; Xie, M.; Tucay, E.S.; Tude Rodrigues, A.C.; Vasquez-Ortiz, Z.Y.; Alizadehasl, A.; Monaghan, M.J.; Ordonez Salazar, B.A.; et al. Echocardiographic Correlates of In-Hospital Death in Patients with Acute COVID-19 Infection: The World Alliance Societies of Echocardiography (WASE-COVID) Study. J. Am. Soc. Echocardiogr. 2021, 34, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, J.; Beck-Friis, J.; Jensen, C.; Dalla, K.; Mårdstam, S.; Christensen, J.; Nordén, N.; Widing, H.; Rosén-Wetterholm, E.; Cavefors, O.; et al. Cardiac dysfunction and mortality in critically ill patients with COVID-19: A Swedish multicentre observational study. Acta. Anaesthesiol. Scand. 2022, 66, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Bui, Q.M.; King, K.; DeMaria, A.; Daniels, L.B. Subclinical left ventricular dysfunction in COVID-19. Int. J. Cardiol. Heart. Vasc. 2021, 34, 100770. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef]
- Silverio, A.; Di Maio, M.; Scudiero, F.; Russo, V.; Esposito, L.; Attena, E.; Pezzullo, S.; Parodi, G.; D’Andrea, A.; Damato, A.; et al. Clinical conditions and echocardiographic parameters associated with mortality in COVID-19. Eur. J. Clin. Investig. 2021, 51, e13638. [Google Scholar] [CrossRef]
- Pishgahi, M.; Karimi Toudeshki, K.; Safari, S.; Yousefifard, M. Echocardiographic Abnormalities as Independent Prognostic Factors of In-Hospital Mortality among COVID-19 Patients. Arch. Acad. Emerg. Med. 2021, 9, e21. [Google Scholar] [CrossRef]
- Gomez, J.M.D.; Zimmerman, A.C.; du Fay de Lavallaz, J.; Wagner, J.; Tung, L.; Bouroukas, A.; Nguyen, T.T.P.; Canzolino, J.; Goldberg, A.; Santos Volgman, A.; et al. Echocardiographic predictors of mortality and morbidity in COVID-19 disease using focused cardiovascular ultrasound. Int. J. Cardiol. Heart Vasc. 2022, 39, 100982. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.P.; Sweitzer, N.K.; Indik, J.H.; Acharya, D.; William, P. SARS-CoV-2 Infection and Cardiovascular Disease: COVID-19 Heart. Heart Lung Circ. 2020, 29, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Holm, A.; Jerkeman, M.; Sultanian, P.; Lundgren, P.; Ravn-Fischer, A.; Israelsson, J.; Giesecke, J.; Herlitz, J.; Rawshani, A. Cohort study of the characteristics and outcomes in patients with COVID-19 and in-hospital cardiac arrest. BMJ Open 2021, 11, e054943. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.M.; Shah, M.; Shah, S.; Li, A.; Jauhar, S. Takotsubo Syndrome and COVID-19: Associations and Implications. Cur. Probl. Cardiol. 2021, 46, 100763. [Google Scholar] [CrossRef]
- Umemura, Y.; Yamakawa, K.; Kiguchi, T.; Nishida, T.; Kawada, M.; Fujimi, S. Hematological Phenotype of COVID-19-Induced Coagulopathy: Far from Typical Sepsis-Induced Coagulopathy. J. Clin. Med. 2020, 9, 2875. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Aslani, M.; Mortazavi-Jahromi, S.S.; Mirshafiey, A. Cytokine storm in the pathophysiology of COVID-19: Possible functional disturbances of miRNAs. Int Immunopharmacol. 2021, 101, 108172. [Google Scholar] [CrossRef]
- Barberato, S.H.; Bruneto, E.G.; Reis, G.S.; Rauen Franco de Oliveira, L.; Possamai, A.F.; Silvestre, O.; Fernandes Silva, M.M. Abnormal Echocardiographic Findings in Hospitalized Patients with Covid-19: A Systematic Review and Meta-analysis. Arq. Bras. Cardiol. 2022, 119, 267–279. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Van den Heuvel, F.M.A.; Vos, J.L.; Koop, Y.; van Dijk, A.P.J.; Duijnhouwer, A.L.; de Mast, Q.; van de Veerdonk, F.L.; Bosch, F.; Kok, B.; Netea, M.G.; et al. Cardiac function in relation to myocardial injury in hospitalised patients with COVID-19. Neth. Heart J. 2020, 28, 410–417. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, G.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intens. Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Soulat-Dufour, L.; Fauvel, C.; Weizman, O.; Barbe, T.; Pezel, T.; Mika, D.; Cellier, J.; Geneste, L.; Panagides, V.; Marsou, W. Prognostic value of right ventricular dilatation in patients with COVID-19: A multicentre study. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 569–577. [Google Scholar] [CrossRef]
- Zou, F.; Qian, Z.; Wang, Y.; Zhao, Y.; Bai, J. Cardiac Injury and COVID-19: A Systematic Review and Meta-analysis. CJC Open 2020, 2, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Mantefardo, B.; Nega, S.; Ali Chekole, Y.; Basu, B.; Ahmed Ali, S.; Taddesse, M. Global burden of acute myocardial injury associated with COVID-19: A systematic review, meta-analysis, and meta-regression. Ann. Med. Surg. 2021, 68, 102594. [Google Scholar] [CrossRef]
- Changal, K.; Veria, S.; Mack, S.; Paternite, D.; Altaf Sheikh, S.; Patel, M.; Mir, T.; Sheikh, M.; Ramanathan, P.K. Myocardial injury in hospitalized COVID-19 patients: A retrospective study, systematic review, and meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 626. [Google Scholar] [CrossRef]
- Figliozzi, S.; Masci, P.G.; Ahmadi, N.; Tondi, L.; Koutli, E.; Aimo, A.; Stamatelopoulos, K.; Dimopoulos, M.-A.; Caforio, A.L.P.; Georgiopoulos, G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13362. [Google Scholar] [CrossRef]
- Demirkıran, A.; Onar, L.; Doğan, M. Decrease of left ventricular ejection fraction in severe illness patients due to COVID-19 may improve as the disease resolves. Turk. J. Med. Sci. 2021, 51, 2861–2869. [Google Scholar] [CrossRef]
- Salah, H.M.; Fudim, M.; O’Neil, S.T.; Manna, A.; Chute, C.G.; Caughey, M.C. Post-recovery COVID-19 and incident heart failure in the National COVID Cohort Collaborative (N3C) study. Nat. Commun. 2022, 13, 4117. [Google Scholar] [CrossRef]
- Van den Heuvel, F.M.A.; Vos, J.L.; van Bakel, B.; Duijnhouwer, A.L.; van Dijk, A.P.J.; Dimitriu-Leen, A.C.; Koopmans, P.C.; de Mast, Q.; van de Veerdonk, F.L.; Bosch, F.H. Comparison between myocardial function assessed by echocardiography during hospitalization for COVID-19 and at 4 months follow-up. Int. J. Cardiovasc. Imaging 2021, 37, 3459–3467. [Google Scholar] [CrossRef]
- Young, K.A.; Krishna, H.; Jain, V.; Hamza, I.; Scott, C.G.; Pellikka, P.A.; Villarraga, H.R. Serial Left and Right Ventricular Strain Analysis in Patients Recovered from COVID-19. J. Am. Soc. Echocardiogr. 2022, 35, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients Without New-Onset Cardiomyopathy 2010 | Patients with New-Onset Cardiomyopathy 209 | p Value | |
|---|---|---|---|---|
| Patient Characteristics | Age (median [IQR]) | 69.72 [60.30, 78.65] | 70.83 [61.83, 78.22] | 0.423 |
| Male sex | 1127 (56.1%) | 143 (68.4%) | 0.001 | |
| Smoking | 1037 (51.6%) | 111 (53.1%) | 0.730 | |
| BMI (median [IQR]) | 28.82 [24.70, 34.54] | 27.87 [24.00, 34.15] | 0.294 | |
| COPD | 457 (22.7%) | 47 (22.5%) | 1.000 | |
| Malignancy | 450 (22.4%) | 44 (21.1%) | 0.723 | |
| Diabetes | 842 (41.9) | 93 (44.5%) | 0.514 | |
| Hypertension | 1516 (75.4%) | 149 (71.3%) | 0.219 | |
| Coronary artery disease | 735 (36.6%) | 83 (39.7%) | 0.411 | |
| Heart failure | 823 (40.9%) | 98 (46.9%) | 0.113 | |
| Pre-hospitalization EF (mean ± SD) | 58% ± 10% | 55% ± 12% | >0.05 | |
| Atrial fibrillation | 937 (46.6%) | 110 (52.6%) | 0.113 | |
| Chronic kidney disease | 1034 (51.4%) | 126 (60.3%) | 0.018 | |
| End-stage renal disease | 233 (11.6%) | 23 (11.0%) | 0.889 | |
| Obstructive sleep apnea | 730 (36.3%) | 79 (37.8%) | 0.728 | |
| ACEi/ARBs | 1051 (52.3%) | 130 (62.2%) | 0.008 | |
| Beta-blockers | 1380 (68.7%) | 161 (77%) | 0.015 | |
| Statin | 1333 (66.3%) | 151 (72.2%) | 0.098 | |
| Aspirin | 1200 (59.7%) | 136 (65.1%) | 0.151 | |
| In-Hospital Treatment | Supplemental Oxygen | 1559 (77.6%) | 161 (77.0%) | 0.931 |
| Glucocorticoids | 1363 (67.8%) | 134 (64.1%) | 0.314 | |
| Convalescent plasma | 51 (2.5%) | 6 (2.9%) | 0.952 | |
| Therapeutic anticoagulation | 1052 (52.3%) | 126 (60.3%) | 0.034 | |
| Vasopressors | 674 (33.5%) | 79 (37.8%) | 0.245 | |
| ACEi/ARBs | 589 (29.3%) | 85 (40.7%) | 0.001 | |
| Beta-blockers | 1325 (65.9%) | 169 (80.9%) | <0.001 | |
| Inpatient Characteristics | Hospital length of stay (median [IQR]) | 11.00 [6.00, 20.00] | 10.00 [5.00, 18.00] | 0.040 |
| Use of non-invasive ventilation | 819 (40.7%) | 90 (43.1%) | 0.566 | |
| Mechanical ventilation | 585 (29.1%) | 67 (32.1%) | 0.417 | |
| Admission to ICU | 1132 (56.3%) | 123 (58.9%) | 0.529 | |
| ICU length of stay (median [IQR]) | 5.00 [3.00, 12.00] | 5.00 [3.00, 11.00] | 0.298 | |
| Variable | Univariate Analysis OR (95% CI) | p Value | Multivariable Analysis OR (97.5% CI) | p Value |
|---|---|---|---|---|
| Age | 1.02 (1.01–1.02) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Female | 0.96 (0.79–1.16) | <0.001 | 0.94 (0.75–1.18) | 0.58 |
| BMI | 0.99 (0.98–1.00) | <0.001 | 0.99 (0.98–1.01) | 0.34 |
| COPD | 1.34 (1.07–1.66) | <0.001 | 1.17 (0.90–1.51) | 0.24 |
| Chronic kidney disease | 1.24 (1.02–1.50) | <0.001 | 1.11 (0.89–1.39) | 0.35 |
| ACEi/ARBs during admission | 0.60 (0.48–0.75) | <0.001 | 0.83 (0.65–1.07) | 0.15 |
| Beta-blockers during admission | 0.69 (0.57–0.84) | <0.001 | 0.80 (0.61–1.00) | 0.05 |
| New-onset cardiomyopathy | 1.32 (0.97–1.79) | <0.001 | 1.13 (0.78–1.65) | 0.51 |
| Glucocorticoid treatment | 3.01 (2.39–3.84) | <0.001 | 1.99 (1.48–2.69) | <0.001 |
| Hospital length of stay | 0.98 (0.97–0.99) | <0.001 | 0.92 (0.91–0.93) | <0.001 |
| Supplemental oxygen | 3.15 (2.39–4.23) | <0.001 | 1.26 (0.87–1.82) | 0.23 |
| Mechanical ventilation | 4.18 (3.42–5.11) | <0.001 | 2.62 (1.85–3.71) | <0.001 |
| Admission to ICU | 3.62 (2.93–4.51) | <0.001 | 1.96 (1.44–2.65) | <0.001 |
| Vasopressor requirement | 4.77 (3.91–5.83) | <0.001 | 3.40 (2.43–4.74) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Shah, G.; Nair, R.; Rikabi, S.; Seif, M.; Ghimire, B.; Griffin, B.; Khot, U.N. Characteristics and Outcomes of New-Onset Cardiomyopathy in Hospitalized COVID-19 Patients. J. Clin. Med. 2025, 14, 3258. https://doi.org/10.3390/jcm14093258
Kumar S, Shah G, Nair R, Rikabi S, Seif M, Ghimire B, Griffin B, Khot UN. Characteristics and Outcomes of New-Onset Cardiomyopathy in Hospitalized COVID-19 Patients. Journal of Clinical Medicine. 2025; 14(9):3258. https://doi.org/10.3390/jcm14093258
Chicago/Turabian StyleKumar, Sachin, Gautam Shah, Raunak Nair, Sarah Rikabi, Mohannad Seif, Bindesh Ghimire, Brian Griffin, and Umesh N. Khot. 2025. "Characteristics and Outcomes of New-Onset Cardiomyopathy in Hospitalized COVID-19 Patients" Journal of Clinical Medicine 14, no. 9: 3258. https://doi.org/10.3390/jcm14093258
APA StyleKumar, S., Shah, G., Nair, R., Rikabi, S., Seif, M., Ghimire, B., Griffin, B., & Khot, U. N. (2025). Characteristics and Outcomes of New-Onset Cardiomyopathy in Hospitalized COVID-19 Patients. Journal of Clinical Medicine, 14(9), 3258. https://doi.org/10.3390/jcm14093258







