Acute Suppurative and Subacute Thyroiditis: From Diagnosis to Management
Abstract
1. Introduction
2. Methods
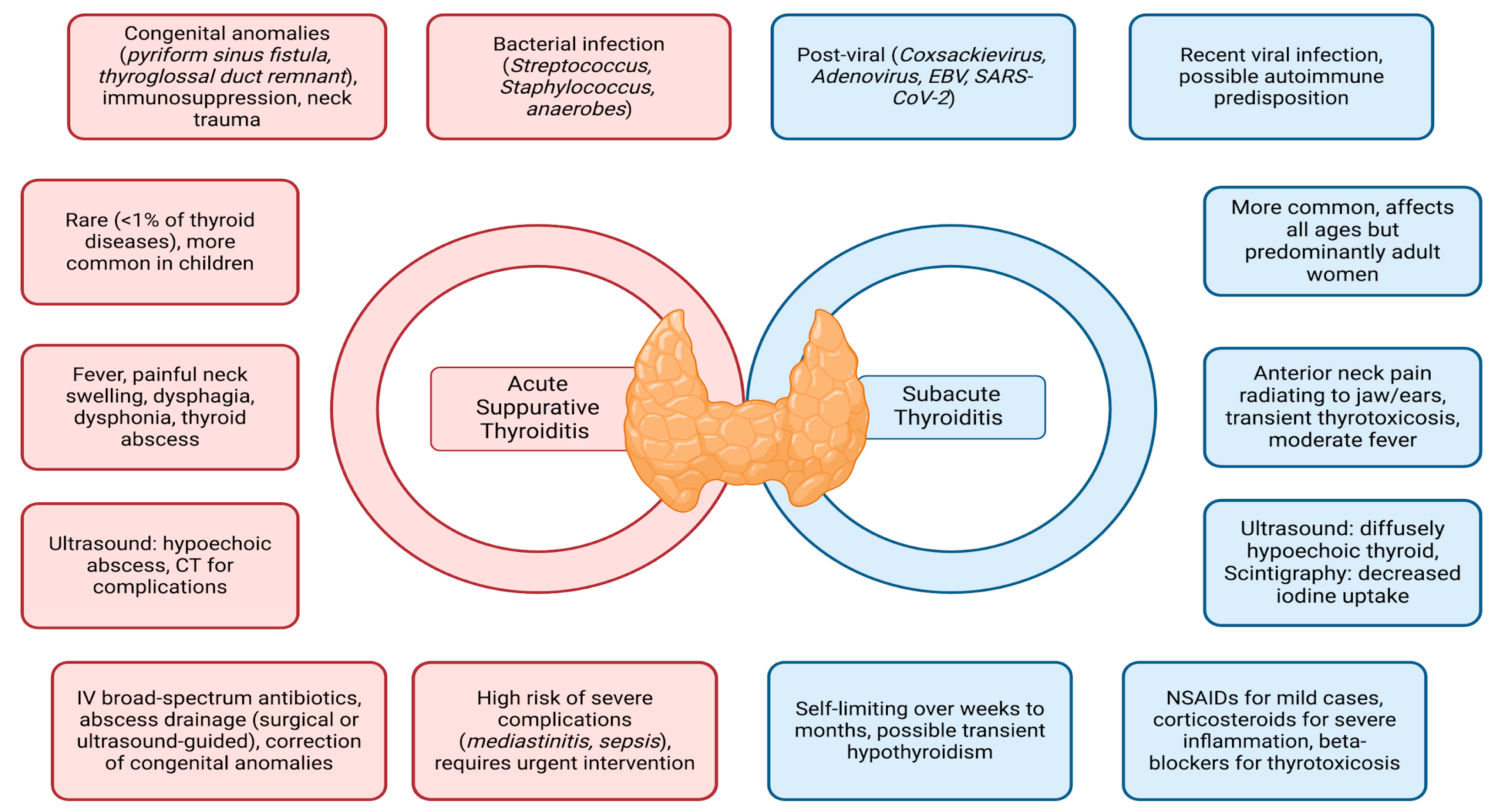
3. Epidemiology and Predisposing Conditions
4. Etiology
Genetic and Epigenetic Considerations
| Category | Infectious Agents | Comments | References |
|---|---|---|---|
| Bacterial | Staphylococcus aureus | The most common bacterial cause: it is often associated with abscess formation. | [34] |
| Streptococcus pyogenes | Can cause severe cases, especially in children. | [21,35] | |
| Streptococcus pneumoniae Escherichia coli | Less common but significant in specific populations. Associated with immunocompromised states or anatomical abnormalities. | [35,36,37] | |
| Klebsiella pneumoniae | Reported in nosocomial infections and immunocompromised patients. | [38] | |
| Salmonella spp. | Rare causes, linked to underlying systemic infections. | ||
| Anaerobic Bacteria | Fusobacterium spp. | Reported in cases associated with dental or oropharyngeal infections. | [39] |
| Bacteroides spp. | They can cause mixed infections with aerobic bacteria. | [40] | |
| Fungal | Candida albicans | Occurs in immunocompromised individuals, such as those undergoing chemotherapy. | [19] |
| Parasitic | Entamoeba histolytica | Extremely rare; reported in endemic areas. | [41] |
| Polymicrobial Infections | Combination of aerobic and anaerobic bacteria | Frequently found in cases with anatomical abnormalities such as pyriform sinus fistula. | [24] |
| Viral | Epstein–Barr virus (EBV) | Rare, usually seen in immunocompromised patients. | [42,43] |
| Cytomegalovirus (CMV) | Similarly to EBV, in cases with compromised immune systems. | [6] |
5. Clinical Presentation
6. Diagnosis
7. Thyroid Function and Management
8. Antibiotic Therapy for Acute Suppurative Thyroiditis (AST) and Treatment of Subacute Thyroiditis
9. Differential Diagnosis
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AST | Acute suppurative thyroidits |
| SAT | Subacute thyroiditis |
| CRP | C-reactive protein |
| ESR | Erythrocyte sedimentation rate |
| IV | Intravenous |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| MRSA | Methicillin-resistan Staphylococcus aureus |
| CMV | Cytomegalovirus |
| EBV | Epstein–Barr Virus |
| HIV | Human Immunodeficiency Virus |
References
- Bilbao, N.A.; Kaulfers, A.-M.D.; Bhowmick, S.K. Subacute Thyroiditis in a Child. AACE Clin. Case Rep. 2019, 5, e184–e186. [Google Scholar] [CrossRef] [PubMed]
- Wongphyat, O.; Mahachoklertwattana, P.; Molagool, S.; Poomthavorn, P. Acute suppurative thyroiditis in young children. J. Paediatr. Child Health 2012, 48, E116–E118. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, N.; Learoyd, D.; Farrell, S.; Wong, R. Suppurative thyroiditis: Systematic review and clinical guidance. Clin. Endocrinol. 2021, 95, 253–264. [Google Scholar] [CrossRef]
- Szabo, S.M.; Allen, D.B. Thyroiditis. Clin. Pediatr. 1989, 28, 171–174. [Google Scholar] [CrossRef]
- Manzano, D.; González, L.; Narváez, L. Acute Suppurative Thyroiditis in a Pediatric Patient: A Case Report. SAS J. Surg. 2023, 9, 618–621. [Google Scholar] [CrossRef]
- Paes, J.E.; Burman, K.D.; Cohen, J.; Franklyn, J.; McHenry, C.R.; Shoham, S.; Kloos, R.T. Acute Bacterial Suppurative Thyroiditis: A Clinical Review and Expert Opinion. Thyroid 2010, 20, 247–255. [Google Scholar] [CrossRef]
- Falhammar, H.; Wallin, G.; Calissendorff, J. Acute suppurative thyroiditis with thyroid abscess in adults: Clinical presentation, treatment and outcomes. BMC Endocr. Disord. 2019, 19, 130. [Google Scholar] [CrossRef]
- Nicoucar, K.; Giger, R.; Pope, H.G.; Jaecklin, T.; Dulguerov, P. Management of congenital fourth branchial arch anomalies: A review and analysis of published cases. J. Pediatr. Surg. 2009, 44, 1432–1439. [Google Scholar] [CrossRef]
- Sai Prasad, T.R.; Chong, C.L.; Mani, A.; Chui, C.H.; Tan, C.E.L.; Tee, W.S.N.; Jacobsen, A.S. Acute suppurative thyroiditis in children secondary to pyriform sinus fistula. Pediatr. Surg. Int. 2007, 23, 779–783. [Google Scholar] [CrossRef]
- Chi, H.; Lee, Y.-J.; Chiu, N.-C.; Huang, F.-Y.; Huang, C.-Y.; Lee, K.-S.; Shih, S.-L.; Shih, B.-F. Acute suppurative thyroiditis in children. Pediatr. Infect. Dis. J. 2002, 21, 384–387. [Google Scholar] [CrossRef]
- She, X.; Zhou, Y.-N.; Guo, J.; Yi, C. Clinical Analysis of Acute Suppurative Thyroiditis in 18 Children. Infect. Drug Resist. 2022, 15, 4471–4477. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Microbiology and management of acute suppurative thyroiditis in children. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 447–451. [Google Scholar] [CrossRef]
- Sheng, Q.; Lv, Z.; Xiao, X.; Zheng, S.; Huang, Y.; Huang, X.; Li, H.; Wu, Y.; Dong, K.; Liu, J. Diagnosis and management of pyriform sinus fistula: Experience in 48 cases. J. Pediatr. Surg. 2014, 49, 455–459. [Google Scholar] [CrossRef]
- Panamonta, O.; Panombualert, S.; Panamonta, M.; Apinives, C. Acute suppurative thyroiditis with thyrotoxicosis. J. Med. Assoc. Thai. 2009, 92, 1370–1373. [Google Scholar]
- Lanzo, N.; Patera, B.; Fazzino, G.; Gallo, D.; Lai, A.; Piantanida, E.; Ippolito, S.; Tanda, M. The Old and the New in Subacute Thyroiditis: An Integrative Review. Endocrines 2022, 3, 391–410. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, R.; Liu, J.Y. Emerging trends and hot spots in subacute thyroiditis research from 2001 to 2022: A bibliometric analysis. Front. Endocrinol. 2023, 14, 1144465. [Google Scholar] [CrossRef]
- Gómez, S.; Mora, C.; López de Suso, D.; Escalada Pellitero, S.; Aragón, P.; Parrón, M.; Bartolomé-Benito, M.; Sanz-Santaeufemia, F.J.; Méndez-Echevarría, A. Acute Suppurative Thyroiditis in Children: Clinical Decision-Making. Pediatr. Infect. Dis. J. 2023, 42, e384–e388. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Gros, D.-A.C.; Gradon, J.D. Thyroid Abscess Due to Acinetobacter calcoaceticus: Case Report and Review of the Causes of and Current Management Strategies for Thyroid Abscesses. South Med. J. 2003, 96, 300–307. [Google Scholar] [CrossRef]
- Niles, D.; Boguniewicz, J.; Shakeel, O.; Margolin, J.; Chelius, D.; Gupta, M.; Paul, D.; King, K.Y.; McNeil, J.C. Candida tropicalis Thyroiditis Presenting With Thyroid Storm in a Pediatric Patient With Acute Lymphocytic Leukemia. Pediatr. Infect. Dis. J. 2019, 38, 1051–1053. [Google Scholar] [CrossRef]
- Yung, B.C.K.; Loke, T.K.L.; Fan, W.C.; Chan, J.C.S. Acute Suppurative Thyroiditis Due to Foreign Body–Induced Retropharyngeal Abscess Presented as Thyrotoxicosis. Clin. Nucl. Med. 2000, 25, 249–252. [Google Scholar] [CrossRef]
- Tien, K.-J.; Chen, T.-C.; Hsieh, M.-C.; Hsu, S.-C.; Hsiao, J.-Y.; Shin, S.-J.; Hsin, S.-C. Acute Suppurative Thyroiditis with Deep Neck Infection: A Case Report. Thyroid 2007, 17, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Yedla, N.; Pirela, D.; Manzano, A.; Tuda, C.; Lo Presti, S. Thyroid Abscess: Challenges in Diagnosis and Management. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618778709. [Google Scholar] [CrossRef]
- Jeng, L.-B.B.; Lin, J.-D.; Chen, M.-F. Acute Suppurative Thyroiditis: A Ten-year Review in a Taiwanese Hospital. Scand. J. Infect. Dis. 1994, 26, 297–300. [Google Scholar] [CrossRef]
- McLaughlin, S.A.; Smith, S.L.; Meek, S.E. Acute Suppurative Thyroiditis Caused by Pasteurella multocida and Associated with Thyrotoxicosis. Thyroid 2006, 16, 307–310. [Google Scholar] [CrossRef]
- Badawy, S.M.; Becktell, K.D.; Muller, W.J.; Schneiderman, J. Aspergillus thyroiditis: First antemortem case diagnosed by fine-needle aspiration culture in a pediatric stem cell transplant patient. Transpl. Infect. Dis. 2015, 17, 868–871. [Google Scholar] [CrossRef]
- Garcia Rueda, J.E.; Monsalve Naranjo, A.D.; Giraldo Benítez, C.; Ramírez Quintero, J.D. Suppurative Thyroiditis: Coinfection by Nocardia spp. and Mycobacterium tuberculosis in an Immunocompromised Patient. Cureus 2024, 16, e65744. [Google Scholar] [CrossRef]
- N’gattia, K.-V.; Kacouchia, N.-B.; Vroh, B.-T.-S.; Kouassi-Ndjeundo, J. Suppurative thyroiditis and HIV infection. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Engkakul, P.; Mahachoklertwattana, P.; Poomthavorn, P. de Quervain thyroiditis in a young boy following hand–foot–mouth disease. Eur. J. Pediatr. 2011, 170, 527–529. [Google Scholar] [CrossRef] [PubMed]
- d’Aniello, F.; Amodeo, M.E.; Grossi, A.; Ubertini, G. Thyroiditis and COVID-19: Focus on pediatric age. A narrative review. J. Endocrinol. Investig. 2024, 47, 1633–1640. [Google Scholar] [CrossRef]
- Brancatella, A.; Ricci, D.; Viola, N.; Sgrò, D.; Santini, F.; Latrofa, F. Subacute Thyroiditis After SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2020, 105, 2367–2370. [Google Scholar] [CrossRef]
- Otani, H.; Notsu, M.; Koike, S.; Morita, M.; Yamamoto, M.; Yamauchi, M.; Fuchiwaki, T.; Morikura, I.; Aoi, N.; Kawauchi, H.; et al. Acute suppurative thyroiditis caused by thyroid papillary carcinoma in the right thyroid lobe of a healthy woman. Thyroid Res. 2018, 11, 4. [Google Scholar] [CrossRef]
- Kalladi Puthanpurayil, S.; Francis, G.L.; Kraft, A.O.; Prasad, U.; Petersson, R.S. Papillary thyroid carcinoma presenting as acute suppurative thyroiditis: A case report and review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Majety, P.; Hennessey, J.V. Acute and Subacute, and Riedel’s Thyroiditis. In Endotext; MDTextCom, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Nishihara, E.; Miyauchi, A.; Matsuzuka, F.; Sasaki, I.; Ohye, H.; Kubota, S.; Fukata, S.; Amino, N.; Kuma, K. Acute Suppurative Thyroiditis After Fine-Needle Aspiration Causing Thyrotoxicosis. Thyroid 2005, 15, 1183–1187. [Google Scholar] [CrossRef]
- Almoffarreh, H.; Alkaabi, N.; Alkhurayji, A. Partially Treated Acute Suppurative Thyroiditis in a Healthy Child Caused by Streptococcus pyogenes. Cureus 2023, 15, e39227. [Google Scholar] [CrossRef] [PubMed]
- Fassih, M.; Moujahid, E.; Abada, R.; Rouadi, S.; Mahtar, M.; Roubal, M.; Essadi, M.; El Kadiri, M.F. Abcès Thyroïdien à Escherichia coli: À Propos d’un cas et Revue de la Littérature [Escherichia coli Thyroid Abscess: Report of a Case and Literature Review]. Pan. Afr. Med. J. 2012, 42. Available online: https://www.ajol.info/index.php/pamj/article/view/81898 (accessed on 4 May 2025). [PubMed] [PubMed Central]
- Adeyemo, A.; Adeosun, A.; Adedapo, K. Unusual cause of thyroid abscess. Afr. Health Sci. 2010, 10, 101–103. [Google Scholar]
- Vengathajalam, S.; Retinasekharan, S.; Mat Lazim, N.; Abdullah, B. Salmonella Thyroid Abscess Treated by Serial Aspiration: Case Report and Literature Review. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 823–826. [Google Scholar] [CrossRef]
- Stavreas, N.P.; Amanatidou, C.D.; Hatzimanolis, E.G.; Legakis, I.; Naoum, G.; Lakka-Papadodima, E.; Georgoulias, G.; Morfou, P.; Tsiodras, S. Thyroid Abscess Due to a Mixed Anaerobic Infection with Fusobacterium mortiferum. J. Clin. Microbiol. 2005, 43, 6202–6204. [Google Scholar] [CrossRef]
- Sun, J.; Chang, H.; Chen, K.; Lin, K.; Lin, J.; Hsueh, C. Anaerobic thyroid abscess from a thyroid cyst after fine-needle aspiration. Head Neck 2002, 24, 84–86. [Google Scholar] [CrossRef]
- Brook, I. Suppurative Thyroiditis in Children and Adolescents. UpToDate. 2024. Available online: https://www.uptodate.com/contents/suppurative-thyroiditis-in-children-and-adolescents#:~:text=Suppurative%20thyroiditis%20(ST)%20is%20caused,threatening%20%5B1%2D3%5D (accessed on 1 March 2025).
- Rafiei, N.; Masoudi, M.; Jadidi, H.; Ghaedi, A.; Jahani, N.; Ebrahimi, S.; Gharei, F.; Amirhoushangi, H.; Bayat, M.; Ansari, A.; et al. The association of subacute thyroiditis with viral diseases: A comprehensive review of literature. Przegl. Epidemiol. 2023, 77, 136–145. [Google Scholar] [CrossRef]
- Frank, T.S.; LiVolsi, V.A.; Connor, A.M. Cytomegalovirus infection of the thyroid in immunocompromised adults. Yale J. Biol. Med. 1987, 60, 1–8. [Google Scholar]
- Giadrosich, R.V.; Hernández, C.M.I.; Izquierdo, Q.C.; Zamora, K.B. Tiroiditis aguda supurada en un paciente pediátrico: Report of a pediatric case. Rev. Med. Chile 2004, 132, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.; Prasad, D.S.; Bal, A.M.; McAteer, D.; Abraham, P. Fatal Descending Necrotizing Mediastinitis Secondary to Acute Suppurative Thyroiditis Developing in an Apparently Healthy Woman. Thyroid 2010, 20, 571–572. [Google Scholar] [CrossRef]
- Lough, D.; Ramadan, H.; Aronoff, S. Acute suppurative thyroiditis in children. Otolaryngol.—Head Neck Surg. 1996, 114, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.R. Serum triiodothyronine, thyroxine, and thyrotropin during hyperthyroid, hypothyroid, and recovery phases of subacute nonsuppurative thyroiditis. Metabolism 1974, 23, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Katsushima, Y.; Fujiwara, I.; Iinuma, K. Subacute Thyroiditis in Children: Patient Report and Review of the Literature. J. Pediatr. Endocrinol. Metab. 2003, 16, 897–900. [Google Scholar] [CrossRef]
- Miyauchi, A.; Tomoda, C.; Uruno, T.; Takamura, Y.; Ito, Y.; Miya, A.; Kobayashi, K.; Matsuzuka, F.; Fukata, S.; Amino, N.; et al. Computed Tomography Scan Under A Trumpet Maneuver to Demonstrate Piriform Sinus Fistulae in Patients with Acute Suppurative Thyroiditis. Thyroid 2005, 15, 1409–1413. [Google Scholar] [CrossRef]
- Masuoka, H.; Miyauchi, A.; Tomoda, C.; Inoue, H.; Takamura, Y.; Ito, Y.; Kobayashi, K.; Miya, A. Imaging Studies in Sixty Patients with Acute Suppurative Thyroiditis. Thyroid 2011, 21, 1075–1080. [Google Scholar] [CrossRef]
- Courtois, M.F.; Colom, N.F.; Rodas, M.A.; Magnanelli, V.; Palmieri, F.; Mirón, L.; Muracciole, B. Tiroiditis aguda supurada en una paciente con fístula del seno piriforme: Presentación de un caso. Arch. Argent Pediatr. 2021, e518–e521. [Google Scholar] [CrossRef]
- Stasiak, M.; Michalak, R.; Lewinski, A. Thyroid primary and metastatic malignant tumours of poor prognosis may mimic subacute thyroiditis—Time to change the diagnostic criteria: Case reports and a review of the literature. BMC Endocr. Disord. 2019, 19, 86. [Google Scholar] [CrossRef]
- Vural, Ç.; Paksoy, N.; Gök, N.D.; Yazal, K. Subacute granulomatous (De Quervain’s) thyroiditis: Fine-needle aspiration cytology and ultrasonographic characteristics of 21 cases. Cytojournal 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- McHan, L.; Gupta, J. Thyrotoxicosis Due to Acute Suppurative Thyroiditis in a Pediatric Patient: A Case Report. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221127841. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, V.; Mezitis, S. A case of acute suppurative thyroiditis complicated by thyrotoxicosis. J. Endocrinol. Investig. 2006, 29, 997–1000. [Google Scholar] [CrossRef]
- Broadney, M.; Senguttuvan, R.; Patel, P.G. Case 3: Anterior Neck Swelling, Fever, and Hypertension in a 3-Year-Old Boy. Pediatr. Rev. 2015, 36, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Onuma, R.; Tonsho, K.; Saito, D.; Sakai, H.; Kamimura, M.; Watanabe, Y.; Kurihara, N.; Ishida, E.; Kumaki, S. Hoarseness as the first symptom in a patient with acute suppurative thyroiditis secondary to a pyriform sinus fistula: A case report. BMC Pediatr. 2023, 23, 273. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, S.; Bhattacharya, B.; Chattopadhay, S. Primary tuberculosis of thyroid gland: A rare case report. Am. J. Otolaryngol. 2007, 28, 267–270. [Google Scholar] [CrossRef]
- McCarty, M.; Coker, R.; Claydon, E. Case report: Disseminated Pneumocystis carinii infection in a patient with the acquired immune deficiency syndrome causing thyroid gland calcification and hypothyroidism. Clin. Radiol. 1992, 45, 209–210. [Google Scholar] [CrossRef]
- Pokhrel, B.; Aiman, W.; Bhusal, K. Thyroid Storm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448095/ (accessed on 1 March 2025).
- De Almeida, R.; Mccalmon, S.; Cabandugama, P.K. Clinical Review and Update on the Management of Thyroid Storm. Mo. Med. 2022, 119, 366–371. [Google Scholar]
- Yamada, H.; Fujita, K.; Tokuriki, T.; Ishida, R. Nine cases of piriform sinus fistula with acute suppurative thyroiditis. Auris Nasus Larynx 2002, 29, 361–365. [Google Scholar] [CrossRef]
- Jee Shee Kim, E.Y. Acute Suppurative Thyroiditis Caused by Methicillin—Resistant Staphylococcus aureus in Healthy Children. J. Korean Soc. Pediatr. Endocrinol. 2011, 16, 128. [Google Scholar] [CrossRef]
- Segni, M.; Turriziani, I.; di Nardo, R.; Pucarelli, I.; Serafinelli, C. Acute Suppurative Thyroiditis Treated Avoiding Invasive Procedures in a Child. J. Clin. Endocrinol. Metab. 2011, 96, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Saklamaz, A. Is There a Drug Effect on the Development of Permanent Hypothyroidism in Subacute Thyroiditis? Acta Endocrinol. 2017, 13, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Yolmo, D.; Madana, J.; Kalaiarasi, R.; Gopalakrishnan, S.; Kiruba Shankar, M.; Krishnapriya, S. Retrospective case review of pyriform sinus fistulae of third branchial arch origin commonly presenting as acute suppurative thyroiditis in children. J. Laryngol. Otol. 2012, 126, 737–742. [Google Scholar] [CrossRef]
- Duan, L.; Feng, X.; Zhang, R.; Tan, X.; Xiang, X.; Shen, R.; Zheng, H. Short-Term Versus 6-Week Prednisone in The Treatment of Subacute Thyroiditis: A Randomized Controlled Trial. Endocr. Pract. 2020, 26, 900–908. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.-P.; Mouton, J.W. Oral amoxicillin and amoxicillin–clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Gonzalez, D.; Melloni, C.; Yogev, R.; Poindexter, B.B.; Mendley, S.R.; Delmore, P.; Sullivan, J.E.; Autmizguine, J.; Lewandowski, A.; Harper, B.; et al. Use of Opportunistic Clinical Data and a Population Pharmacokinetic Model to Support Dosing of Clindamycin for Premature Infants to Adolescents. Clin. Pharmacol. Ther. 2014, 96, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Choonara, I.; Zhang, L.; Xue, S.; Chen, Z.; He, M. Safety of ceftriaxone in paediatrics: A systematic review protocol. BMJ Open 2017, 7, e016273. [Google Scholar] [CrossRef]
- Caloza, D.L.; Semar, R.W.; Bernfeld, G.E. Intravenous Use of Cephradine and Cefazolin Against Serious Infections. Antimicrob. Agents Chemother. 1979, 15, 119–122. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Pramar, Y.V.; Mandal, T.K.; Bostanian, L.A.; Le, G.; Morris, T.C.; Graves, R.A. Physicochemical and Microbiological Stability of Compounded Metronidazole Suspensions in PCCA SuspendIt. Int. J. Pharm. Compd. 2021, 25, 169–175. [Google Scholar]
- Volta, C.; Carano, N.; Street, M.E.; Bernasconi, S. Atypical Subacute Thyroiditis Caused by Epstein-Barr Virus Infection in a Three-Year-Old Girl. Thyroid 2005, 15, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Sanker, V.; Mohamed, A.; Jadhav, C. Acute Suppurative Thyroiditis (AST) with Thyroid Abscess: A Rare and Potentially Fatal Neck Infection. Cureus 2022, 14, e29062. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, R.A. Inflammatory swellings of the head and neck. Surgery 2012, 30, 597–603. [Google Scholar] [CrossRef]
- Smith, S.L.; Pereira, K.D. Suppurative Thyroiditis in Children. Pediatr. Emerg. Care 2008, 24, 764–767. [Google Scholar] [CrossRef]
- da Fonseca, I.F.A.; Avvad, C.K.; Sanchez, E.G.; Henriques, J.L.M.; Leão, L.M.C.S.M. Tireoidite supurativa aguda com múltiplas complicações. Arq. Bras. De Endocrinol. Metabologia 2012, 56, 388–392. [Google Scholar] [CrossRef]
- Imai, C.; Kakihara, T.; Watanabe, A.; Ikarashi, Y.; Hotta, H.; Tanaka, A.; Uchiyama, M. Acute Suppurative Thyroiditis as a Rare Complication of Aggressive Chemotherapy in Children with Acute Myelogeneous Leukemia. Pediatr. Hematol. Oncol. 2002, 19, 247–253. [Google Scholar] [CrossRef]
| Study Types | Articles n | Description |
|---|---|---|
| Case Reports | 34 | Description of clinical cases of AST/SAT, with emphasis on pediatric presentations, rare pathogens, complications as abscess or thyrotoxicosis |
| Case Series/Retrospective Analyses | 16 | Small- to medium-sized cohorts focusing on clinical features, management, imaging, recurrence |
| Systematic/Narrative Reviews | 11 | Comprehensive overviews on pathogenesis, diagnosis, imaging, or therapy (pediatric and general) |
| Original Research Articles | 8 | Prospective studies, cytopathologic analyses, drug dosing, imaging, and pharmacokinetics |
| Guidelines/Clinical Recommendations/Expert Opinions | 6 | Practical management tools, treatment algorithms, or expert statements |
| Letters/Editorials/Conference Notes/Book Sections | 4 | Commentary, update papers, educational or textbook-style summaries |
| Antibiotic | Dosage Range (Pediatric) | Administration Frequency | Coverage | References |
|---|---|---|---|---|
| Amoxicillin-clavulanate | 20–40 mg/kg/day of amoxicillin component | Subdivided; every 8 h | Broad-spectrum (Gram-positive, anaerobes) | [68] |
| Clindamycin | 20–40 mg/kg/day | Subdivided; every 6–8 h | Gram-positive, anaerobes | [69] |
| Ceftriaxone | 50–75 mg/kg/day | Once daily | Broad-spectrum (Gram-negative, Gram-positive) | [70] |
| Cefazolin | 50–100 mg/kg/day | Subivided; every 8 h | Gram-positive | [71] |
| Vancomycin | 40 mg/kg/day | Subdivided every 6–8 h | MRSA, Gram-positive | [72] |
| Metronidazole | 15–30 mg/kg/day | Subdivided; every 8 h | Anaerobes | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toschetti, T.; Parenti, C.; Ricci, I.; Addati, I.; Diona, S.; Esposito, S.; Street, M.E. Acute Suppurative and Subacute Thyroiditis: From Diagnosis to Management. J. Clin. Med. 2025, 14, 3233. https://doi.org/10.3390/jcm14093233
Toschetti T, Parenti C, Ricci I, Addati I, Diona S, Esposito S, Street ME. Acute Suppurative and Subacute Thyroiditis: From Diagnosis to Management. Journal of Clinical Medicine. 2025; 14(9):3233. https://doi.org/10.3390/jcm14093233
Chicago/Turabian StyleToschetti, Tommaso, Cecilia Parenti, Ilaria Ricci, Irene Addati, Sonia Diona, Susanna Esposito, and Maria Elisabeth Street. 2025. "Acute Suppurative and Subacute Thyroiditis: From Diagnosis to Management" Journal of Clinical Medicine 14, no. 9: 3233. https://doi.org/10.3390/jcm14093233
APA StyleToschetti, T., Parenti, C., Ricci, I., Addati, I., Diona, S., Esposito, S., & Street, M. E. (2025). Acute Suppurative and Subacute Thyroiditis: From Diagnosis to Management. Journal of Clinical Medicine, 14(9), 3233. https://doi.org/10.3390/jcm14093233







